Abstract
Objective
To explore strategies for detecting childhood hearing loss, aside from newborn hearing screening.
Design
A retrospective review of medical records on the modes of detection of hearing loss, risk factors for late-onset hearing loss, hearing loss degree, aetiology, additional disabilities, and timelines from referral to intervention.
Study sample
Children, born 2006 to 2015, enrolled for intervention whose hearing loss was detected up to age 7 years but not from newborn hearing screening (n = 326).
Results
Universal pre-school hearing screening detected 38% of the cohort at 4–5 years of age. Risk factors for late-onset hearing loss were present in 36% of children, 80% of whom had a reported family history. Sixty-nine percent had mild bilateral or unilateral hearing loss. Children with additional disabilities faced significantly longer delays from referral to intervention. Children self-referred due to parent concern had more severe degree of hearing loss than those referred from screening.
Conclusion
Most children with hearing loss detected after the newborn period do not have any known risk factors for late-onset hearing loss. Pre-school hearing screening is needed for comprehensive detection of hearing loss in early childhood. More work is needed towards improving timely diagnosis and intervention for children with additional disabilities.
Introduction
Early detection and intervention of hearing loss among newborns and infants is a high priority in most high-income countries. Early detection and intervention programs strive to detect hearing loss by 1 month of age and begin intervention by 6 months of age at the latest, optimally by 3 months of age (Joint Committee on Infant Hearing Citation2019). Early intervention of hearing loss leads to better outcomes for language (Ching et al. Citation2017), education (Tomblin et al. Citation2020), and socioemotional development (Wong et al. Citation2017).
However, not all children with hearing loss are detected by newborn hearing screening. The prevalence of permanent hearing loss of all types and degrees increases from 1 per 1000 by 1 year of age, to 3–4 per 1000 by 9 years of age (Uhlén, Mackey, and Rosenhall Citation2020). The increase in prevalence can be attributed to multiple causes. First, not all childhood hearing loss is present at birth. Some pre- and perinatal factors increase the risk for late-onset or progressive hearing loss. These include congenital infection from cytomegalovirus (cCMV) (Lanzieri et al. Citation2017), extracorporeal membrane oxygenation (ECMO) in infancy (Lasky, Wiorek, and Becker Citation1998) and family history of childhood hearing loss (Dedhia et al. Citation2013). Hearing loss can also be acquired from head trauma, post-natal infection, chronic ear disease, or ototoxic medication. Second, some children with hearing loss at birth may not be detected by newborn hearing screening. For example, the protocols used for newborn hearing screening are typically not sensitive enough to detect some hearing loss less than 40 dB HL (Harrison and Norton Citation1999; Johnson et al. Citation2005). The most common tool for screening, the otoacoustic emissions test, also cannot detect hearing loss caused by auditory neuropathy (Starr et al. Citation1996). Third, universal newborn hearing screening is still not available in many countries. In Sweden, 9% of children are foreign born (Statistics Sweden Citation2023); and in the U.S. this figure is around 3–4% (U.S. Census Bureau, 2017–Citation2022). Some children born outside the country may never have been screened as a newborn. Finally, hearing loss may be missed by newborn hearing screening because of an error by the screening device or screener, or because the family was lost to follow-up after they were referred from screening.
To detect hearing loss after the newborn period, further hearing checks are required. Some studies have advocated for universal hearing screening of pre-school-aged children (Lü et al. Citation2011). However, unlike newborn hearing screening, the benefits of universal pre-school hearing screening have not yet been established (Fortnum et al. Citation2016). Despite the encouragement from the World Health Organisation to initiate pre-school hearing screening programs (World Health Organization Citation2021), there is limited evidence showing its effectiveness (Gumbie et al. Citation2022). In a 2019 survey, only 17 of 45 European countries had a universal pre-school hearing screening program in place (Bussé et al. Citation2021). While some countries have implemented new programs in recent years, others have discontinued or scaled back existing ones.
Another mode of detecting hearing loss after the newborn period is targeted (risk factor) surveillance. For this strategy, risk factors for late-onset hearing loss are logged in a database after newborn hearing screening, and children with identified risk factors for late-onset hearing loss are rescreened at regular intervals (Joint Committee on Infant Hearing Citation2019). A targeted surveillance program may detect children with hearing loss sooner than pre-school hearing screening (Beswick, Driscoll, and Kei Citation2012; Wood, Davis, and Sutton Citation2013). However, as it requires that families return to the audiology clinic for follow-up screening, there is a risk for lost to follow-up. According to reports from Australia and England, follow-up rates to targeted surveillance appointments were only 68% and 55% (Beswick, Driscoll, and Kei Citation2012; Wood, Davis, and Sutton Citation2013). Furthermore, with targeted surveillance alone, children with hearing loss and no identifiable risk factors will go undetected (Lü et al. Citation2011). Like pre-school hearing screening, the use of a targeted surveillance programme varies across countries (Beswick, Driscoll, and Kei Citation2012).
In Region Stockholm, there is a well-established universal pre-school hearing screening program for 4-year-olds embedded in the well-child health program, plus regular checks for speech and language at 6 months, 8 months, 18 months, and 2.5–3 years of age. Depending on the school district, hearing screening may be administered again in school at 6 years of age. There is no formal targeted surveillance program; however, if a clear risk factor is present at or soon after birth (e.g. a craniofacial abnormality or syndrome), children are referred to the audiology clinic from their birth hospital, at which point the family will be called in for regular hearing checks.
The evidence is lacking to determine which mode is optimal to detect children with hearing loss (Beswick, Driscoll, and Kei Citation2012). If targeted surveillance is the primary strategy for detecting postnatal hearing loss, will some children with hearing loss be missed? If pre-school hearing screening is the primary strategy for detecting postnatal hearing loss, will children with hearing loss go undetected for too long?
One subgroup of children with hearing loss who are particularly at risk for later detection are those with additional disabilities (Bonino and Mood Citation2023). This group of children constitute approximately 20–40% of all children with hearing loss (Picard Citation2004). Regardless of hearing screening system, these children often have many other clinical visits of which hearing appointments may not always be prioritised, and the referral pathways and timelines for these children warrant exploration.
Without evidence on the optimal strategy for detecting hearing loss after the newborn period, decision makers cannot make informed choices about how to implement or modify strategies for postnatal hearing loss detection in their country or region. This study used eight years of registry data extracted for all children with hearing loss who are receiving intervention in Region Stockholm. The primary aim of this study was to explore the modes by which childhood hearing loss is detected after the newborn period up to 7 years of age. The secondary aim was to assess the timelines from referral to intervention between children with and without additional disabilities.
Methods
Study group
The study group included children with hearing loss who were born from 1 January 2006 to 31 December 2014 and enrolled for intervention in Region Stockholm by 31 December 2020 at the age of 0–7 years. A total of 257,232 children were born in Region Stockholm during the period from 1 January 2006 to 31 December 2014. Because the purpose of this study was to explore and compare the modes of referral for detection of hearing loss up to 7 years of age, children born after 2014 were not included in the cohort. This was to ensure that all children with hearing loss present from birth to age 7 would be included. Hearing loss was defined by hearing thresholds being ≥25 dB HL at any frequency from 250 to 8000 Hz. The group did not include children with auditory processing disorder and normal hearing thresholds. Children whose hearing loss was detected by newborn hearing screening were also excluded, except for those who were lost to follow-up after screening referral. This study group was ascertained by linking registry data from two registries, AudioHab and AudioScreen (described below), using unique personal identification numbers assigned to each individual living in Sweden.
Data collection
All children enrolled for hearing loss intervention in Region Stockholm are entered into a registry, AudioHab. AudioHab covers the entirety of Region Stockholm. Audiological intervention includes amplification, but it also includes other family-centred care such as access to a special education teacher, counselling, speech-language therapy, sign-language support, or sound-field technology. Any child who could benefit from one of these interventions is enrolled, even if amplification is not provided or eventually returned. Children with hearing loss who are not receiving audiological intervention are not included; this includes, for example families who refuse intervention or children with temporary conductive hearing loss. The AudioHab registry is managed by the Department of Child Hearing Habilitation at Karolinska University Hospital and used for both clinical quality control and research purposes.
Newborn hearing screening data are managed in a database AudioScreen, which stores the result of each screening test for all infants born in Region Stockholm. AudioScreen is managed by the Department of Hearing and Balance at Karolinska University Hospital. It is used by clinical staff to track infants who have not been screened, as well as to perform quality assessments and research.
Audiogram data were extracted from the audiogram database Auditbase (Auditdata A/S). Medical record data were extracted for each child in the study group. The medical record system was accessed by two authorised clinical staff. The data extracted for each child included the modes of referral for detection of hearing loss, the timeline from referral to intervention, type of amplification, the aetiology of the hearing loss, and any identified and documented risk factors for late-onset hearing loss. Data on the presence of the risk factors were extracted based on the list from the Joint Committee on Infant Hearing (Citation2019) of risk factors for late-onset hearing loss. This included family history of hearing loss from childhood, ECMO treatment, hyperbilirubinemia, asphyxia, known congenital infection, craniofacial malformation, or known syndrome associated with hearing loss. Whether or not the infant was admitted to the neonatal intensive care unit (NICU) shortly after birth was also extracted as a variable; however, data were missing regarding the length of NICU stay from hospitals outside the university hospital network, and therefore this variable was removed from analysis. From the AudioHab registry, data were also extracted on whether children have an additional disability.
Analysis
Modes of referral for detection of hearing loss were coded and categorised into the following groups: risk factor follow-up, 6-month check-up, 8-month check-up, 18-month check-up, 3-year check-up, 4-year hearing screening, 6-year hearing screening, new arrival hearing test, neuropediatric referral, family doctor referral, speech-language pathologist referral, ear, nose and throat (ENT) referral, parent/teacher concern for hearing, parent/teacher concern for speech, and other. Some example referrals for “other” included the surgery division due to cleft-lip and palate operation, endocrinology department, or allergen specialist. In many cases, two or more modes of referral were reported for a child. A suspected hearing loss may start at a regular check-up and then proceed through a family doctor or ENT. Therefore, the main mode of referral for hearing loss detection was identified by first determining if hearing loss was suspected at an age-defined check-up (18-month check-up, 4-year hearing screening, etc.). If the referral was not directly associated with a check-up, the location from which the referral was provided (for example, ENT or family doctor) was identified as the main source. For cases where no medical referral source was identified, self-referrals were the main source either because of concern regarding speech and language or concern regarding hearing.
The degree of hearing loss was determined by selecting the first two audiograms on file. These audiograms were taken at the age when hearing loss was diagnosed, and therefore the age of the child varies across the cohort. Standard clinical practice at Karolinska University Hospital is that thresholds for young children are rarely tested below 10 dB HL. As part of the hearing assessment on children, audiologists indicate whether they believe a threshold is reliable or unreliable. In cases where the first audiogram was determined unreliable or if thresholds were missing, the second audiogram was used. In this study, only air-conduction thresholds were assessed and only at one time point. Because of this, some children may have fluctuating degrees of hearing loss which were not considered in the analysis of hearing loss degree. Pure-tone averages were calculated from hearing thresholds at 500, 1000, 2000 and 4000 Hz in each ear. Unilateral hearing loss was defined as normal hearing in one ear (pure-tone average of less than 20 dB HL) and a hearing loss in the opposite ear (pure-tone average of 20 dB HL or more). Hearing loss classifications were based on the World Health Organization (Citation2021) in which mild hearing loss is defined as pure-tone average thresholds from 20 to 34 dB HL, moderate is 35–49 dB HL and moderately severe or worse is from 50 dB HL and worse. The classification of minimal hearing loss was used for cases where pure-tone average was less than 20 dB HL but hearing thresholds were over 20 dB HL for one or more frequencies.
Descriptive statistics were performed in SPSS version 28 to explore the modes of hearing loss detection, ages of referral, risk factors, and degree of hearing loss. Pearson correlations, Fisher exact tests, Kruskal–Willis and Mann–Whitney U tests were performed. p-Values were tested against an alpha of 0.05 but adjusted using the Bonferroni correction for multiple comparisons.
Results
Study cohort
From the AudioHab registry, 899 children born from 1 January 2006 to 31 December 2014 were enrolled for intervention by December 2020 and by age 7 years. Of these, 204 had passed newborn hearing screening in both ears; 70 moved into Region Stockholm and did not have a known hearing loss at intake; and 52 had failed newborn hearing screening but were discharged due to normal auditory brainstem responses or were lost to follow-up. Because of technicalities in the registry, it is impossible to separate these two groups. In total, 326 of the 899 children were included in the final cohort.
The cohort (n = 326) was made up of 44 children born in 2006, 35 in 2007, 39 in 2008, 33 in 2009, 31 in 2010, 35 in 2011, 41 in 2012, 37 in 2013 and 30 in 2014. Females made up 49% (n = 159) of the cohort. A total of 56 children (17%) were foreign born, of which five had been screened as a newborn, 13 children were not screened as a newborn, and no information was available for the remaining 38.
Main mode of referral for hearing loss detection
As described, many children had multiple reasons for a referral documented in the medical records; for example, they may have been referred from the 4-year-old hearing screening and parental concern was expressed with regards to hearing. For each child, a main mode of referral was selected. Details on how the main mode of referral was determined are described in the methods. illustrates the main mode of referral for all 326 children across age of referral to the audiology clinic.
Figure 1. Cumulative frequency of children whose hearing loss was detected through each mode or referral across the age of referral to the audiology clinic, rounded to the nearest half-year. SLP: speech-language pathologist; ENT: ear, nose and throat physician.
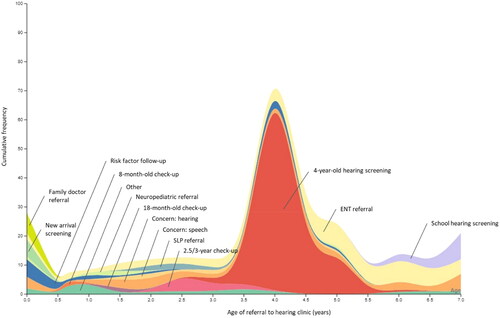
Four-year old hearing screening was the most common main mode of referral for detecting hearing loss after newborn hearing screening, comprising 38% of the cohort (n = 124). An additional 20% (n = 62) were referred to the audiology clinic through an ENT referral, and 11% (n = 35) were self-referred for concern regarding hearing. Ten children who were referred via ENT had chronic middle ear problems, such as stapes fixation or chronic otitis media; most referrals from ENT had an unknown cause of hearing loss. Other less prevalent main modes of referral included risk factor follow-up (n = 13) and school screening (n = 14). Eight children were referred directly to the audiology clinic from the 2.5-to-3-year health care check-up for speech and language development, an additional five children were referred from a speech-language pathologist, and six were self-referred by the family to the audiology clinic because of concern regarding speech or language development. Of the 56 children who were foreign born, the most common mode for referral was pre-school hearing screening (n = 16), followed by family concern (n = 9), ENT referral (n = 8) and new arrival health check-up (n = 5). No children from this subgroup were referred due to risk factors.
Note that these are distributions for the main mode or referral. Many children had multiple modes of referral (e.g. screening and parent concern). To see an overview of the total number of children referred from all applicable modes, see .
Table 1. The number and percentage of children with various risk factors for late-onset hearing loss who were referred to the audiology clinic by various modes.
Hearing loss, aetiology, additional disabilities, and amplification
Of 326 children, 200 had bilateral hearing loss, pure-tone averages for the better ear were mild for 54%, moderate for 23%, moderately severe or worse for 15%, and minimal for 5%. The remaining 3% had missing or incomplete threshold data which made it not possible to compute pure-tone averages. Thirty-six percent of the cohort (n = 117) had unilateral hearing loss and the remaining 81 had missing threshold data. For children with unilateral hearing loss, pure-tone averages in the worse ear were mild for 38% of children, moderate for 17% and moderately severe or worse for 45%.
The age at which children were referred to the audiology clinic was correlated to the severity of hearing loss for children with bilateral hearing loss. Children who were referred to the audiology clinic at an earlier age showed poorer hearing thresholds (pure-tone averages) for both the better ear, r(191)= −0.329, p < 0.001, and for the worse ear, r(191)= −0.334, p < 0.001. Observe, however, that the audiogram data are taken at the age of hearing loss diagnosis. Similar trends were not observed for children with unilateral hearing loss. There were no differences in age of referral between sex (p = 0.92).
The aetiology of the hearing loss was not available in the case files for 64% of children. For mild bilateral or unilateral hearing loss investigation of aetiology is not routinely offered to parents. It may also be that the parents were not interested in seeking the cause, or because no cause was found after investigation. Twelve different syndromes accounted for the hearing loss in 10% of the study group, including 10 children (3%) with large vestibular aqueduct syndrome. Middle ear malformation or stapes fixation was present in 6%, and cCMV was determined to be the cause of hearing loss for 3% of the study group. Other less prevalent causes of hearing loss included chronic middle ear disease, cholesteatoma, other cochlear malformations, heredity, herpes virus, auditory neuropathy, meningitis, ototoxicity, and trauma.
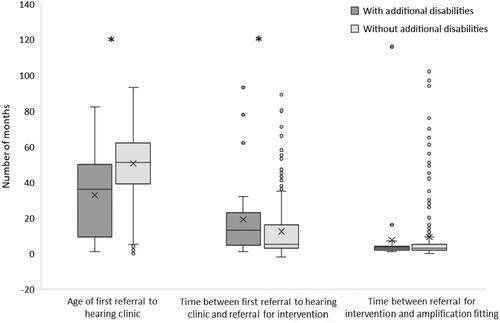
Additional disabilities were, for example, Down’s Syndrome, autism, or other non-specific developmental disabilities. In the registry data, only the presence or absence of additional disabilities (yes/no) was available, not the specific diagnosis. Of the 326 children in the cohort, 33 had additional disabilities, 274 children had no additional disabilities, and data were missing for the remaining 19. Because of the highly skewed data, Mann-Whitney U tests were performed, and medians are reported. shows that children with additional disabilities were referred to the audiology clinic at a significantly younger age (median 3.0 years or 36 months) than children without additional disabilities (median 4.2 years or 51 months; p < 0.001). Despite the earlier age at referral, it took a significantly longer time to confirm a hearing loss and start intervention among children with additional disabilities (median 9.7 months), compared to children without additional disabilities (median 3.3 months; p = 0.007). Once a diagnosis and decision were made on their hearing loss, the time to the hearing aid fitting appointment was similar between groups (median 2.7 months for both groups), despite a lot of individual variability.
Figure 2. Box plots showing the quadrants, medians, means (X), and range for the age and time delays (in months) from detection to intervention for children with hearing loss, with and without additional disabilities, whose hearing loss was detected after newborn hearing screening. Single points show the skewed distribution and wide variability in time delays from referral to intervention. *Significant difference at p < 0.01.
As described, most of the children whose hearing loss was detected after neonatal hearing screening had mild bilateral hearing loss or unilateral hearing loss. The majority of children in the study group received amplification, although the time from habilitation enrolment to amplification fitting varied greatly (). Of the children with unilateral hearing loss, 86% were fitted with amplification. Of the children with bilateral hearing loss, 96% were fitted, including 93% of children with a mild hearing loss in both ears. Among the children whose hearing loss was detected by the 4-year-old screening, 90% were fitted with hearing aids. Data were not collected on the percentage of children who eventually returned their amplification devices, nor the amount of hearing aid usage for these subgroups of children.
Hearing loss and mode of referral
Pure-tone averages were compared across the main mode or referral. Some groups had very low sample sizes and were excluded from this analysis. These included 6-, 8- and 18-month check-up, new arrival screening, and neuropediatric referral. It was also necessary to collapse the main mode or referral into fewer groups. To assess referrals due to language concerns, the referrals from the language screening at 3-years-old was combined with referrals from a speech language pathologist and self-referrals due to parent/teacher language concern. To assess referrals from a physician, ENT and family doctor referrals were combined into one group. As specified earlier, referrals from, for example, endocrinologist, plastic surgeon, or allergy specialist were classified as “other”. A total of 297 children remained in the analysis.
Unilateral versus bilateral hearing loss was compared across the collapsed seven main modes of referral using a Fisher exact test. Hearing loss data were missing for seven of the 297 children. A significant difference in distributions were shown (p = 0.014). The categories showing significant differences (p ≤ 0.05) between bilateral and unilateral hearing loss were for the categories of pre-school hearing screening and other. Specifically, of the 112 children with unilateral hearing loss, 55% (n = 61) were detected by pre-school hearing screening, compared to 35% (n = 62) of the 179 children with bilateral hearing loss. Fifteen children with bilateral hearing loss were referred within the category “Other” compared to only two children with unilateral hearing loss. As indicated, “other” are referrals from department of surgery, endocrinology, or allergy.
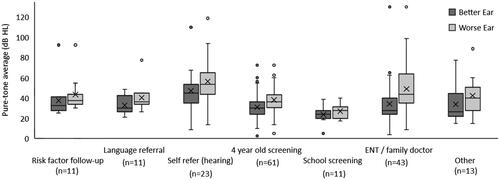
To compare hearing thresholds across main mode of referral, Kruskal Wallis tests were performed, as assumptions of normality were violated for ANOVA. For children with bilateral hearing loss, illustrates how pure-tone averages in the better and worse ear differed across each main mode of referral. The rank differences were significant across groups for both the pure-tone average in the worse ear, H(6, n = 173) = 30.6, p < 0.001, and the better ear, H(6, n = 173) = 22.4, p = 0.001. For the better ear, significant differences in pure-tone average rank were observed between the group of children who were self-referred because of family/teacher concern regarding hearing (median= 45 dB HL, n = 23) and children referred from their ENT or family doctor median= 27.5 dB HL, n = 43; p = 0.022), children referred from school screening (median = 23.7 dB HL, n = 11; p = 0.001), and children referred from the 4-year-old hearing screening (median= 30 dB HL, n = 61; p = 0.005). Similarly, for the worse ear, pure-tone average rank was significantly higher for children whose families self-referred because of concern regarding hearing (median= 53.7 dB HL, n = 23), compared to children referred from their 4-year-old hearing screening (median= 36.2 dB HL, n = 61; p = 0.004) and from school screening (median = 27.5 dB HL, n = 11; p < 0.001). For children with unilateral hearing loss, no significant differences were found between those who were self-referred and those referred from screening when comparing the pure-tone average of the worse ear.
Risk factors
A total of 118 children in the cohort (36%) showed evidence of one or more risk factors for late-onset hearing loss upon intake to the audiology clinic. There were also other documented causes for acquired hearing loss such as ototoxicity and meningitis among children in our sample, which are known risk factors for acquired hearing loss. Meningitis was identified as a cause of hearing loss for four children and ototoxicity for six children. The remaining 61% of the cohort had no identifiable risk factor for hearing loss. No differences in the proportion of children with or without risk factors were observed between sex (p = 0.36). It is important to note that a syndrome or congenital infection can only be classified as a risk factor if it is known prior to hearing loss diagnosis. For example, children with hearing loss caused by congenital CMV did not have congenital CMV as a risk factor if it was first discovered after the hearing loss was identified.
There were no differences in the degree of hearing loss across risk factors for late-onset hearing loss, nor were there differences in the percentage of bilateral versus unilateral hearing loss. For all risk factors, a mild degree in the better ear was most prevalent for bilateral hearing loss. On average, children with at least one risk factor for late-onset hearing loss were referred to the audiology clinic by 3.6 years of age (SD: 2.0) which is significantly earlier than the mean of 4.2 years (SD: 1.8) for children without any risk factors for late-onset hearing loss, t(181)= −2.8, p = 0.005.
Targeted surveillance vs 4-year-old hearing screening
Risk factors for this study were ascertained through medical records after children were identified with hearing loss. shows the distribution of risk factors for late-onset hearing loss across the mode of referral for suspected hearing loss. Among the 124 children with hearing loss who were referred to the audiology clinic from the 4-year-old hearing screening, 40 children had at least one risk factor for late-onset hearing loss, 32 of whom had a reported family member with hearing loss from childhood. Eighty-four (68%) had no reported risk factor for late-onset hearing loss at the time that the hearing loss was diagnosed. This may be because children did not have any risk factors, or because the families are not aware of risk factors that may be present prior to the hearing loss identification.
Summary of results
This study retrospectively analysed the audiological and medical record data of a cohort of 326 children with hearing loss who were enrolled for intervention by 7 years of age and whose hearing loss had not been detected through newborn hearing screening. The most common mode of referral was from universal pre-school hearing screening, which detected 124 children with hearing loss. The most common risk factor for late-onset hearing loss among the children detected after newborn hearing screening was family history of hearing loss from childhood. Most children, however, had no documented risk factors for late-onset hearing loss. Children with multiple disabilities made up 10% of the cohort, and these children were faced with a longer delay between initial referral and enrolment for intervention compared to children without additional disabilities. The degree of hearing loss among children in the study group was mostly mild bilateral and unilateral hearing loss. Finally, only a small percentage of parents expressed concern regarding their children’s hearing, and this concern was related to the severity of the child’s hearing loss.
Discussion
Results from this study suggest that pre-school hearing screening is necessary to detect hearing loss in young children after newborn hearing screening. Among the children whose hearing loss was detected by pre-school hearing screening, 68% had no documented risk factors for late-onset hearing loss, according to the patient file. Had no screening been performed, most of these children would have likely entered school with undetected hearing loss. Based on the number of children without reported risk factors in our cohort, a targeted surveillance program alone would not be sufficient for detecting all children with hearing loss.
Findings from this study can be compared to recent studies from England (Wilding et al. Citation2023) and Australia (Fitzgibbons et al. Citation2023). In both of these studies, the targeted surveillance program was assessed. Wilding et al. (Citation2023), with a 6-year cohort of almost 4 million children, found that targeted hearing surveillance among children with risk factors detected only 12% of hearing loss among children up to age 5 who were not referred from newborn hearing screening (Wilding et al. Citation2023). Fitzgibbons et al. (Citation2023) reported that targeted surveillance was the mode of detection for about half of their cohort of 385 children with hearing loss. Only 19 children were referred from pre-school and school hearing screening. However, this result was not surprising given the fragmented coverage of their hearing screening opportunities. Both studies concluded that targeted surveillance is not sufficient for detecting all children with hearing loss (Fitzgibbons et al. Citation2023; Wilding et al. Citation2023).
Another advantage of universal pre-school hearing screening is that it does not exclude children who are adopted or foreign-born, who may not have their birth history documented in the local medical record system. Only 14 additional children with hearing loss were detected from school screening, which takes place 2–3 years after universal pre-school hearing screening. This speaks to the potential effectiveness of pre-school hearing screening. Coverage rates of pre-school hearing screening are estimated to be 95–99%, though data are not available on the coverage rate of the school hearing screening, the referral rate from pre-school or school screening, nor the loss to follow-up to the hearing clinic. Therefore, it is not possible to speculate about the sensitivity or specificity from screening nor its cost-effectiveness.
A choice between pre-school hearing screening and targeted risk factor surveillance may be a choice between detecting all hearing loss versus detecting those with risk factors earlier. Despite the high number of children with hearing loss detected by pre-school hearing screening in Region Stockholm, the average age of detection for children with risk factors was 3.6 years. There is no standardised protocol in place in Region Stockholm for regular hearing loss surveillance due to risk factors. Referral for surveillance is at the discretion of the hearing clinic and paediatrician. In a study from Queensland, Australia with a structured risk factor surveillance program, the average age of detection for children with risk factors was 2.7 years (Fitzgibbons et al. Citation2023). Therefore, while pre-school hearing screening may detect more hearing loss, a targeted surveillance program may reduce the length of time children with hearing loss go without intervention.
Most of children who were detected by pre-school hearing screening had mild bilateral or unilateral hearing loss, and it may be questioned what impact the detection of their hearing loss ultimately had on their language, academic and socioemotional development. The results of this study show the wide variability in time from detection through to amplification, suggesting a clinical uncertainty in how the hearing loss for these children should be managed. More evidence is becoming available on the importance of intervention for children with mild hearing loss (Walker et al. Citation2015). While the benefits of amplification for unilateral hearing loss are still uncertain (Johansson, Asp, and Berninger Citation2020), it is important to recognise that family centred intervention for hearing loss extends beyond personal amplification devices. Early intervention also includes guiding parents and other important caregivers how to help the child develop speech and language skills and strategies that promotes communication in challenging listening situations, both at home, in pre/school and other activities. Therefore, despite the lack of empirical evidence, it seems clinically justified to offer children with mild and unilateral hearing loss the help they may need before they enter the challenging listening environment of a primary-school classroom (McPherson et al. Citation2022).
Only 11% of children self-referred to the hearing clinic because of parent or teacher concern. Although low, this result is consistent with previous studies (Swierniak et al. Citation2021), and shows that the level of awareness regarding the signs of childhood hearing loss is quite poor, or that parents may depend on childhood hearing screening programs to detect any hearing loss if present. For children with mild or unilateral hearing loss, parents may not disassociate the behavioural nuances of a hearing loss with other typical behavioural variations of a toddler (Fitzpatrick et al. Citation2016). Despite the poor capacity for parents to suspect hearing loss, it has been shown that those who are suspicious are highly accurate in their assessment (Thompson and Thompson Citation1991). Therefore, we cannot rely on parental suspicion alone for the detection of hearing loss after newborn hearing screening, though parents that do express concern should be taken very seriously.
Furthermore, this study shows that children with hearing loss who also have additional needs have a longer delay for starting their intervention after being referred. Similarly, Fitzpatrick et al. (Citation2017) showed that additional medical or developmental diagnoses was the cause for delayed hearing loss identification in over 70% of children who had more than a three month delay in receiving a diagnosis after referral. In general, children with multiple disabilities require more clinical visits, and often need to reschedule appointments due to other medical priorities, which may postpone the timepoint of hearing loss detection. Balancing pressure for early intervention with the complex needs of children with multiple disabilities is an issue that requires further investigation. Individuals with complex needs and their families can provide insightful perspectives on this matter and should be included in the development of practice guidelines to better meet the needs for this target group.
This study was a retrospective analysis of medical record data, which has drawbacks. Data collection relies on the interdisciplinary team of nurses, audiologists and physicians to collect and record information on the case and medical history of children seen in the audiology clinic. The clinical routine in Region Stockholm places weight on the accurate and thorough medical record reporting. There is always a risk of human error in documentation, or a lack of thorough case history, which would have impacted the results of the study. However, this issue even highlights another difficulty for targeted surveillance programs: achieving complete documentation for all risk factors, or even coming to a consensus on which risk factors are relevant to report.
In conclusion, based on the data from our cohort, universal pre-school hearing screening is the recommended strategy for detecting all children with hearing loss at age 4 years. A targeted surveillance program that relies on medical records and parent reporting is only effective at identifying a small portion of children with hearing loss, although earlier detection for this small group may be possible. In the cohort studied, a quarter of the children whose hearing loss was detected by pre-school hearing screening had a family history of hearing loss. If primary health care providers are aware of this risk and monitor changes in hearing more carefully, hearing loss may be detected earlier. As hearing is one of our most important senses affecting communication at various levels in all children, the importance of both early and comprehensive detection of hearing loss is vital, with future attention specific to those having additional disabilities and those with mild bilateral and unilateral hearing loss.
Ethical approval
Ethical approval for this study was granted by the Swedish Ethical Review Authority (2020-07302). Due to the nature of the registry-based study design, informed consent was waived by the ethical authority.
Acknowledgements
We thank Johanna Hjort and Martin Eklöf for support with extracting journal data.
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Additional information
Funding
References
- Beswick, R., C. Driscoll, and J. Kei. 2012. “Monitoring for Postnatal Hearing Loss Using Risk Factors: A Systematic Literature Review.” Ear and Hearing 33 (6): 745–756. https://doi.org/10.1097/AUD.0b013e31825b1cd9.
- Beswick, R., C. Driscoll, J. Kei, and S. Glennon. 2012. “Targeted Surveillance for Postnatal Hearing Loss: A Program Evaluation.” International Journal of Pediatric Otorhinolaryngology 76 (7): 1046–1056. https://doi.org/10.1016/j.ijporl.2012.04.004.
- Bonino, A. Y., and D. Mood. 2023. “Identifying Reduced Hearing in Children Who Have Developmental Disabilities: Insights for Inclusive Research Practices with Electronic Health Records.” Frontiers in Psychology 14:1134034. https://doi.org/10.3389/fpsyg.2023.1134034.
- Bussé, A. M. L., A. R. Mackey, G. Carr, H. L. J. Hoeve, I. M. Uhlén, A. Goedegebure, and H. J. Simonsz, EUS€REEN Foundation. 2021. “Assessment of Hearing Screening Programmes Across 47 Countries or Regions III: Provision of Childhood Hearing Screening After the Newborn Period.” International Journal of Audiology 60 (11): 841–848. https://doi.org/10.1080/14992027.2021.1897170.
- Ching, T. Y. C., H. Dillon, L. Button, M. Seeto, P. Van Buynder, V. Marnane, L. Cupples, and G. Leigh. 2017. “Age at Intervention for Permanent Hearing Loss and 5-Year Language Outcomes.” Pediatrics 140 (3): e20164274. https://doi.org/10.1542/peds.2016-4274.
- Dedhia, K., D. Kitsko, D. Sabo, and D. H. Chi. 2013. “Children with Sensorineural Hearing Loss After Passing the Newborn Hearing Screen.” JAMA Otolaryngology–Head Neck Surgery 139 (2): 119–123. https://doi.org/10.1001/jamaoto.2013.1229.
- Fitzgibbons, E. J., S. Keszegi, C. Driscoll, and R. Beswick. 2023. “Childhood Hearing Loss Detected Beyond the Newborn Screen.” International Journal of Audiology 62 (3): 278–285. https://doi.org/10.1080/14992027.2022.2042606.
- Fitzpatrick, E., V. Grandpierre, A. Durieux-Smith, I. Gaboury, D. Coyle, E. Na, and N. Sallam. 2016. “Children With Mild Bilateral and Unilateral Hearing Loss: Parents’ Reflections on Experiences and Outcomes.” Journal of Deaf Studies and Deaf Education 21 (1): 34–43. https://doi.org/10.1093/deafed/env047.
- Fitzpatrick, E. M., J. C. dos Santos, V. Grandpierre, and J. Whittingham. 2017. “Exploring Reasons for Late Identification of Children with Early-Onset Hearing Loss.” International Journal of Pediatric Otorhinolaryngology 100: 160–167. https://doi.org/10.1016/j.ijporl.2017.06.039.
- Fortnum, H., O. C. Ukoumunne, C. Hyde, R. S. Taylor, M. Ozolins, S. Errington, Z. Zhelev, et al. 2016. “A Programme of Studies Including Assessment of Diagnostic Accuracy of School Hearing Screening Tests and a Cost-Effectiveness Model of School Entry Hearing Screening Programmes.” Health Technology Assessment 20 (36): 1–178. https://doi.org/10.3310/hta20360.
- Gumbie, M., B. Parkinson, H. Dillon, R. Bowman, R. Song, and H. Cutler. 2022. “Cost-Effectiveness of Screening Preschool Children for Hearing Loss in Australia.” Ear and Hearing 43 (3): 1067–1078. https://doi.org/10.1097/AUD.0000000000001134.
- Harrison, W. A., and S. J. Norton. 1999. “Characteristics of Transient Evoked Otoacoustic Emissions in Normal-Hearing and Hearing-Impaired Children.” Ear and Hearing 20 (1): 75–86. https://doi.org/10.1097/00003446-199902000-00007.
- Johansson, M., F. Asp, and E. Berninger. 2020. “Children With Congenital Unilateral Sensorineural Hearing Loss: Effects of Late Hearing Aid Amplification—A Pilot Study.” Ear and Hearing 41 (1): 55–66. https://doi.org/10.1097/AUD.0000000000000730.
- Johnson, J. L., K. R. White, J. E. Widen, J. S. Gravel, M. James, T. Kennalley, A. B. Maxon, L. Spivak, M. Sullivan-Mahoney, B. R. Vohr, et al. 2005. “A Multicenter Evaluation of How Many Infants With Permanent Hearing Loss Pass a Two-Stage Otoacoustic Emissions/Automated Auditory Brainstem Response Newborn Hearing Screening Protocol.” Pediatrics 116 (3): 663–672. https://doi.org/10.1542/peds.2004-1688.
- Joint Committee on Infant Hearing. 2019. “Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs.” The Journal of Early Hearing Detection and Intervention, 4 (2): 1–44.
- Lanzieri, T. M., W. Chung, M. Flores, P. Blum, A. C. Caviness, S. R. Bialek, S. D. Grosse, J. A. Miller, and G. Demmler-Harrison, Congenital Cytomegalovirus Longitudinal Study Group. 2017. “Hearing Loss in Children with Asymptomatic Congenital Cytomegalovirus Infection.” Pediatrics 139 (3): e20162610. https://doi.org/10.1542/peds.2016-2610.
- Lasky, R. E., L. Wiorek, and T. R. Becker. 1998. “Hearing Loss in Survivors of Neonatal Extracorporeal Membrane Oxygenation (ECMO) Therapy and High-Frequency Oscillatory (HFO) Therapy.” Journ of the American Academy of Audiology 9: 47–58.
- Lü, J., Z. Huang, T. Yang, Y. Li, L. Mei, M. Xiang, Y. Chai, et al. 2011. “Screening for Delayed-Onset Hearing Loss in Preschool Children Who Previously Passed the Newborn Hearing Screening.” International Journal of Pediatric Otorhinolaryngology 75 (8): 1045–1049. https://doi.org/10.1016/j.ijporl.2011.05.022.
- McPherson, A., G. Webb, C. Miniscalco, and T. Flynn. 2022. “Language and Quality of Life in Swedish Children With Mild Hearing Loss.” Deafness & Education International 25 (4): 309–326. https://doi.org/10.1080/14643154.2022.2158522.
- Picard, M. 2004. “Children with Permanent Hearing Loss and Associated Disabilities: Revisiting Current Epidemiological Data and Causes of Deafness.” Volta Review 104 (4): 221–236.
- Starr, A., T. W. Picton, Y. Sininger, L. J. Hood, and C. I. J. B. Berlin. 1996. “Auditory Neuropathy.” Brain 119 (3): 741–753.
- Statistics Sweden. 2023. “Children and Young Persons Aged 0–21 with Swedish and Foreign Background by Sex and Age. Year 2002–2022.” Retrieved Sept 12 from https://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__LE__LE0102__LE0102A/LE0102T15/.
- Swierniak, W., E. Gos, P. H. Skarzynski, N. Czajka, and H. Skarzynski. 2021. “The Accuracy of Parental Suspicion of Hearing Loss in Children.” International Journal of Pediatric Otorhinolaryngology 141: 110552. https://doi.org/10.1016/j.ijporl.2020.110552.
- Thompson, M. D., and G. Thompson. 1991. “Early Identification of Hearing Loss: Listen to Parents.” Clinical Pediatrics 30 (2): 77–80. https://doi.org/10.1177/000992289103000202.
- Tomblin, J. B., J. Oleson, S. E. Ambrose, E. A. Walker, and M. P. Moeller. 2020. “Early Literacy Predictors and Second-Grade Outcomes in Children Who Are Hard of Hearing.” Child Development 91 (1): e179–e197. https://doi.org/10.1111/cdev.13158.
- U.S. Census Bureau. 2017–2022. American Community Survey 5-Year Estimates. Table S0901: Children Characteristics. Accessed 16 June, 2024. https://data.census.gov/table/ACSST5Y2022.S0901?q=S0901.
- Uhlén, I. M., A. R. Mackey, and U. Rosenhall. 2020. “Prevalence of Childhood Hearing Impairment in the County of Stockholm – a 40-Year Perspective from Sweden and Other High Income Countries.” International Journal of Audiology 59 (11): 866–873. https://doi.org/10.1080/14992027.2020.1776405.
- Walker, E. A., L. Holte, R. W. McCreery, M. Spratford, T. Page, and M. P. Moeller. 2015. “The Influence of Hearing Aid Use on Outcomes of Children With Mild Hearing Loss.” Journal of Speech, Language, and Hearing Research 58 (5): 1611–1625. https://doi.org/10.1044/2015_JSLHR-H-15-0043.
- Wilding, M. R., J. S. R. Hibbert, J. A. Tucker, C. E. Magee, C. Bauer-Staeb, and S. A. Wood. 2023. “Prevalence of Moderate or Greater Permanent Childhood Hearing Impairment and Effectiveness of Targeted Surveillance for Babies Who Pass Newborn Hearing Screening.” International Journal of Audiology:(published online ahead of print). https://doi.org/10.1080/14992027.2023.2227763.
- Wong, C. L., T. Y. C. Ching, L. Cupples, L. Button, G. Leigh, V. Marnane, J. Whitfield, M. Gunnourie, and L. Martin. 2017. “Psychosocial Development in 5-Year-Old Children With Hearing Loss Using Hearing Aids or Cochlear Implants.” Trends in Hearing 21: 2331216517710373. https://doi.org/10.1177/2331216517710373.
- Wood, S. A., A. C. Davis, and G. J. Sutton. 2013. “Effectiveness of Targeted Surveillance to Identify Moderate to Profound Permanent Childhood Hearing Impairment in Babies with Risk Factors Who Pass Newborn Screening.” International Journal of Audiology 52 (6): 394–399. https://doi.org/10.3109/14992027.2013.769067.
- World Health Organization. 2021. World report on hearing. Geneva, Switzerland: World Health Organization.