Abstract
Objective
To evaluate the short- and long-term effect of remote synchronous fine-tuning and follow-up visits on hearing-related problems and hearing aid (HA) benefits for first-time HA users.
Design
A randomised controlled trial.
Study sample
Patients at public tax-funded HA clinics in Sweden due for aural rehabilitation (AR) were randomised to either an intervention group (n = 33) or a control group (n = 36). Both groups completed the conventional AR process, but the intervention group received synchronous remote fine-tuning of HAs and online follow-up visits. Outcome measures were used before and after intervention, and 6 months and 1 year after intervention.
Results
Both groups improved hearing-related problems measured with the Hearing Handicap Inventory for the Elderly/Adults over time, and no significant differences were found between the groups. Such improvements were also found for the Abbreviated Profile of Hearing Aid Benefit except for the subscale aversiveness. Both groups decreased the use of HAs in hours/day over time. The intervention group reported significant improvements in activity limitation when measured directly after the intervention, compared to the control group.
Conclusion
Synchronous remote fine-tuning and follow-ups for first-time HA users did not influence the outcomes of hearing-related problems and HA benefits differently from standard care at our clinic.
Introduction
The objectives of aural rehabilitation (AR) are to reduce activity and participation restrictions for hard-of-hearing persons in everyday life (Gotowiec et al. Citation2022). In addition to sensory management (i.e. hearing aid fitting), AR needs to be person-centered and include informational and psychosocial counselling (Basura et al. Citation2023; Bennett et al. Citation2021). A person-centred approach includes, for example, addressing individual experiences of the impact of hearing loss (HL) on daily activities, and hearing aid (HA) fitting and fine-tuning performed with the individual’s subjective experience of an acoustic situation. Informational and psychosocial counselling addresses the psychosocial, social, and emotional acceptance of HL, and guides hard-of-hearing persons in finding self-management solutions related to hearing-related problems (Ferguson et al. Citation2019). However, various factors can affect the outcomes of AR. These are, for example, the degree of HL, gender, motivation, and HA usage time (Houmøller et al. Citation2022). According to Houmøller et al. (Citation2022), motivation may negatively affect experienced limitations related to HL, even if it positively influences the perceived benefit of AR. However, high HA usage can positively influence residual limitations. Furthermore, factors such as not addressing patient concerns and self-reported psychosocial problems can negatively affect the outcomes of AR (Bennett et al. Citation2021).
Remote services can enhance access to care and potentially boost patient interactivity, thereby influencing engagement in AR and self-management (Gomez et al. Citation2022). Previous research suggests including the internet and electronic devices to deliver healthcare, i.e. telehealth, to enhance AR services and to allow patients to manage their HL (Gomez et al. Citation2022; Malmberg et al. Citation2022; Malmberg and Hagberg Citation2023). When including self-management support in AR the HA users are encouraged to develop skills that could help them to successfully manage their hearing health (Convery et al. Citation2019). For example, internet-based interventions in general clinical practice that include psychosocial support may improve perceived hearing difficulties, with long-term effects for experienced HA users, and improve HA users’ self-reported communication skills (Malmberg et al. Citation2022). Furthermore, including remote follow-up consultations in AR has shown to be as appreciated as standard consultations among HA users (Tao et al. Citation2020). When assessing the perspectives of audiologists, it is suggested that telehealth could enhance the comprehension of hearing function and AR options among HA users (Bennett et al. Citation2022). However, audiologists generally believe in accomplishing better patient outcomes when engaging in face-to-face appointments (Bennett et al. Citation2022). A study that explored both patients’ and audiologists’ perspectives of telehealth revealed benefits such as enhanced patient readiness and involvement in decision-making (Heffernan, Maidment, and Ferguson Citation2023).
Remote services may also include remote fine-tuning of HAs (Ba Mashmous Citation2022; Duckworth, Beckman, and Heinrich Citation2022), which could encourage HA users to recognise individual needs directly or soon after experiencing hearing-related problems in a specific situation. Using HAs underpins aided personal experiences of the everyday environment and may result in personal preferences when fine-tuning. Such individual needs and personal communication goals are usually recalled by the HA users when reported to the audiologist during follow-up appointments at a clinic. Fine-tuning of HAs is a complex process where the audiologist must interpret patient preferences to improve sound quality (Bennett et al. Citation2018). Previous research reveals that audiologists depend on their expert knowledge to do so, but often rely on manufacturers’ default settings for HA fitting and fine-tuning (Anderson, Arehart, and Souza Citation2018). Also, HA users may present delayed reports during follow-up appointments due to recall biases when describing personal experiences that may vary over time (Stull et al. Citation2009). According to Stull et al. (Citation2009), such biases may affect treatment outcomes.
Both synchronous (direct communication) and asynchronous (saved information utilised later) modes are applicable for remote fine-tuning of HAs. Smartphone-connected HAs using asynchronous fine-tuning have shown to be beneficial for experienced HA users (Convery et al. Citation2020). A recent systematic review reveals positive outcomes when including remote synchronous HA fitting performed by an assisting facilitator acting as an intermediator between the patient and the audiologist (Ba Mashmous Citation2022). Nevertheless, Duckworth, Beckman, and Heinrich (Citation2022) found that remote HA fittings (consisting of telephone consultations and posting HAs to the patient) were less effective for first-time users compared to face-to-face or hybrid modes, with the latter requiring more follow-up visits. However, the blended mode maintained equivalent patient outcomes in terms of benefit. Blyth and Saunders (Citation2024) evaluated the perspectives of patients and healthcare providers on remote HA delivery (HAs were adjusted synchronously/asynchronously/by posting HAs to the clinic) and found that while patients generally expressed satisfaction with the service, approximately two in three expressed a preference for future HA fittings to be conducted in-person rather than remotely. The authors suggest that telehealth services should be tailored to individual preferences as there is no universal approach that will meet the needs of all patients. Our earlier study revealed positive short-term effects when involving remote synchronous fine-tuning and remote follow-up consultations for experienced HA users in AR (Malmberg and Hagberg Citation2023). The results also showed that virtually entering the daily lives of HA users enables the HA user, and the audiologist to specify personal hearing-related problems that could be addressed by fine-tuning. This suggests that fine-tuning for experienced HA users can be related to an individual and an immediate, subjective experience of an acoustic situation rather than a recall usually reported in a clinic. However, compared to experienced HA users, first-time HA users might not have had the chance to explore effective communication strategies on their own, and therefore may report different hearing-related problems. Preminger (Citation2007) suggests that the duration of HA use may influence the psychosocial benefits of interventions. For further evaluation of expanding the AR services with synchronous remote fine-tuning, more research is required to explore the patient outcomes for first-time HA users. Synchronous remote fine-tuning and gaining closer access to HA users’ everyday communication may have the potential to positively influence first-time HA users in identifying and reporting psychosocial hearing-related problems, and subsequently the outcomes of AR.
The main objective of the current study was to examine the short- and long-term effects of enabling follow-ups with synchronous fine-tuning and online communication between the patient and the audiologist, on the outcomes of AR for first-time HA users. We wanted to assess whether the synchronous mode can influence HA benefits and self-perceived hearing-related problems, in addition to involvement and participation in AR, compared to a control group receiving standard care.
Materials and methods
Participant recruitment and study procedure
This was a randomised controlled trial (RCT) with a parallel group design, approved by the Swedish Ethics Review Authority (Dnr. 2020-06658) and registered at ClinicalTrials.gov, NCT04843124 (August 2021). The recruitment letter was sent to patients >20 years of age (eligibility criteria one, i.e. the patient category at our clinics for adults) on a waiting list for AR (eligibility criteria two, i.e. the patient is experiencing HL and not using HAs) at five different public tax-funded clinics within the Hearing Organisation, Västra Götaland County (VGC). The five clinics were chosen due to having the largest catchment area within VGC. The letter included a consent form, information on the study procedure, the randomisation process, and the requirement to install and manage a mobile application, audio/video calls, and messaging. To participate, the patient was required to return a signed consent form and to understand spoken and written Swedish. Interested participants who returned a signed consent form were randomised into two groups, an intervention group receiving two synchronous remote fine-tuning and online communication appointments within standard care (group 1, n = 33) and a control group receiving standard care (group 2, n = 36), see . A simple randomisation procedure was used according to the method stipulated in the trial’s online registration record. An independent audiologist not involved in the recruitment process generated the random allocation sequence using a computer software program. The independent audiologist was not aware of the participant’s age, gender, or degree of HL. HA selection was made from a premium segment of HAs at our clinics based on the patient’s individual needs and in collaboration with the patient. The participants in the intervention group who chose HAs incompatible with synchronous mobile applications were excluded from the study (see ).
Figure 1. Flowchart of the study procedure. BOCF: Baseline Observation Carried Forward; HHIE/A: Hearing Handicap Inventory for the Elderly/Adults; IOI-HA: International Outcome Inventory for Hearing Aids; APHAB: Abbreviated Profile of Hearing Aid Benefit; AR: Aural rehabilitation.
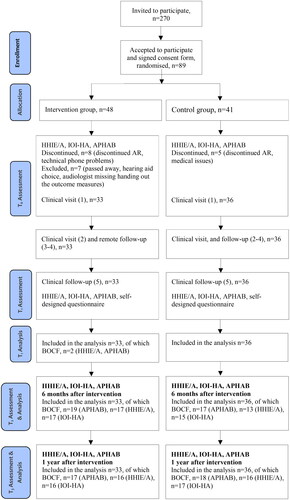
Standard care in VGC clinics includes up to four clinical visits; visit 1) diagnostic tests, informational counselling, and gathering information, visit 2) sensory management including HA fitting (compatible/incompatible with synchronous mobile applications) and psychosocial counselling, visit 3-4) HA maintenance, fine-tuning, and continued psychosocial counselling. There is approximately 1-2 months between each visit.
Altogether, sixteen (16) different audiologists provided standard care for both the intervention and control groups. Both groups received two initial clinical visits (visits 1 and 2). In addition to standard care during visit 1 the participants in both groups were verbally informed about the study and had the opportunity to ask questions regarding participation. They were also asked to respond to preintervention outcome measures. During visit 2 the intervention group was required to install and test-run an HA-specific synchronous mobile application, that was free of charge, and available for iPhone and Android mobile phones. Mobile applications depended on the HA choice made that was based on the participant’s individual needs (in total, five different applications were handled). Subsequently, the intervention group received two remote follow-ups, while the control group received two clinical follow-ups (visits 3-4), and both groups received the final visit at the clinic (visit 5) and responded to postintervention outcome measures, see . The same outcome measures were also deployed for both groups 6 months and 1 year after the intervention.
Outcome measures
The following tools were used:
The Hearing Handicap Inventory for the Elderly (HHIE; Ventry and Weinstein Citation1982) and the Hearing Handicap Inventory for the Adults (HHIA; Newman et al. Citation1990) were used as primary outcome measures, targeting HA users >65/<65 years of age and evaluating their self-perceived psychosocial and emotional effects of HL. Both HHIE and HHIA include two subscales, respectively, where higher points equal greater self-perceived hearing problems: 12 questions addressing social consequences and 13 questions addressing emotional.
The Abbreviated Profile of Hearing Aid Benefit (APHAB; Cox and Alexander Citation1995) and the International Outcome Inventory for Hearing Aids (IOI-HA; Cox et al. Citation2000) were used as secondary outcome measures. The APHAB consists of 24 questions and four subscales: ease of communication, reverberation, background noise, and aversiveness. This outcome measure addresses activity limitations associated with HL that are experienced with- compared to without wearing HA (i.e. HA benefit; higher points equal greater limitation). The IOI-HA measures seven different variables where higher points equal better outcomes. The variables are use, benefit, activity limitation, satisfaction, participation restriction, impact on environment, and quality of life.
The primary outcome measure HHIE/A and the secondary outcome measure APHAB were used at four different time points, before intervention (visit 1), after intervention (visit 5), and 6 months and 1 year after intervention (postal). The secondary outcome measure IOI-HA was only used after intervention (visit 5, and postal; 6 months-, and 1 year after intervention) since it measures self-perceived experiences after HA fitting.
In addition, the final clinical visits for both groups (visit 5) also included a self-designed questionnaire that evaluated perceived experiences of and participation in AR (see ). Questions asked in the self-designed questionnaire are, for example: “Are you satisfied with the information you were given during your aural rehabilitation?”, “How motivated are you to change your hearing situation?, and, “Did you experience occasions where you desired higher involvement in decision-making during your aural rehabilitation but did not get the opportunity to participate?” All questions are presented in Supplementary Appendix 1. The questionnaire included nine questions with a response scale from 1 (totally disagree) to 4 (totally agree) and three free-text questions inquiring about personal perspectives on AR and patient participation.
Analysis
Initially, 89 participants signed the consent form, 48 in the intervention and 41 in the control group (see ). Eight participants in the intervention and five in the control group discontinued (e.g. due to technical phone problems or medical issues). Seven participants in the intervention were excluded due to HA choice (e.g. choosing HA incompatible with synchronous mobile applications) or to the audiologist missing handing out the outcome measures (see ). Consequently, 33 participants were included in the intervention and 36 in the control group (see ).
Table 1. Demographics of the participants, showing the mean and standard deviations in brackets, or number (n) and % in brackets.
The power calculation showed that 60 participants needed to be included in the study to ensure a between-group effect of 80% at the 5% significance level (measured on the expected standardised mean difference for the HHIE-total scale). No statistically significant baseline differences were found between the intervention and control groups for all outcome measures, except for the HHIE emotional subscale (χ2 [20, n = 69] = 31.83, p = 0.05).
Both groups were examined at four measurement time points: before intervention (T0), after intervention (T1), 6 months after intervention (T2), and 1 year after intervention (T3). Not all participants returned the postal outcome measures at T2/T3 (see ). If post-observation (T1/T2/T3) is missing, the baseline observation was carried forward (BOCF). For the HHIE/A, the main differences between T0 to post-observations (T1/T2/T3) were evaluated using Analysis of Variance (ANOVA). T-tests with Bonferroni correction were applied when statistically significant differences were found T0 to post-observations (T1/T2/T3) within groups (main effect of time) or between time and groups (interaction effect). To study the within-group differences between the measurement time points for APHAB and IOI-HA, the Mann-Whitney U test was used.
In addition, a per-protocol analysis was performed to strengthen the interaction between the chosen intervention and the primary and secondary outcome measures by excluding participants who did not complete all post-measurement time points (T1/T2/T3).
Also, clinically relevant within-group changes before-to-directly-after-intervention for HHIE/A and APHAB, and the between-group differences in the self-designed questionnaire and IOI-HA, were evaluated using Chi2 tests. The Bonferroni correction was applied when statistically significant differences were found. Finally, within-group and between-group effect sizes (Cohen’s d) for HHIE/A, APHAB, and IOI-HA are calculated and presented with a 95% confidence interval for both groups. No interim analyses were performed during the trial.
Results
The results for the primary outcome measure HHIE/A are presented in . Both intervention and control groups significantly improved their total and subscale within-group scores from T0 to T1, T0 to T2, and T0 to T3. No statistically significant differences were found between the groups. A per-protocol analysis was performed excluding all data from participants who did not complete post-measurements (T1/T2/T3). The per-protocol results did not show statistically significant differences between the groups and showed significant improvements within each group from T0 to post-observations (T1/T2/T3) (see Supplementary Appendix 2). However, the groups are small which makes the results from per-protocol analysis underpowered. Supplementary Appendix 3 presents only medium within- and between-group effect sizes for HHIE/A, and both groups.
Table 2. Observed means (OM) and standard deviations (SD) for HHIE/A, APHAB, and IOI-HA are presented for the intervention (I-gr), n = 33, and control (C-gr) group, n = 36, before intervention (T0), directly after intervention (T1), 6 months (T2), and 1 year (T3) after the intervention. The main effect of time, the interaction effect between time and group, and the Mann-Whitney U test, are presented with a p-value.
According to Weinstein, Spitzer, and Ventry (Citation1986), a 36% improvement for an individual participant in HHIE/A scores is clinically meaningful. Examining clinically relevant individual changes showed that in the intervention group, 70% improved their score by more than 36% from T0 to T1, 70% showed clinically relevant changes from T0 to T2, and 67% from T0 to T3. In the control group, 67% showed such improvement from T0 to T1, 64% from T0 to T2, and 70% from T0 to T3. No significant difference was found between the groups (χ2 [1, N = 69] = 0.56, p = 0.45 for T0-to-T1; χ2 = 0.26, p = 0.61 for T0-to-T2; χ2 = 0.06, p = 0.81 for T0-to-T3).
The results of the APHAB analysis are presented in where the T0 measurement point represents unaided values and T1, T2, and T3 aided values. Both intervention and control groups showed statistically significant improvements for all measurement points and all subscales except for the aversiveness subscale (see ). The per-protocol analysis confirmed these results (see Supplementary Appendix 2). Mostly large and some medium within-group effect sizes, and only medium between-group effect sizes were found (see Supplementary Appendix 3).
For an individual change to be considered clinically relevant, subscales ease of communication, background noise, and reverberation need to have a score of 22 before-to-after intervention (unaided to aided), and 31 for the subscale aversiveness (Cox Citation1997). In the intervention group, 52% of the participants showed such a change for the ease of communication subscale, 58% of the participants for the background noise subscale, and 55% for the reverberation subscale. The corresponding results for the control group were 50% for the ease of communication subscale, 64% for the background noise subscale, and 44% for the reverberation subscale. These results remained almost the same 6 months and 1 year after intervention for both groups and no significant changes were found between the groups evaluated using Chi2 tests. For the subscale aversiveness, 15% in the intervention and 33% in the control group showed clinically relevant changes from T0 to T1. These changes varied over time (12%/28% at T2 and 21%/19% at T3) though Chi2 tests showed no statistically significant differences between the groups.
Furthermore, presents the values for each IOI-HA question at post-observations (T1/T2/T3). No significant differences were found between the groups at T1 except for the variable activity limitation (χ2 [2, N = 69] = 9.34, p = 0.009). Both groups showed high mean values for all seven variables directly after AR. The intervention group kept these results at 6 months and 1 year after intervention except for the variable activity limitation, showing statistically significant lower within-group scores 1 year after AR. The control group showed a similar within-group decrease indicating that the participants experienced more activity limitations 1 year after intervention. The control group also showed a within-group decrease in HA use (variable: daily use), HA benefit (variable: benefit), and satisfaction (variable: satisfaction) 6 months and 1 year after intervention; see , and no significant differences were found at T2/T3 between the groups. The control group also showed a within-group decrease in variable participation restriction 1 year after the intervention. The intervention group showed similar, but not significant, within-group changes when evaluating mean differences with the per-protocol analyses, and the control group showed within-group decreases in HA benefit at T2 and T3, and satisfaction at T2 (see Supplementary Appendix 2). Finally, medium within- and between-group effect sizes were found for IOI-HA (see Supplementary Appendix 3).
The self-designed questionnaire was used at one measurement point (directly after AR; T1) for both groups, evaluating different aspects, including perceived usability and participation in AR (see Supplementary Appendix 1). The response scale was from 1 to 4 and the average score ranged from 3.8 to 4.0 for the intervention group and from 3.7 to 3.9 for the control group. No significant differences were found between the groups. The first free-response question that considers the most important information during AR was addressed by 65% in the intervention group and 75% in the control group. Both groups highlighted the importance of getting information on how to manage and utilise their HA. Many participants responded that “all” information was equally important and valuable, and some expressed an appreciation for being included in every part of the AR. When addressing patient involvement in the second free-response question (84% responders in the intervention group and 81% in the control group), many participants valued the opportunities to get responses to personal inquiries. Also, many reported a feeling of being heard and understood. For example, one participant expressed the importance of being viewed as a person with significant individual needs related to hearing difficulties. Another participant described the experience of patient involvement as a time when it’s all about the person and everything a person experiences counts. The third question required the participant to state an example of experiencing being involved in AR. In total, 74% responded to this question in the intervention group and 78% in the control group. Many participants expressed that they valued every part of the AR and appreciated the meetings with the audiologist. Also, many reported a high presence of shared decision-making, e.g. in HA choice/fitting/fine-tuning.
Discussion
Expanding AR services using synchronous online appointments that may encourage self-management may increase access to hearing care. The purpose of this study was to evaluate if synchronous fine-tuning and online communication for first-time HA users can influence HA benefits and self-perceived hearing-related problems compared to a control group receiving standard care. The results showed that the synchronous mode did not influence the outcomes of AR, nevertheless, the intervention group showed significantly better improvements in activity limitations measured with one variable of the IOI-HA directly after the intervention, compared to the control group. The results can support hearing care professionals in considering remote fine-tuning and self-management when developing AR services.
The primary findings of the study that concern subjective hearing-related problems as measured by HHIE/A showed that both groups improved significantly directly after participation, and the results remained 6 months and 1 year after intervention (see ). Furthermore, both groups showed highly clinically relevant individual changes. AR benefits individuals with HL by improving communication skills and reducing deficits in function, activity, participation, and quality of life (Ferguson et al. Citation2019). The current study results from before- to directly after AR are in line with our previous study showing significant benefits in AR when including remote intervention for experienced HA users (Malmberg and Hagberg Citation2023), indicating that remote fine-tuning and follow-ups are as effective as in-person appointments when addressing hearing-related problems measured by the HHIE/A. A study by Tao et al. (Citation2020) presented a comparable statement when comparing remote HA follow-up consultations with conventional hearing care, showing improvements in outcomes for communication, fitting, and quality of life for both delivery modes. A closer look at the HHIE/A results shows that both groups initially benefitted from the psychosocial support provided by the audiologists, but slightly increased their scores 6 months and 1 year after AR (see ). It may be that the psychosocial counselling provided is not sufficient for the HA user to continuously benefit from the support or that there is a need to develop the AR services after completing the HA fitting. Previous research shows fewer subjective hearing-related problems when providing psychosocial support after completing the AR (Malmberg et al. Citation2022). Furthermore, both the intervention and the control group benefitted from emotional support during the AR in the current study, and while the intervention group’s scores were stable 6 months and 1 year after completing AR, the control group’s scores increased 6 months and 1 year after AR (see ). On the contrary, significant baseline differences were identified between the groups regarding the emotional subscale, and it might be more challenging for the control group to maintain positive changes when starting with higher baseline scores. Addressing emotional problems in clinical practice is necessary to reduce the impact of health-related quality of life. A recent literature overview recognises the importance of audiologists identifying emotional needs and addressing the emotional well-being of HA users (Timmer et al. Citation2023).
The secondary findings of the study showed that both groups had significant within-group improvements in limitations experienced with wearing HAs compared to without it measured by APHAB, except for the aversiveness subscale. The aversiveness subscale measures negative reactions to environmental sounds, and HA fitting is usually associated with higher aided scores for this subscale (Brännström et al. Citation2020). Compared to our previous study, the participants in the current study showed fewer clinically relevant individual changes on the aversiveness subscale, and first-time HA users rated unaided hearing experiences higher than experienced HA users (Malmberg and Hagberg Citation2023). On the contrary, Brännström et al. (Citation2020) showed generally low scores for the aversiveness subscale when examining first-time HA users, arguing that first-time HA users might rate unaided hearing experiences lower than experienced HA users, who rely on their experienced benefits of HAs. Also, the degree of HL might be crucial when experiencing negative reactions to environmental sounds. The current study participants have better PTA4 thresholds compared to the experienced HA users in our previous study (Malmberg and Hagberg Citation2023), and it might be that higher PTA4 thresholds lead to greater annoyance or negative reactions to environmental sounds. However, it is interesting that neither the intervention nor the control group significantly improved their scores for the aversiveness subscale in the current study. Dawes and Munro (Citation2017) showed that first-time HA users need to learn how to “tune out” undesirable sounds to adjust to HAs, suggesting consistent HA use to facilitate acclimatisation. Examining the daily use of HAs for both groups in the current study, measured with the IOI-HA variable daily use (see ), it is obvious that both groups decreased their HA use over time which could explain the lack of improvement for the aversiveness subscale.
The IOI-HA presents within-group measurements directly after completed AR, 6 months-, and 1 year after AR (see ). Overall, high scores are seen for all seven variables when measured directly after the participants completed AR, for both groups, although some variables changed within each group over time. For example, longer hours of daily HA use (variable: daily use) are observed directly after completing the AR, but then both groups decreased in HA use, though not statistically different from each other. Less frequent use of HAs within 6 months after AR is associated with HA-related issues such as sound quality and handling (Solheim, Gay, and Hickson Citation2018). It might be that the sound quality was not sufficiently addressed in both groups. Anderson, Arehart, and Souza (Citation2018) reveal that audiologists often rely on the default settings for fine-tuning and the authors emphasise the need for addressing person-specific characteristics. Additionally, Swanepoel et al. (Citation2023) used IOI-HA in a study to evaluate reported HA outcomes for two service delivery models: over-the-counter (OTC) and conventional hearing care professionals (HCP). In contrast, the authors found longer hours of daily use for participants receiving HA fitting from HCP compared to OTC. Furthermore, both groups in the current study experienced great HA benefits measured with the variable benefit directly after completing AR, but the within-group scores changed differently at 6 months and 1 year after AR, with no significant differences between the groups (see ). As HA outcomes are affected by factors such as motivation and HA usage (Houmøller et al. Citation2022), a decrease in HA use may also affect HA benefits. It could be that experiencing higher motivation is related to longer hours of daily HA use.
Both groups reported a within-group decrease in activity limitations and participation restrictions (IOI-HA variable: activity limitations, and participation restrictions) when measured directly after completing AR (see ), and the intervention group showed a significantly higher between-group decrease in activity limitations compared to the control group. However, the within-group scores for both variables and groups increased over time and no significant differences remained between the groups 6 months and 1 year after the intervention. Different explanations for this increase could be considered. Firstly, HAs provide limited benefits in, for example, noisy environments (Lesica, Citation2018), and HA fitting should be accompanied by sufficient adjustment counselling addressing the psychosocial impacts of HL. Such self-management solutions may empower HA users to manage their hearing health (Convery et al. Citation2019). In line with the results presented for HHIE/A, it might be that the adjustment counselling during the AR in the current study was insufficient for the HA users to maintain long-term self-managing solutions. Adjusting to HL is a continuous process, as well as acquiring knowledge about the condition and finding skills and strategies to manage good hearing health (Gotowiec et al. Citation2022). HA users may improve their use of communication strategy skills when participating in interventions provided after completing AR (Malmberg et al. Citation2022). Another explanation for the within-group increase in activity limitations and participation restrictions over time could be that the participants in the current study are active HA users who are coping with work-life or leisure activities and thus have impractical expectations of their listening environments. Working-age first-time HA users with mild-to-moderate sensorineural HL report better AR outcomes and improved social-communicative functioning, with younger age and positive expectations being key factors (Laakso et al. Citation2022). Finally, the significance of between-group differences in activity limitations measured directly after the intervention may be explained by the synchronous fine-tuning in the current study, allowing HA users to more effectively acknowledge individual needs related to everyday situations, which may result in enhanced communication strategy skills. Hence, the online mode may be one of the key concepts for audiologists to better address the psychosocial support needed to be done in a clinical setting (Bennett et al. Citation2021). However, these between-group differences in activity limitations only highlight the potential of the online synchronous mode, and this topic requires further evaluation.
Furthermore, both groups reported high satisfaction directly after the intervention when measured with the variable satisfaction of the IOI-HA (see ), emphasising the benefits of participating in AR, regardless of the delivery mode. Participation, self-efficacy, and feeling of control of hearing health care are prominent components that influence an individual’s use of HAs (Gotowiec et al. Citation2022). In contrast, Tao et al. (Citation2020) showed that online HA follow-up consultations can negatively affect satisfaction compared to conventional hearing care. However, the patient-provider hierarchy that could affect HA users’ participation in hearing care may be toned down in online follow-ups. Online technology use strengthens patient-provider relationships and patient empowerment (Zoghlami and Rached Citation2022). Convery et al. (Citation2020) assessed a smartphone app to facilitate remote patient-provider communication and found that numerous of the requests concerned personal hearing-related challenges, rather than fine-tuning. According to the practice guidelines on person-centred AR, in addition to providing sensory management, informational and psychosocial counselling should be combined and available to HA users (Basura et al. Citation2023). Person-centred approach and self-management are necessary components of online interventions that provide personalisation and interactivity (Ferguson et al. Citation2019). The interactivity might be the one that empowers the HA user and not the HA technology itself (Gomez et al. Citation2022), regardless of the delivery mode. Usability is an additional component of online interventions that might affect HA outcomes (Convery et al. Citation2020). In the current study, the self-designed questionnaire showed positive outcomes in usability and involvement in AR for both groups.
Limitations, strengths, and future directions
Participants in the intervention group were required to have access to a mobile phone, install an application, and have certain skills to use technology, all variables that could be considered limitations in the current study. In Sweden, 85% of the population has access to a smartphone, and 82% of smartphone users between 65 to 75 years old use their smartphone daily (Internetstiftelsen Citation2023). However, the proportion of users decreases with age. Furthermore, a recent research review identified variables such as health status, knowledge and perception, motivation, and social influences as factors that influence older people’s adaptation to digital technologies (Schroeder et al. Citation2023). These variables were not measured in the current study and could have influenced who chose to participate in the study, the study attrition and adherence, and accordingly the study results. No other demographics were obtained except for age, gender, and HL, which could be considered a limitation of this study. For example, education and health literacy predict intention to adopt technology (Schroeder et al. Citation2023), and these variables may have also influenced who consented to participate in the current study. Hence, the findings may not be generalised to a wider population. An additional limitation in the study is that the only difference between control and intervention groups was that two sessions relating to HA fine-tuning and follow-up were delivered either in person (standard care) or remotely (synchronous mode). This might be reflected in the study results, where no significant differences were found between the groups. Lastly, limitations such as lack of blinding and high drop-out rates at T2/T3 may have influenced the study outcomes. Discontinuing long-term follow-up may be related to decreased HA use due to experiencing handling issues or no perceived need (Solheim, Gay, and Hickson Citation2018).
The authors recognise synchronous remote fine-tuning and follow-ups within online services as a significant prospect. One of the strengths of this study is that few earlier reports are found regarding synchronous remote fine-tuning for first-time HA users. Another strength is that this randomised controlled trial is conducted by 16 audiologists within our county, who throughout the study completion became familiar with the synchronous remote appointments and can continuously provide this online mode to our clinic’s future patients. Previous research shows that clinicians are generally positive about the provision of telehealth and believe it may result in improved patient outcomes (Bennett et al. Citation2022). As remote appointments may strengthen patient-provider relationships (Zoghlami and Rached Citation2022), future research should evaluate the accessibility and patient/clinician acceptability of remote appointments in hearing clinics not providing HA fine-tuning and follow-ups as standard practice.
Future challenges of synchronous remote fine-tuning and online communication lie in how to prepare the audiologist and the HA user to identify the emotional and psychosocial variables that can source significant support provided by the audiologist using synchronous online services. Recent research presents valuable evidence-based recommendations on how to include emotional and psychosocial support in clinical practice (Timmer et al. Citation2023), and suggests recognising the social-emotional well-being of the HA user and integrating social-emotional needs and objectives into a personalised management plan. Furthermore, it would be beneficial to assess what personal and environmental resources are needed to identify individual goals in AR when providing remote fine-tuning and follow-ups that include emotional and psychosocial support. Consequently, it would be valuable to examine the factors influencing audiologists’ uptake and use of, and perspectives on synchronous remote fine-tuning.
Conclusions
This study has provided additional perspectives on the potential of extending AR services by including synchronous remote fine-tuning and follow-ups for first-time HA users. Regardless of the delivery mode, the results of the study revealed short-term and long-term improvements in self-rated hearing-related problems, recognising the benefits of AR. However, the synchronous delivery mode might have the potential to acknowledge HA user’s activity limitations. Audiologists may consider including remote fine-tuning and self-management in the expanded AR services. Yet, there is a need to further evaluate the opportunities to approach the HA user’s daily communication and to address individual hearing-related challenges experienced by the HA user in everyday life.
| Abbreviations | ||
| APHAB | = | Abbreviated Profile of Hearing Aid Benefit |
| AR | = | Aural Rehabilitation |
| HA | = | Hearing aid/s |
| HHIE/A | = | Hearing Handicap Inventory for the Elderly/Adults |
| HL | = | Hearing loss |
| IOI-HA | = | International Outcome Intervention for Hearing Aid Users |
| RCT | = | Randomised controlled trial |
| VGC | = | Västra Götaland County. |
Supplemental Material
Download MS Word (36.4 KB)Supplemental Material
Download MS Word (35.2 KB)Supplemental Material
Download MS Word (29.6 KB)Acknowledgments
The authors would like to thank the participants in this study and the clinicians and operation managers at the Hearing Organisation, VRG, Sweden.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Anderson, M., K. Arehart, and P. Souza. 2018. “Survey of Current Practice in the Fitting and Fine-Tuning of Common Signal-Processing Features in Hearing Aids for Adults.” Journal of the American Academy of Audiology 29 (2):118–124. https://doi.org/10.3766/jaaa.16107.
- Ba Mashmous, M. H. A. 2022. “Efficacy of Remote Hearing Aids Programming Using Teleaudiology: A Systematic Review.” E-Health Telecommunication Systems and Networks 11 (01):14–33. https://doi.org/10.4236/etsn.2022.111002.
- Basura, G., K. Cienkowski, L. Hamlin, C. Ray, C. Rutherford, G. Stamper, T. Schooling, and J. Ambrose. 2023. American Speech-Language-Hearing Association Clinical Practice Guideline on Aural Rehabilitation for Adults With Hearing Loss. American Journal of Audiology, 32(1), 1–51. https://doi.org/10.1044/2022_AJA-21-00252.
- Bennett, R. J., A. Laplante-Lévesque, M. Carly, and R. H. Eikelboom. 2018. “Exploring Hearing Aid Problems: Perspectives of Hearing Aid Owners and Clinicians.” Ear and Hearing 39 (1):172–187. https://doi.org/10.1097/AUD.0000000000000477.
- Bennett, R. J., C. Barr, J. Montano, R. H. Eikelboom, G. H. Saunders, M. Pronk, J. E. Preminger, M. Ferguson, B. Weinstein, E. Heffernan, et al. 2021. “Identifying the approaches used by audiologists to address the psychosocial needs of their adult clients.” International Journal of Audiology 60 (2):104–114. https://doi.org/10.1080/14992027.2020.1817995.
- Bennett, R. J., I. Kelsall-Foreman, C. Barr, E. Campbell, T. Coles, M. Paton, and J. Vitkovic. 2022. “Barriers and facilitators to tele-audiology service delivery in Australia during the COVID-19 pandemic: perspectives of hearing healthcare clinicians.” International Journal of Audiology 62 (12):1145–1154. https://doi.org/10.1080/14992027.2022.2128446.
- Blyth, M., and G. H. Saunders. 2024. “Remote hearing-aid delivery and support: perspectives of patients and their hearing care providers.” International Journal of Audiology :1–9. https://doi.org/10.1080/14992027.2024.2304585.
- Brännström, K. J., K. Andersson, O. Sandgren, and S. Whitling. 2020. “Clinical Application and Psychometric Properties of a Swedish Translation of the Abbreviated Profile of Hearing Aid Benefit.” Journal of the American Academy of Audiology 31 (9):656–665. https://doi.org/10.1055/s-0040-1718702.
- Convery, E., G. Keidser, M. McLelland, and J. Groth. 2020. “A Smartphone App to Facilitate Remote Patient-Provider Communication in Hearing Health Care: Usability and Effect on Hearing Aid Outcomes.” Telemedicine Journal and e-Health: The Official Journal of the American Telemedicine Association 26 (6):798–804. https://doi.org/10.1089/tmj.2019.0109.
- Convery, E., L. Hickson, C. Meyer, and G. Keidser. 2019. “Predictors of Hearing Loss Self-Management in Older Adults.” Disability and Rehabilitation 41 (17):2026–2035. https://doi.org/10.1080/09638288.2018.1457091.
- Cox, R. 1997. “Administration And Application of the APHAB.” The Hearing Journal 50 (4):32–48. https://doi.org/10.1097/00025572-199704000-00002.
- Cox, R., and G. Alexander. 1995. “Abbreviated Profile of Hearing Aid Benefit.” Ear and Hearing 16 (2):176–186. https://doi.org/10.1097/00003446-199504000-00005.
- Cox, R., M. Hyde, S. Gatehouse, W. Noble, H. Dillon, R. Bentler, and L. Hallberg. 2000. “Optimal Outcome Measures, Research Priorities, and International Cooperation.” Ear & Hearing 21 (4):106–115.
- Dawes, P., and K. Munro. 2017. “Auditory Distraction and Acclimatization to Hearing Aids.” Ear and Hearing 38 (2):174–183. https://doi.org/10.1097/AUD.0000000000000366.
- Duckworth, Z., A. Beckman, and A. Heinrich. 2022. “Did Changes to Adult Hearing Aid Pathways Due to COVID-19 Affect Patient Outcomes? A Service Evaluation.” American Journal of Audiology 31 (3S):876–891. https://doi.org/10.1044/2022_AJA-21-00195.
- Ferguson, M., D. Maidment, H. Henshaw, and E. Heffernan. 2019. “Evidence-Based Interventions for Adult Aural Rehabilitation: That Was Then, This is Now.” Seminars in Hearing 40 (1):68–84. https://doi.org/10.1055/s-0038-1676784.
- Gomez, R., A. Habib, D. W. Maidment, and M. A. Ferguson. 2022. “Smartphone-Connected Hearing Aids Enable and Empower Self-Management of Hearing Loss: A Qualitative Interview Study Underpinned by the Behavior Change Wheel.” Ear and Hearing 43 (3):921–932. https://doi.org/10.1097/AUD.0000000000001143.
- Gotowiec, S., J. Larsson, P. Incerti, T. Young, K. Smeds, F. Wolters, P. Herrlin, and M. Ferguson. 2022. “Understanding Patient Empowerment Along the Hearing Health Journey.” International Journal of Audiology 61 (2):148–158. https://doi.org/10.1080/14992027.2021.1915509.
- Heffernan, E., D. W. Maidment, and M. A. Ferguson. 2023. “A Qualitative Study Showing that a Telecare Tool can Have Benefits Before and During the Initial Hearing Assessment Appointment.” International Journal of Audiology 62 (4):295–303. https://doi.org/10.1080/14992027.2022.2041740.
- Houmøller, S. S., A. Wolff, S. Möller, V. K. Narne, C. S. Narayanan, K. Godballe, D. D. Hougaard, G. Loquet, M. Gaihede, D. Hammershøi, et al. 2022. “Prediction of Successful Hearing Aid Treatment in First-Time and Experienced Hearing Aid Users: Using the International Outcome Inventory for Hearing Aids.” International Journal of Audiology 61 (2):119–129. https://doi.org/10.1080/14992027.2021.1916632.
- Internetstiftelsen. 2023. The Swedes and the Internet. Internetstiftelsen. https://svenskarnaochinternet.se/english/.
- Laakso, M., J. Lipsanen, K. Pajo, I. Salmenlinna, T. Aaltonen, J. Ruusuvuori, and A. Aarnisalo. 2022. “Working-Age First-Time Hearing Aid Users’ Self-Reported Outcomes.” International Journal of Audiology 62 (9):877–885. https://doi.org/10.1080/14992027.2022.2106454.
- Lesica, N.A. 2018. “Why Do Hearing Aids Fail to Restore Normal Auditory Perception?” Trends in Neurosciences, 41 (4):174–185. https://doi.org/10.1016/j.tins.2018.01.00
- Malmberg, M., and H. Hagberg. 2023. “Synchronous Remote Finetuning and Follow-Up Within Aural Rehabilitation: A Randomised Controlled Trial.” International Journal of Audiology 63 (6):458–466. https://doi.org/10.1080/14992027.2023.2188437.
- Malmberg, M., K. Anióse, J. Skans, and M. Öberg. 2022. “A Randomised, Controlled Trial of Clinically Implementing Online Hearing Support.” International Journal of Audiology 62 (5):472–480. https://doi.org/10.1080/14992027.2022.2059712.
- Newman, C. W., B. E. Weinstein, G. P. Jacobson, and G. A. Hug. 1990. “The Hearing Handicap Inventory for Adults; Psychometric Adequacy and Audiometric Correlates.” Ear and Hearing 11 (6):430–433. https://doi.org/10.1097/00003446-199012000-00004.
- Preminger, J. E. 2007. “Issues Associated with the Measurement of Psychosocial Benefits of Group Audiologic Rehabilitation Programs.” Trends in Amplification 11 (2):113–123. https://doi.org/10.1177/1084713807301084.
- Schroeder, T., L. Dodds, A. Georgiou, H. Gewald, and J. Siette. 2023. “Older Adults and New Technology: Mapping Review of the Factors Associated With Older Adults’ Intention to Adopt Digital Technologies.” JMIR Aging 6:e44564. https://doi.org/10.2196/44564.
- Solheim, J., C. Gay, and L. Hickson. 2018. “Older Adults’ Experiences and Issues With Hearing Aids in the First Six Months After Hearing Aid Fitting.” International Journal of Audiology 57 (1):31–39. https://doi.org/10.1080/14992027.2017.1380849.
- Stull, D. E., N. K. Leidy, B. Parasuraman, and O. Chassany. 2009. “Optimal Recall Periods for Patient-Reported Outcomes: Challenges and Potential Solutions.” Current Medical Research and Opinion 25 (4):929–942. https://doi.org/10.1185/03007990902774765.
- Swanepoel, D., I. Oosthuizen, M. Graham, and V. Manchaiah. 2023. “Comparing Hearing Aid Outcomes in Adults Using Over-the-Counter and Hearing Care Professional Service Delivery Models.” American Journal of Audiology 32 (2):314–322. https://doi.org/10.1044/2022_AJA-22-00130.
- Tao, K. F. M., T. C. Moreira, D. M. P. Jayakody, D. W. Swanepoel, C. G. Brennan-Jones, L. Coetzee, and R. H. Eikelboom. 2020. “Teleaudiology Hearing Aid Fitting Follow-Up Consultations for Adults: Single Blinded Crossover Randomised Control Trial and Cohort Studies.” International Journal of Audiology 60 (sup1):S49–S60. https://doi.org/10.1080/14992027.2020.1805804.
- Timmer, B. H. B., R. J. Bennett, J. Montano, L. Hickson, B. Weinstein, J. Wild, M. Ferguson, J. A. Holman, V. LeBeau, and L. Dyre. 2023. “Social-Emotional Well-Being and Adult Hearing Loss: Clinical Recommendations.” International Journal of Audiology 63 (6):381–392. https://doi.org/10.1080/14992027.2023.2190864.
- Ventry, I. M., and B. E. Weinstein. 1982. “The Hearing Handicap Inventory for the Elderly: A New Tool.” Ear and Hearing 3 (3):128–134. https://doi.org/10.1097/00003446-198205000-00006.
- Weinstein, B. E., J. B. Spitzer, and I. M. Ventry. 1986. “Test–Retest Reliability of the Hearing Handicap Inventory for the Elderly.” Ear and Hearing 7 (5):295–299. https://doi.org/10.1097/00003446-198610000-00002.
- Zoghlami, M., and K. Rached. 2022. “From Physician’s Authority to Patient Expertise: The Effects of e-Health Technology Use on Patient’s Behavior and Physician-Patient Relationship.” VINE Journal of Information and Knowledge Management Systems 54 (3):597–615. https://doi.org/10.1108/VJIKMS-07-2021-0106.
