Abstract
Female Rainbow Trout Oncorhynchus mykiss were cultured within a freshwater recirculating aquaculture system under 24-h constant lighting in 13°C water and fed every 6 h to near satiation. An opaque roof allowed surface light intensity to vary between <200 and about 1,500 lx. During months 14–26 posthatch we examined changes in plasma concentrations of testosterone (T), estradiol-17β (E2), the maturation inducing steroid (MIS) 17α,20β-dihydroxy-4-pregnen-3-one (17,20βP), growth hormone (GH), and insulin-like growth factor-I (IGF-I). Oocyte diameter was variable at the start of the study, most averaging <1 mm; diameter increased to above 3.2 mm in the final 2 months, with migrating germinal vesicles indicating they were postvitellogenic. Some ovaries exhibited atresia, and no fish ovulated, suggesting some reproductive dysfunction. Testosterone and E2 began increasing between months 16 and 18, and although T continued to increase throughout the study, E2 changed little after month 20. The MIS 17,20βP, remained near or below detection. Plasma GH remained relatively unchanged although values trended slightly higher during the final 4 months, GH being significantly greater at months 22, 24 and 26 than at month 16. Plasma IGF-I was higher at the first time point, month 14 posthatch than at months 20, 24, 25, and 26. In summary, gradual changes in growth and fillet quality attributes during gonadal development were accompanied by gradual changes in hormone levels, but no clear changes in hormones were associated with rapid changes in product quality traits observed around months 24–26.
Received August 21, 2014; accepted November 8, 2014
Water recirculating aquaculture systems (RAS) allow control over environmental conditions such as water temperature, water quality, lighting, and feeding regimen, which in turn affect production traits including reproduction, growth, and fillet quality (Wedemeyer Citation1996; Summerfelt et al. Citation2001). To maximize production potential from RAS, we must understand how the aforementioned environmental variables affect the physiology behind these production traits. Production performance and product quality attributes are particularly affected by environmental factors when fish are undergoing sexual maturation. Salmonids undergo substantial repartitioning of nutrients and energy from somatic growth to gonadal growth during sexual maturation. In maturing female Rainbow Trout Oncorhynchus mykiss and depending on plane of nutrition, muscle and visceral lipid stores are reduced, growth rate is slowed, and flesh or fillet quality can be compromised (Nassour and Leger Citation1989; Shearer Citation1994; Kiessling et al. Citation1995; Salem et al. Citation2006; 2010; Aussanasuwannakul et al. Citation2011, 2012; Manor et al. Citation2012). Changes in hormone profiles during sexual maturation have been characterized and there is some understanding of how gonadal sex steroids and growth-axis hormones regulate the utilization and partitioning of nutrients and energy (see reviews; Le Gac et al. Citation1993; Taranger et al. Citation2010; Reindl and Sheridan Citation2012). However, it remains poorly understood how sex steroid and the growth hormones, growth hormone (GH), and insulin-like growth factor-I (IGF-I), interact or are coordinately regulated to affect nutrient partitioning during sexual maturation.
Growth is primarily under the control of the GH-IGF-I axis in fish, as in mammals. In turn, circulating GH and IGF-I levels appear to be largely determined by nutritional status, but they are also influenced by other environmental factors, including temperature and photoperiod (Björnsson Citation1997; Reinecke Citation2010; Reindl and Sheridan Citation2012). Studies suggest GH and IGF-I may also participate in the regulation of sexual maturation in salmonids (Björnsson Citation1997; Taylor et al. Citation2008; Benedet et al. Citation2010). Reproduction in salmonids is principally under photoperiod control, and the sex steroids—estradiol-17β (E2), testosterone (T), and 17α,20β-dihydroxy-4-pregnen-3-one (17,20βP)—are primary regulators of ovarian development (Bromage et al. Citation1992; Bromage et al. Citation2001; Lubzens et al. Citation2010). Altering environmental conditions such as nutritional, photoperiod, and temperature cues are expected to impact reproductive and growth-axis hormone levels, which in turn should impact reproduction, growth, and fillet quality. Nevertheless, the complexity of these anticipated interactions makes it difficult to predict the outcomes in terms of changes in hormone levels and effects on growth and fillet quality traits.
We examined interactions among the environment and the reproduction and growth axes in an RAS, which need to be understood to define environmental conditions and diet formulations for optimal production of food fish. We cultured an August–September breeding strain of female Rainbow Trout in a RAS under 24-h constant lighting but with exposure to shaded natural lighting. Water temperature was about 13°C, and fish were fed to apparent satiation, feed being delivered at least every 6 h. Characterized from month 14 through 26 posthatch were plasma profiles of the sex steroids—E2, T, and the maturation inducing steroid (MIS) 17,20βP—and plasma profiles of growth-axis hormones GH and IGF-I. Ovarian follicle development was also recorded. This paper supplements Davidson et al. (Citation2014), in which we described growth performance, fillet yield, and fillet quality attributes for fish from this study and showed female Rainbow Trout raised under the described conditions can reach 4.8 kg by 22 months posthatch, at which point reproductive development negatively affects growth, yield, and fillet quality. Here, we provide physiological context for the production traits reported in (Davidson et al. Citation2014).
METHODS
Fish and culture system.—The animal care, water quality variables, and culture system design we employed are presented in detail in Davidson et al. (Citation2014). Briefly, fish were female Kamloop-strain Rainbow Trout purchased as eyed eggs from Troutlodge (Sumner, Washington). Fish were maintained under 24-h light from hatching through the end of the study. Fish were raised at a mean temperature of about 13°C, ranging between 12°C and 15°C, from first feeding until 12 months posthatch. At 12 months posthatch, when the fish averaged about 1.4 kg, 330 fish were transferred to an 11-m3 tank with a partial water reuse system (60%), and raised until 26 months posthatch. Fish were removed from the tank as needed to maintain density at 55–80 kg/m3 and maintain water quality metrics within strict limits. Lighting for the tank was provided by constant (24 h/d) overhead fluorescent lights, although the canvas housing of the building allowed penetration of natural light (). Fish were raised at a mean monthly temperature of 12.9°C (range, 12.5–13.6°C) while in this tank. Fish were fed a standard trout feed (42% protein,16% fat) produced by Zeigler Brothers (Gardners, Pennsylvania) every 6 h based on a feeding schedule (see Davidson et al. Citation2014). Weight and length of 60–100 fish were recorded and returned to the tank monthly as reported in Davidson et al. (Citation2014). Ten fish were randomly sampled at bimonthly intervals from months 14–24 posthatch, and then monthly from months 24–26 posthatch for additional analyses that included collecting plasma samples for hormone measurement, ovarian tissue for oocyte diameter and staging, and viscera for proximate composition. These same fish or some of these fish were also analyzed for measures of growth, yield, muscle proximate composition, and fillet quality, the results of which are reported in Davidson et al. (Citation2014).
Table 1 Light intensity measured on August 13, 2008, where A–D are different indoor locations, all at arm's length from the edge of the tank wall and 30 cm above the water surface, and outdoor locations were N (north) and S (south) of the building.
Light intensity measurement.—Light intensity was measured on August 13, 2008, the second year of the study, with a Lutron LX-102 digital light meter (Taipei, Taiwan) at four times during the day: 0510, 0635, 1350, and 1610 hours. Light was recorded at four locations around the tank with the meter positioned at arm's length from the edge of the tank, 30 cm above the water surface. Light intensity was also recorded outdoors, north and south of the building.
Follicle diameter measurement and staging, and viscera proximate analysis.—An estimate of follicle diameter for each fish was obtained by measuring the diameter of 10–12 follicles (nearest 100 μm) using a dissecting stereomicroscope fitted with an ocular micrometer. During months 24, 25, and 26, 10 or more follicles per fish were fixed and cleared in Davidson's solution (2 parts 37% formaldehyde : 3 parts 95% EtOH : 1 part acetic acid : 3 parts deionized ultrafiltered H2O) for staging maturation based on migration and breakdown of the germinal vesicle (GV). Viscera used in proximate analysis included gastrointestinal tract, liver, heart, gallbladder, and spleen, but not digesta and gonads. Moisture and crude fat analyses were completed according to AOAC methods (AOAC 1990) and using an Ankom XT10 Extractor (Ankom Technology, Macedon, New York) and crude fat method, AOCS procedure Am 5-04. All collected tissues were frozen in liquid nitrogen and then powdered. An aliquot of the powdered sample was used for analyses.
Plasma hormone concentration measurement.—Plasma levels of T, E2 and 17,20βP were measured by specific radioimmunoassay (RIA) according to Woods and Sullivan (Citation1993). Antisera were validated for use in salmonid species (Young et al. Citation1983; Fitzpatrick et al. Citation1986). Plasma IGF-I was measured by RIA methods described by Moriyama et al. (Citation1994) as modified by Shimizu et al. (Citation2000) and using recombinant trout IGF-I and anti-barramundi IGF-I antiserum (GroPep, South Australia, Australia). Plasma GH was measured by the RIA methods described by Peterson et al. (Citation2003), as modified by Shepherd et al. (Citation2005), and via recombinant salmon/trout GH and anti-salmon/trout primary antiserum (GroPep, South Australia, Australia).
Statistical analysis.—Statistical analyses were performed using SigmaPlot 12 (Systat Software, San Jose, California) software. Data were analyzed using one-way ANOVA to test for main effects. When main effects were significant, differences among means were determined using the Holm–Sidak multiple comparisons test. If normality and equal variance assumptions were not met, data were analyzed using Kruskal–Wallis one-way ANOVA on ranks to test for main effects, followed by the Tukey multiple comparison test to determine differences among means. Differences were considered significant at α = 0.05.
RESULTS
Light Intensity
Light intensity measured 30 cm above the surface of the tank was strongly affected by outdoor sunlight conditions (). Light intensity at night measured at 0510 hours ranged from 38 to 195 lx directly under the tank light. Maximum intensity observed over the tank was 1,943 lx, measured at 1350 hours. On this day and time outdoor light intensity was >80,000 lx. Light intensity outdoors at night (0510 hours) was measured at 1 lx, increasing to about 1,400 lx by 0635 hours.
Follicle Diameter and Staging, and Viscera Proximate Analysis
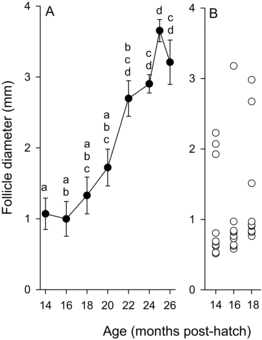
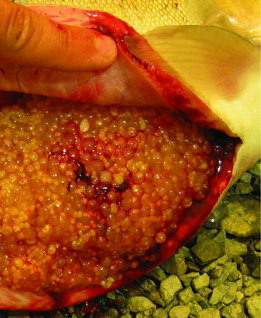
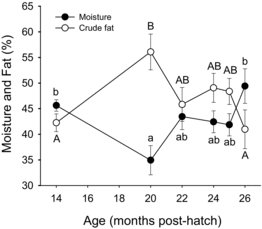
At 14 months posthatch, the first sampling of the study, the diameter of the follicles had reached a mean of 1.1 mm, which suggests most had reached early vitellogenesis (a; Lankford and Weber Citation2010). Oocyte diameter varied considerably among fish during months 14–18 (b). The follicles reached about 3.6 mm during month 25 (a), and all 10 fish had oocytes in which the GV reached the midmigration or later stage, one having several oocytes that completed GV breakdown (data not shown). During month 26, however, follicle diameters of fish sampled were smaller, about 3.2 mm, and although GV migration was observed in all fish, only four fish had reached the midmigration stage. Extensive follicle atresia was noted in 3 of 10 fish at month 24, 1 at month 25, and 2 at month 26 (see image, ). No fish ovulated during the study or in the month following the study. Fat content of the viscera on a percent wet weight basis increased and moisture content decreased from months 14 to 20 posthatch; thereafter fat content of the viscera generally decreased from month 20 posthatch through the end of maturation whereas moisture content of the viscera increased ().
Plasma Hormone Concentration Measurement
Plasma T were at about 7 ng/mL and E2 levels at 5 ng/mL from months 14 to 16 (), although hormone concentrations varied considerably with follicle diameter. Plasma concentrations of both hormones were more than sixfold greater on average in the fish with mean follicle diameters >1 mm than with those <1 mm during these months (data not shown). Plasma T increased to about 30 ng/mL by month 26. Plasma E2 increased to about 17 ng/mL by month 20 and plateaued for the next 5 months before increasing slightly to 24 ng/mL at month 26. The MIS 17,20βP remained low, never exceeding 0.4 ng/mL in any fish (data not shown). Growth hormone remained relatively unchanged although slightly higher overall during the last 5 months (a). Plasma IGF-I was higher at the first time point compared with values at later time points, although levels remained generally stable during the final seven months (b).
DISCUSSION
Rainbow Trout held under 24-h light conditions for at least part of their reproductive cycle and exposed to static temperatures around 13°C will still produce viable eggs (Bromage et al. Citation1984; Davies and Bromage Citation2002). However, exposure to 24-h light for about a year prior to normal spawning time resulted in early ovulation and a loss of synchronicity in a winter-spawning strain (Bromage et al. Citation1984). We previously reported (Davidson et al. 2014) changes in fillet quality traits and reductions in growth and fillet yield coincided with rapid ovarian growth, occurring mainly at or after month 22 posthatch. It is therefore notable that ovarian development may have been impeded. Mean follicle diameter was variable among fish during most months. There were 7 of 30 fish across months 14, 16, and 18 that had mean follicle diameters about threefold greater and T and E2 levels more than sixfold greater than the means of the remaining fish, suggesting a lack of synchronicity in recruitment into secondary or vitellogenic growth. The seven fish that had advanced follicle development also had among the highest values for viscerosomatic index (VSI), calculated as [(viscera weight − gonad weight)/whole body weight] × 100 (data not shown). This perhaps contributed to their early development since elevated lipid stores has been suggested to support the onset of puberty (see Taranger et al. Citation2010). It is not known, however, if such asynchrony is uncommon for this line of fish under normal broodstock culture conditions.
Ovaries were probably near to attaining full mass by the end of the study. The gonadosomatic index (GSI), calculated as (gonad weight/whole body weight) × 100, only reached 7% by month 26 (Davidson et al. Citation2014), and mean follicle diameter was about 3.6 mm which is less than would be expected for preovulatory fish of many strains of Rainbow Trout (Bromage et al. Citation1992). The strain we used, however, have relatively small egg diameters and GSI of about 10% at spawning (K. Martin, Troutlodge, personal communication). Consequently, the GSI and follicle diameter values are within expectations for preovulatory females of this line of fish, taking into account the large body size they attained in our study. Furthermore, most of the fish in the last 2 months of the study possessed oocytes that had initiated GV migration, an early step in ovarian follicle maturation (Nagahama and Yamashita Citation2008), which indicates the follicles had completed or nearly completed vitellogenic growth. The absence of any ovulating fish throughout the study, given that all fish examined had initiated GV migration by month 25, together with 6 out of 30 fish in the last 3 months having shown signs of extensive follicle atresia, suggests reproductive failure late in the reproductive cycle. The similarity between the ratio of fish exhibiting early follicle development and those with atresia is consistent with the notion that the fish exhibiting atresia may have been fish with early follicle recruitment.
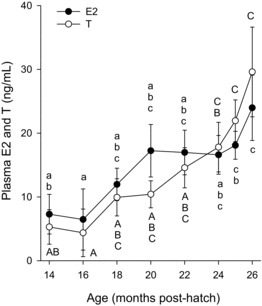
Plasma T and E2 concentrations were within normal ranges for maturing Rainbow Trout although reported levels are quite variable among studies (e.g., Scott and Sumpter Citation1983; Scott et al. Citation1983; Lankford and Weber Citation2010; Wilkinson et al. Citation2010). As mentioned, mean value for T and E2 in the early months were skewed upwards by a small number of advanced fish and, thus, the relative increase in the steroids over the year preceding spawning time are lower than what is often reported. Concentrations of 17,20βP were always very low, usually below detection and never exceeded 0.4 ng/mL in any fish. In salmonids, the MIS, 17,20βP, induces oocyte maturation, including the resumption of meiosis, as indicated by GV breakdown. Usually follicles with GV in midmigration become competent to produce and respond to the MIS, and this increase in plasma 17,20βP is usually preceded by a decrease in E2 and the E2:T ratio (Scott and Sumpter Citation1983; Scott et al. Citation1983; Bobe et al. Citation2003; Nakamura et al. Citation2005; Lankford and Weber Citation2010). Steroid profiles together with only one fish exhibiting GV breakdown, suggest the Rainbow Trout we studied either did not reach this stage of follicle maturational competence or did not receive a sufficient increase in gonadotropin to elicit increased MIS production for ovarian follicle maturation and ovulation (Scott et al. Citation1983; Bobe et al. Citation2003; Nagahama and Yamashita Citation2008). Weekly water quality measurements (Davidson et al. Citation2014) suggest reproductive problems did not derive from poor water quality.
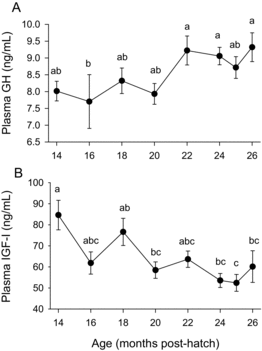
Plasma GH and IGF-I levels changed little throughout the study. Overall GH levels were slightly increased and IGF-I was slightly decreased from month 22 through 26 compared with the previous time points. The GSI increased from 3.5% to 7% over this period (Davidson et al. Citation2014). Previous studies of females from winter-spawning or autumn-spawning strains of Rainbow Trout exposed to ambient photoperiod and temperature conditions showed either no change in plasma GH before ovulation (Sumpter et al. Citation1991; Gomez et al. Citation1999) or slightly greater GH concentrations during the second half of ovarian growth (Holloway et al. Citation1999). The small or nonexistent changes in plasma GH in female Rainbow Trout going through vitellogenesis and approaching spawning is inconsistent with changes observed over the same developmental period in Atlantic Salmon Salmo salar (Björnsson et al. Citation1994; Benedet et al. Citation2010), suggesting differences in the roles of GH during reproduction among salmonids. Taylor et al. (Citation2008) reported substantial changes in plasma IGF-I in response to photoperiod manipulations during the reproductive cycle of female Rainbow Trout exposed to wide seasonal fluctuations in water temperature. They found peak levels of IGF-I at about 4–5 months prior to the onset of spawning. Plasma IGF-I levels changed little during our study, which covered at least 8 months prior to the expected spawning time at about month 24. However, the IGF-I peak in the study by Taylor et al. (Citation2008) occurred prior to the increase in T, which occurred about 4 months before spawning time in their study but earlier in ours (i.e., month 18). Furthermore, the first time point in our study, month 14, had the highest IGF-I levels. Therefore it is possible IGF-I levels were higher earlier in our study, prior to our collecting samples for hormone measurements, albeit fish that had T levels below detection limits (0.4 ng/mL) at months 14 and 16 did not have higher IGF-I than those with higher T levels in these same months (data not shown). In both the Taylor et al. (Citation2008) and our study, plasma IGF-I concentrations changed little between the onset of the rise in T concentrations and ovulation.
Other than a failure to see an increase in the MIS and a decrease in E2 late in our study, effects of the culture conditions on hormone profiles were not established. As previously noted, significant anabolic and catabolic processes are coincident during the later stages of maturation complicating interpretations of plasma sex steroid and growth axis hormone concentrations (Holloway et al. Citation1999). Plasma concentrations of E2, T, and GH levels were lower and IGF-I was higher before months 20–22, when GSI was low and more favorable growth and product quality measures were observed. Gains in product quality traits, including increased fillet thickness, percent separable muscle, and fillet yield, ended at about months 20–22, and visceral fat content and fillet firmness began to decrease (Davidson et al. Citation2014). These changes in traits related to decreases in growth are consistent with the increase in GH and reduction in IGF-I concentrations. Plasma GH has been shown to negatively correlate and IGF-I to positively correlate with growth rate in Rainbow Trout (Pérez-Sánchez and Le Bail Citation1999; Lankford and Weber Citation2006). However, bidirectional regulation of the sex steroids E2 and T with GH has also been described in fish including Rainbow Trout (see Le Gac et al. Citation1993; Holloway and Leatherland Citation1998). In this regard, Holloway and Leatherland (Citation1997) showed in vivo treatment with E2 and T increased GH in Rainbow Trout; they suggested that increased GH later in the reproductive cycle, as observed in our study, may be a response to increases in these steroids.
Notably, rapid changes in growth and fat mobilization, which took place late in the study, did not associate with changes in hormone levels. Body weight gain and thermal growth coefficient reached their lowest values by month 25 before reversing at month 26 (Davidson et al. Citation2014). At month 24, fillet firmness and VSI (excluding gonad) reached their lowest levels, and fillet fat content reached its highest level at this time before all reversed directions (Davidson et al. Citation2014). On the other hand, the high fat content of the viscera, which was always greater than 40%, together with the high VSI indicates that high levels of stored energy were available for tissue growth throughout the study. Furthermore, although viscera crude fat content and VSI started decreasing after month 20, fillet fat was increasing until month 24, indicating energy was still going to intramuscular fat stores even though visceral fat stores were decreasing, presumably to support gonad growth. Nevertheless, growth had slowed between months 23 and 24, therefore supporting that signaling for muscle growth might have been reduced after the initiation of visceral fat mobilization but before initiation of fillet fat mobilization or utilization and, therefore, before energy restrictions in the muscle. Although reductions in growth and a shift from using visceral adipose tissue lipid stores to using intramuscular adipose lipid stores occurred without detectable proximate changes in plasma sex steroid or growth axis hormone concentrations, changes in clearance, availability, or sensitivity to the hormones measured, particularly those of the growth axis, may still have been affected by the culture conditions and contributed to changes we observed in growth and nutrient utilization (as supported by Benedet et al. Citation2010; Norbeck and Sheridan Citation2011; Reindl and Sheridan Citation2012). In addition, plasma sampling did not address possible changes in diurnal hormone patterns that might have resulted from changing lighting or feeding every 6 h.
Unlike in most previous studies in salmonids examining changes in hormone levels over multiple seasons or with sexual maturation, the hormone patterns we observed cannot be attributed to changes in temperature. The influence of photoperiod signals is less certain. Photoperiod, including constant lighting, has been shown to have strong effects on reproduction and growth in Rainbow Trout (Bromage et al. Citation2001; Taylor et al. Citation2006). This includes light provided 24-h to fish maintained outdoors (Taylor et al. Citation2006). Previous studies have shown night-time light levels as low as 10 lx can affect plasma melatonin in Rainbow Trout (Alvarino et al. Citation1993; Bromage et al. Citation2001), and spectrum and intensity matters to reproduction, growth, and stress response (Karakatsouli et al. Citation2008; Falcón et al. Citation2010). We recorded a minimum of 38 lx in our tanks and at least 10-fold changes in light intensity during the course of the day, which surely also changed in intensity with seasonal changes in ambient light intensities. Together, these measures and the related studies suggest the fish we studied received photoperiod stimuli that probably affected multiple endocrine systems including disruption of normal reproduction cycles. Furthermore, both the reproduction and growth axes crosstalk with the thyroid system, which is directly responsive to environmental signals (including temperature and photoperiod) and changes in metabolic state, and thyroid hormones directly regulate metabolism (Larsen et al. Citation2001; Blanton and Specker Citation2007; Reindl and Sheridan Citation2012). It is not clear to what extent the observed changes in hormones of either the reproductive or growth axes might be responses to each other, some other hormone system, changing metabolic state, or environmental signals.
In summary, gradual changes in growth and fillet-quality attributes during gonadal development were accompanied by gradual changes in hormone levels but no clear changes in hormones were associated with rapid changes in product-quality traits observed around months 24–26. In addition, the culture conditions we used appeared to lead to reproductive dysfunction, particularly as the follicles approached maturation and ovulation. Our findings provide important data to compare with other studies examining the impact of culture conditions on interactions among reproduction and growth that affect fillet yield and quality traits.
ACKNOWLEDGMENTS
The authors thank Lisa Radler, Jill Birkett, and David Payne for their technical expertise during sample collection and analysis. This research was supported by the U.S. Department of Agricuture (USDA), Agricultural Research Service under Agreement 59-1930-5-510 and 59-1930-0-046 and from the Agricultural Research Service Project 1930-31000-010-000D. All experimental protocols and methods were in compliance with the Animal Welfare Act (9CFR) requirements and were approved by the Freshwater Institute's Institutional Animal Care and Use Committee. Mention of trade names is solely for the purpose of providing accurate information and should not imply product endorsement by the USDA. The USDA is an equal opportunity provider and employer.
REFERENCES
- Alvarino, J.M., C.F. Randall, and N. Bromage. 1993. Pattern of melatonin secretion in the Rainbow Trout exposed to light pulses of different duration and intensity. Pages 191–196 in A. Cerviño, A. Landin, A. de Coo, A. Guerra, and M. Torre, editors. Actas del IV Congreso Nacional de Acuicultura. [Proceedings of the Fourth National Congress on Aquaculture.] Villanova de Arosa, Spain.
- AOAC. 1990. Official methods of analysis of the Association of Official Analytical Chemists, 15th edition. AOAC, Arlington, Virginia.
- Aussanasuwannakul, A., P.B. Kenney, G.M. Weber, J.B. Yao, S.D. Slider, M.L. Manor, and M. Salem. 2011. Effect of sexual maturation on growth, fillet composition, and texture of female Rainbow Trout (Oncorhynchus mykiss) on a high nutritional plane. Aquaculture 317:79–88.
- Aussanasuwannakul, A., G.M. Weber, M. Salem, J.B. Yao, S. Slider, M.L. Manor, and P.B. Kenney. 2012. Effect of sexual maturation on thermal stability, viscoelastic properties, and texture of female Rainbow Trout, Oncorhynchus mykiss, fillets. Journal of Food Science 77(Supplement):S77–S83.
- Benedet, S., E. Andersson, C. Mittelholzer, G.L. Taranger, and B.T. Björnsson. 2010. Pituitary and plasma growth hormone dynamics during sexual maturation of female Atlantic Salmon. General and Comparative Endocrinology 167:77–85.
- Björnsson, B.T. 1997. The biology of salmon growth hormone: from daylight to dominance. Fish Physiology and Biochemistry 17:9–24.
- Björnsson, B.T., G.L. Taranger, T. Hansen, S.O. Stefansson, and C. Haux. 1994. The interrelation between photoperiod, growth hormone, and sexual maturation of adult Atlantic Salmon (Salmo salar). General and Comparative Endocrinology 93:70–81.
- Blanton, M.L., and J.L. Specker. 2007. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Critical Reviews in Toxicology 37:97–115.
- Bobe, J., G. Maugars, T. Nguyen, H. Rime, and B. Jalabert. 2003. Rainbow Trout follicular maturational competence acquisition is associated with an increased expression of follicle stimulating hormone receptor and insulin-like growth factor 2 messenger RNAs. Molecular Reproduction and Development 66:46–53.
- Bromage, N., J. Jones, C. Randall, M. Thrush, B. Davies, J. Springate, J. Duston, and G. Barker. 1992. Broodstock management, fecundity, egg quality and the timing of egg production in the Rainbow Trout (Oncorhynchus mykiss). Aquaculture 100:141–166.
- Bromage, N., M. Porter, and C. Randall. 2001. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98.
- Bromage, N.R., J.A. K. Elliott, J.R. C. Springate, and C. Whitehead. 1984. The effects of constant photoperiods on the timing of spawning in the Rainbow Trout. Aquaculture 43:213–223.
- Davidson, J.W., P.B. Kenney, M.L. Manor, C.M. Good, G.M. Weber, A. Aussanasuwannakul, P.J. Turk, C. Welsh, and S.T. Summerfelt. 2014. Growth performance, fillet quality, and reproductive maturity of Rainbow Trout (Oncorhynchus mykiss) cultured to 5 kilograms within freshwater recirculating systems. Journal of Aquaculture Research and Development [online serial] 5:238.
- Davies, B., and N. Bromage. 2002. The effects of fluctuating seasonal and constant water temperatures on the photoperiodic advancement of reproduction in female Rainbow Trout, Oncorhynchus mykiss. Aquaculture 205:183–200.
- Falcón, J., H. Migaud, J.A. Muñoz-Cueto, and M. Carrillo. 2010. Current knowledge on the melatonin system in teleost fish. General and Comparative Endocrinology 165:469–482.
- Fitzpatrick, M.S., G. Van Der Kraak, and C.B. Schreck. 1986. Profiles of plasma sex steroids and gonadotropin in Coho Salmon, Oncorhynchus kisutch, during final maturation. General and Comparative Endocrinology 62:437–451.
- Gomez, J.M., C. Weil, M. Ollitrault, P.Y. Le Bail, B. Breton, and F. Le Gac. 1999. Growth hormone (GH) and gonadotropin subunit gene expression and pituitary and plasma changes during spermatogenesis and oogenesis in Rainbow Trout (Oncorhynchus mykiss). General and Comparative Endocrinology 113:413–428.
- Holloway, A.C., and J.F. Leatherland. 1997. Effect of gonadal steroid hormones on plasma growth hormone concentrations in sexually immature Rainbow Trout, Oncorhynchus mykiss. General and Comparative Endocrinology 105:246–254.
- Holloway, A.C., and J.F. Leatherland. 1998. Neuroendocrine regulation of growth hormone secretion in teleost fishes with emphasis on the involvement of gonadal sex steroids. Reviews in Fish Biology and Fisheries 8:409–429.
- Holloway, A.C., M.A. Sheridan, G. Van Der Kraak, and J.F. Leatherland. 1999. Correlations of plasma growth hormone with somatostatin, gonadal steroid hormones and thyroid hormones in Rainbow Trout during sexual recrudescence. Comparative Biochemistry and Physiology B 123:251–260.
- Karakatsouli, N., S.E. Papoutsoglou, G. Panopoulos, E.S. Papoutsoglou, S. Chadio, and D. Kalogiannis. 2008. Effects of light spectrum on growth and stress response of Rainbow Trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacultural Engineering 38:36–42.
- Kiessling, A., L. Larsson, K.H. Kiessling, P.B. Lutes, T. Storebakken, and S.S. S. Hung. 1995. Spawning induces a shift in energy metabolism from glucose to lipid in Rainbow Trout white muscle. Fish Physiology and Biochemistry 14:439–448.
- Lankford, S.E., and G.M. Weber. 2006. Associations between plasma growth hormone, insulin-like growth factor-I, and cortisol with stress responsiveness and growth performance in a selective breeding program for Rainbow Trout. North American Journal of Aquaculture 68:151–159.
- Lankford, S.E., and G.M. Weber. 2010. Temporal mRNA expression of transforming growth factor-beta superfamily members and inhibitors in the developing Rainbow Trout ovary. General and Comparative Endocrinology 166:250–258.
- Larsen, D.A., B.R. Beckman, and W.W. Dickoff. 2001. The effect of low temperature and fasting during the winter on metabolic stores and endocrine physiology (insulin, insulin-like growth factor-I, and thyroxine) of Coho Salmon, Oncorhynchus kisutch. General and Comparative Endocrinology 123:308–323.
- Le Gac, F.O. Blaise, A. Fostier, P.Y. Le Bail, M. Loir, B. Mourot, and C. Weil. 1993. Growth hormone (GH) and reproduction: a review. Fish Physiology and Biochemistry 11:219–232.
- Lubzens, E., G. Young, J. Bobe, and J. Cerdà. 2010. Oogenesis in teleosts: how fish eggs are formed. General and Comparative Endocrinology 165:367–389.
- Manor, M.L., G.M. Weber, M. Salem, J.B. Yao, A. Aussanasuwannakul, and P.B. Kenney. 2012. Effect of sexual maturation and triploidy on chemical composition and fatty acid content of energy stores in female Rainbow Trout, Oncorhynchus mykiss. Aquaculture 364:312–321.
- Moriyama, S., P. Swanson, M. Nishii, A. Takahashi, H. Kawauchi, W.W. Dickhoff, and E.M. Plisetskaya. 1994. Development of a homologous radioimmunoassay for Coho Salmon insulin-like growth factor-I. General and Comparative Endocrinology 96:149–161.
- Nagahama, Y., and M. Yamashita. 2008. Regulation of oocyte maturation in fish. Development Growth and Differentiation 50(Supplement):S195–S219.
- Nakamura, I., J.C. Evans, M. Kusakabe, Y. Nagahama, and G. Young. 2005. Changes in steroidogenic enzyme and steroidogenic acute regulatory protein messenger RNAs in ovarian follicles during ovarian development of Rainbow Trout (Oncorhynchus mykiss). General and Comparative Endocrinology 144:224–231.
- Nassour, I., and C.L. Leger. 1989. Deposition and mobilisation of body fat during sexual maturation in female trout (Salmo gairdneri Richardson). Aquatic Living Resources 2:153–159.
- Norbeck, L.A., and M.A. Sheridan. 2011. An in vitro model for evaluating peripheral regulation of growth in fish: effects of 17β-estradiol and testosterone on the expression of growth hormone receptors, insulin-like growth factors, and insulin-like growth factor type 1 receptors in Rainbow Trout (Oncorhynchus mykiss). General and Comparative Endocrinology 173:270–280.
- Pérez-Sánchez, J., and P.Y. Le Bail. 1999. Growth hormone axis as marker of nutritional status and growth performance in fish. Aquaculture 177:117–128.
- Peterson, B.C., P.R. Simpson, K.D. Cain, R.H. Hardy, G.T. Schelling, and T.L. Ott. 2003. Effects of administration of somatostatin-14 and immunoneutralization of somatostatin on endocrine and growth responses in Rainbow Trout. Journal of Fish Biology 63:506–522.
- Reindl, K.M., and M.A. Sheridan. 2012. Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comparative Biochemistry and Physiology A 163:231–245.
- Reinecke, M. 2010. Insulin-like growth factors and fish reproduction. Biology of Reproduction 82:656–661.
- Salem, M., P.B. Kenney, C.E. Rexroad III, and J. Yao. 2006. Microarray gene expression analysis in atrophying Rainbow Trout muscle: a unique nonmammalian muscle degradation model. Physiological Genomics 28:33–45.
- Salem, M., P.B. Kenney, C.E. Rexroad III, and J. Yao. 2010. Proteomic signature of muscle atrophy in Rainbow Trout. Journal of Proteomics 73:778–789.
- Scott, A.P., and J.P. Sumpter. 1983. A comparison of the female reproductive cycles of autumn-spawning and winter-spawning strains of Rainbow Trout (Salmo gairdneri Richardson). General and Comparative Endocrinology 52:79–85.
- Scott, A.P., J.P. Sumpter, and P.A. Hardiman. 1983. Hormone changes during ovulation in the Rainbow Trout (Salmo gairdneri Richardson). General and Comparative Endocrinology 49:128–134.
- Shearer, K.D. 1994. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 119:63–88.
- Shepherd, B.S., K. Drennon, J. Johnson, J.W. Nichols, R.C. Playle, T.D. Singer, and M.M. Vijayan. 2005. Salinity acclimation affects the somatotropic axis in Rainbow Trout. American Journal of Physiology Regulatory Integrative and Comparative Physiology 288:R1385–R1395.
- Shimizu, M., P. Swanson, H. Fukada, A. Hara, and W.W. Dickhoff. 2000. Comparison of extraction methods and assay validation for salmon insulin-like growth factor-I using commercially available components. General and Comparative Endocrinology 119:26–36.
- Summerfelt, S., J. Bebak-Williams, and S. Tsukuda. 2001. Controlled systems: water reuse and recirculation. Pages 285–395 in G.A. Wedemeyer, editor. Fish hatchery management, 2nd edition. American Fisheries Society, Bethesda, Maryland.
- Sumpter, J.P., R.F. Lincoln, V.J. Bye, J.F. Carragher, and P.Y. Le Bail. 1991. Plasma growth hormone levels during sexual maturation in diploid and triploid Rainbow Trout (Oncorhynchus mykiss). General and Comparative Endocrinology 83:103–110.
- Taranger, G.L., M. Carrillo, R.W. Schulz, P. Fontaine, S. Zanuy, A. Felip, F.A. Weltzien, S. Dufour, Ø. Karlsen, B. Norberg, E. Andersson, and T. Hansen. 2010. Control of puberty in farmed fish. General and Comparative Endocrinology 165:483–515.
- Taylor, J.F., B.P. North, M.J. R. Porter, N.R. Bromage, and H. Migaud. 2006. Photoperiod can be used to enhance growth and improve feeding efficiency in farmed Rainbow Trout, Oncorhynchus mykiss. Aquaculture 256:216–234.
- Taylor, J.F., M.J. R. Porter, N.R. Bromage, and H. Migaud. 2008. Relationships between environmental changes, maturity, growth rate and plasma insulin-like growth factor-I (IGF-I) in female Rainbow Trout. General and Comparative Endocrinology 155:257–270.
- Wedemeyer, G.A. 1996. Physiology of fish in intensive culture systems. Chapman and Hall, New York.
- Wilkinson, R.J., R. Longland, H. Woolcott, and M.J. R. Porter. 2010. Effect of elevated winter-spring water temperature on sexual maturation in photoperiod manipulated stocks of Rainbow Trout (Oncorhynchus mykiss). Aquaculture 309:236–244.
- Woods, C.L., and C.V. Sullivan. 1993. Reproduction of Striped Bass, Morone saxatilis (Walbaum), broodstock: monitoring maturation and hormonal induction of spawning. Aquaculture Research 24:211–222.
- Young, G., L.W. Crim, H. Kagawa, A. Kambegawa, and Y. Nagahama. 1983. Plasma 17α,20β-dihydroxy-4-pregnen-3-one levels during sexual maturation of Amago Salmon (Oncorhynchus rhodurus): correlation with plasma gonadotropin and in vitro production by ovarian follicles. General and Comparative Endocrinology 51:96–105.