Abstract
In order to select appropriate amendments for cropping hyperaccumulator or normal plants on contaminated soils and establish the relationship between Cd sorption characteristics of soil amendments and their capacity to reduce Cd uptake by plants, batch sorption experiments with 11 different clay minerals and organic materials and a pot experiment with the same amendments were carried out. The pot experiment was conducted with Sedum alfredii and maize (Zea mays) in a co-cropping system. The results showed that the highest sorption amount was by montmorillonite at 40.82 mg/g, while mica was the lowest at only 1.83 mg/g. There was a significant negative correlation between the n value of Freundlich equation and Cd uptake by plants, and between the logarithm of the stability constant K of the Langmuir equation and plant uptake. Humic acids (HAs) and mushroom manure increased Cd uptake by S. alfredii, but not maize, thus they are suitable as soil amendments for the co-cropping S. alfredii and maize. The stability constant K in these cases was 0.14–0.16 L/mg and n values were 1.51–2.19. The alkaline zeolite and mica had the best fixation abilities and significantly decreased Cd uptake by the both plants, with K ≥ 1.49 L/mg and n ≥ 3.59.
INTRODUCTION
Heavy metal pollution of croplands in China has recently intensified due to mining activity, and the application of sewage sludge and heavy metal-containing fertilizers. This has resulted in a significant challenge for food security and ecological safety in China (Chen et al. Citation2000).
At present, remediation methods for heavy metal contaminated soil include physical, biological and chemical treatments. Among them, chemical remediation typically employs soil amendments which can reduce their bioavailability through a variety of reactions. The technology relies on choosing an economic and effective amendment (Mercier and Detellier Citation1995). Many scientists have studied the adsorption of heavy metals on clay minerals including zeolite, sepiolite, attapulgus, illite, montmorillonite and other natural clay minerals or modified forms, and have found that they have great potential to remove heavy metals from water (Liu and Gonzalez Citation1999; Liu Citation2007; Missana, Gutierrez, and Alonso Citation2008; Bailey et al. Citation1999; Gworek Citation1992a, 1992b). These clay minerals are low cost materials and offer an attractive and inexpensive remediation option. They are abundant and cheap, with negatively charged layered aluminosilicates that make them good cationic adsorbents because of their relatively large surface areas (Wu et al. Citation2009). In recent years, researchers have used clay minerals in the remediation of heavy metal contaminated soil and achieved positive results (Haidouti Citation1997; Zorpas et al. Citation2000; Nissen, Lepp, and Edwards Citation2000; Garćla-Sánchez, Alastuey, and Querol Citation1999).
Organic amendments have also been used in the remediation of heavy metal contaminated soils (Herwijnen et al. Citation2007). Application of organicmatter in forms such as cattle manure, pig manure, chicken manure, peat and crop straw are inexpensive, highly available and feasible in the restoration of heavy metal contaminated soils. It was shown by Sauvé et al. (Citation2003) that the adsorption ability of heavy metals on soil organic matter is 30 times that of the clay minerals, so soils with high organic matter content had a higher adsorption capacity and could effectively reduce the mobilityofheavymetals. The stability of the complex will influence the bioavailability and extraction of heavy metals by plants. In addition, the organic materials can affect the acidity and redox properties of the soil (Walker et al. Citation2003; Walker, Clemente, and Bernal Citation2004). Although in-situ remediation with amendments for heavy metal contaminated soils is widely studied, the impact of OM on the element retention is case specific and generalizations are difficult to make (Kumpiene, Lagerkvist, and Maurice Citation2008). The relationship between the sorption characteristics of soil amendments and subsequent effects in reducing plant uptake has not been established.
Among common remediation methods of heavy metal contaminated soils, phytoremediation has the advantages of in situ remediation, low cost, and the retention of soil biological function (McGrath, Zhao, and Lombi Citation2002). Conversely, it presents disadvantages including the problem that the hyper accumulators often have a small biomass and thus low extraction rate, and so a long time is required for soil clean up. Therefore some studies have used natural and/or synthetic chelators such as EDTA, EGTA, EDDS to increase the uptake and translocation of heavy metals from soil, thus achieving higher removal rates (Doong, Wu, and Lei Citation1998; Tandy et al. Citation2004; Zheng et al. Citation2007). Unfortunately, most of these chelators showed some negative effects to the soil environment such as elevated toxicity to both plant and the groundwater. Therefore, it is crucial to develop a method to enhance phytoremediation efficiency and minimize adverse effects. Alternatively, some researchers proposed a co-crop system of a hyperaccumulator with a low metal crop, which ensures production of animal feeds whilst remediating the soil (Liu, Wu, and Banks Citation2005; Wu, Wei, and Ouyang Citation2007). In this system, animal manure applications are required with successive croppings to maintain the crop yield, but the application of amendments should ideally not decrease the metal uptake by the co-planted hyperaccumulator.
A pot experiment was conducted in this study to screen for environmentally friendly and low cost soil amendments which will not inhibit the remediation efficiency of Sedum alfredii yet reduce metal uptake by co-cropped maize. Sorption experiments of Cd on the same soil amendments were also conducted. The results from both experiments will provide probably suitable sorption parameters which can help in predicting the effects of amendments on phytoavailability of heavy metals for two different plants in amended soil.
MATERIALS AND METHODS
Materials
Kaolinite, montmorillonite, zeolite, diatomite, and micas were obtained from Guangying Co. LTD (Guangdong province, China). Pigmanure, chickenmanure, wormcast, mushroom residue and peatwere purchased from the Xuneng Biotechnology Co. LTD (Guangdong province, China). Humicacids (HAs, secondgenerationproduct) were obtained from Wanguo Biochemical Group R&D Center (Hebei province, China). All other chemicals used in this study (NaNO3, Cd(NO3)2·4H2O, HNO3 and NaOH) were analytical reagent grade. Stock solution of Cd2+ was prepared by dissolving cadmium nitrate in deionized water. The characteristics of the clay minerals and organic materials are shown in .
Table 1 Chemical characteristics of the clay minerals and organic materails used as amendments in this study
Heavy metal contaminated soil was collected from 0–20 cm in Shangba, an agricultural field adjacent to a mine in Shaoguan in the Guangdong province of China. The basicphysiochemicalproperties and total contents of nutrients and heavy metals of the soil were as follows: clay (<0.001 mm) 250 g/kg, pH 4.43, CEC 3.3 cmol/kg, organic matter 15.7 g/kg, N 1.37 g/kg, P 0.58 g/kg, K 16.2 g/kg, Cd 0.69 mg/kg, Zn 355.7 mg/kg, Pb 512.4 mg/kg and Cu 517.7 mg/kg. The soil parameters were determined using the methods proposed by the Soil Science Society of China (Lu Citation2000). For determining the total contents of heavy metals, the soil samples were digested using HNO3, HF, HClO4 in the ratio 5:5:3 (Lu Citation2000) and the concentrations of heavy metals in the solutions were determined by Atomic Absorption Spectrometry (AAS) (Hitachi Z-2300, Z-2700).
The Cd/Zn hyperaccumulator used was Sedum alfredii, which was collected from an old Pb/Zn mine area in Zhejiang province, China. The crop plant used was maize (Zea mays, CV. Yunshi-5) which was obtained from the Chunxi SeedIndustry (Yunnan province, China).
Sorption Tests
According to the method of Kooner et al. (Citation1992), sorption tests were performed by batch technique in three replicates. 0.125 g of the adsorbent was weighed into 100 mL centrifuge tubes containing 25 mL of known concentration of Cd2+ solution (0.4–160 mg/L, 9 concentrations). The experiments were conducted with 0.01 mol/L NaNO3 as the background electrolyte and the pH was adjusted to 5.0 by 0.1 M NaOH or 0.1 M HNO3. The suspension was shaken at 160 rpm for 24h at 25°C. After centrifugation at 5000 rpm for 10 min, the concentration of Cd2+ in the supernatant was analyzed with AAS (Hitachi Z-2300, Z-2700). The amount of Cd2+ adsorbed was calculated by the difference between the amount of Cd2+ added and that remaining in the solution.
Pot Experiment
The pot culture was conducted in a greenhouse at the South China Agricultural University. The soil samples were air dried and ground to pass through a 5 mm sieve. A total of 5 kg of dry soil was used in each pot (19 × 21 × 23 cm3). Eleven different amendments were separately added to the soil at the same level (50 g/pot). A control without amendment was also set up. In order to avoid the variability of soil fertility, all 12 treatments were fertilized with 100, 100 and 80 mg N, K and P per kg soil, respectively (added with urea and KH2PO4).
Seeds of maize were sterilized in 2% (v/v) hydrogen peroxide for 15 min, washed with tap water and soaked in water for one day, then sowed directly into prepared soils in October 2011. Five seedlings of S. alfredii (5 cm in height) were transplanted into each pot. After three weeks, maize seedlings were thinned to one seedling per pot. Tap water was added throughout the experiment to sustain 60% water holding capacity. Each treatment was performed in four replicates. Maize was harvested after reaching maturity (100 days) and S. alfredii one week later.
Determination of Plant Heavy Metals
The plant tissues were dried at 65°C for 76 h to obtain the dry weights. The oven dried materials were finely ground in an agate mill. The plant samples were incinerated smokelessly on a hotplate and placed in a muffle furnace at 550°C for 5.5 h. The dry ash was then dissolved by 2 mL of 1:1 (v:v) HCl and adjusted to a final volume of 25 mL for total metal analysis. The concentrations of Zn, Cd and Pb were determined with AAS.
Statistical Analysis
The means and standard deviations (SD) were calculated by Microsoft Office Excel 2003. The sorption isotherm simulation was conducted with Origin 7.05. Analysis of variance and comparison between means by Duncan's test were carried out with SAS 8.1 (SAS Institute Inc., Cary, NC, USA) (Hong and Hou Citation2004).
RESULTS
Sorption Isotherms
shows parameters determined from the sorption isotherms of Cd2+ on the various clay minerals and organic materials. The sorption data were fit with the Langmuir (1) and Freundlich (2) models:
Table 2 Simulated parameters of Cd2+ adsorption on soil amendments by the Langmuir and Freundlich isotherm models. (25°C, 9 Cd concentrations; significant R value = 0.666)
The sorption parameters obtained from the two models () showed that the Freundlich model did not fit the Cd sorption data for the amendments as well as the Langmuir except for kaolinite and diatomite. However, the Freundlich equation displayed a wide adaptability with significant correlations for all amendments. Maximum sorption amount of Cd2+, was highest for montmorillonite at 40.82 mg/g, followed by pig manure and wormcast manure at 37.04 mg/g and 36.10 mg/g, respectively. The lowest was mica at 1.83 mg/g.
Many studies have reported the sorption of Cd2+ on organic materials and clay minerals. Wu et al. (Citation2011) studied the sorption ability of Cd2+ from aqueous solutions on humic acid modified Ca-montmorillonite, and the results showed that the Langmuir model provided a better fit for adsorption of Cd2+ on the modified clay than the Freundlich model, as well as for the adsorption of Cd2+ by the raw clay. Karapinar and Donat (Citation2009) reported the Langmuir model effectively described the sorption data of Cd2+ on bentonite with R2 values >0.99 with the maximum adsorption of 7.64 mg/g (0.068 mmol/g). However, Gupta and Bhattacharyya (Citation2006) reported that the adsorption of Cd2+ on kaolinite and montmorillonite was well fitted with both the Langmuir and Freundlich isotherms (R2 values of >0.96). Ibrahim et al. (Citation2010) showed similar results for zeolites prepared from local Egyptian clay (kaolin). Safa et al. (Citation2012) studied the adsorption of Cd2+ from aqueous solution by Algerian raw diatomite and the results showed that the adsorption equilibrium was well described by both model isotherms, while the maximum adsorption capacities reached 20.2 mg/g (0.18 mmol/g). Moreover, Kalmykova et al. (Citation2008) investigated the adsorption of metal ions on Sphagnum peat and the results showed the adsorption data fitted the Freundlich equation with R2 >0.96. Zhu et al. (Citation2008) studied the adsorption behavior of Cd2+ on pig manure and wormcast manure, and found that the adsorption isotherm conformed to the Freundlich and Henry equations, and a much higher adsorption capacity of Cd2+ was found in wormcast manure than in pig manure. Compared to these studies, the results of the present experiment are normal.
Effects of Soil Amendments on Plant Growth and Soil pH
illustrates the effects of amendments on soil pH and maize biomass. At the end of the experiment, no significant change in soil pH was detected for amended soils except in those treated with zeolite and mica. The soil pH in the alkaline zeolite and mica treatments increased by 3.07 and 2.76 compared to the control. Soil pH is considered to be one of the most important chemical factors controlling the availability of heavy metals (Li et al. Citation2011). The increase in soil pH could decrease the phytoavailability of the soil. Zhang et al. (Citation2006) found that soil pH increased by 1.2 with addition of manures. The results of this experiment showed only a 0.04–0.29 pH increase with the application of organic materials.
Table 3 Final soil pH, biomass production and Cd uptake by maize in response to application of different amendments
Maize grain production among treatments of mushroom manure, humic acids, wormcast manure, pig manure, chicken manure, peat soil, mica and zeolite were significantly higher than that of the control (). Grain production increased 47.9%, 35.4%, 73.5%, 84.5%, 87.6%, 74.0%, 92.7%, and 70.1%, relative to the control, respectively. Maize straw biomasses among soils treated with wormcast manure, chicken manure, mica, peat and zeolite were significantly higher compared with the control (16.7–53.4% higher than control). This suggests that organic materials can effectively promote the growth of maize in this Cd treated soil and that maize grain and straw biomass can increase with the application of organic materials, especially maize grain. Liu et al. (Citation2009) also reported that chicken manure compost significantly increased wheat seed yield in Cd treated soils. For the clay minerals, only the alkaline mica and zeolite treatments significantly increased maize yield, probably due to the pH increase, which was more suitable for the growth of maize than the non-amended soil.
Effects of Soil Amendments on Cd Uptake by Maize
The applications of amendments did not significantly decrease the Cd content of maize grain (). In fact, mushroom manure, HAs, pig manure and diatomite amendments increased grain Cd compared with the control. However, the Cd concentrations in maize grain for all the treatments were still below the limit required by the Chinese food standards (GB 2762-2005).
It was found that mushroom manure, HAs, mica and zeolite significantly diminished Cd concentration in maize straw compared with the control (), by 32.3%, 24.7%, 56.9%, and 53.0%, respectively. This indicates that the translocation of Cd from maize straw to grain may be increased with the application of HAs and mushroom manure, similar to the study of Wang et al. (Citation1999) who reported that rice straw and Chinese mile vetch promoted the transport of Cu from straw to grain of rice. The other amendments did not significantly decrease Cd concentrations in maize straw.
The results showed that the total uptake of Cd significantly decreased in the mica and zeolite treatments compared with that of control, while it significantly increased in the chicken manure and diatomite treatments ().
It appears that application of mica and zeolite can greatly decrease Cd phytoavailability in soil by decreasing not only its uptake by maize, but also the Cd contents in grain and straw. Mushroom manure and HAs can decrease Cd in maize straw and the uptake by maize but may increase the translocation of Cd to grain. Diatomite can increase Cd phytoavailability in the tested soil indicated by increased Cd total uptake by maize and Cd contents in straw and grain.
Effects of Amendments on the Phytoextraction Rate of Heavy Metals by Sedum alfredii
shows the effect of amendments on the phytoextraction rate of Cd and Zn by S. alfredii. Compared to the control, phytoextraction rate of Cd significantly increased by 47.5% and 48.5% in the mushroom manure and HAs treatments, respectively while the mica and zeolite treatments significantly decreased by 80.5% and 83.2%, respectively. The other amendments did not significantly influence the phytoextraction rate.
Table 4 Phytoextraction rate of Cd and Zn by Sedum alfredii among soils treated with different amendments
S. alfredii is also a hyperaccumulator of Zn. The phytoextraction rate of Zn was significantly increased by HAs, similar to Cd, but Mushroom manure did not have a significant influence on Zn phytoextraction. Mica and zeolite significantly decreased the phytoextraction of Zn by S. alfredii.
Relationship Between Cd Sorption Constant on Amendments and Plant Uptake
Significant negative correlations were found between Cd concentration in maize grain and isotherm model components, n (Freundlich) and K (Langmuir; ). Similar trends were observed for Cd concentration in maize straw (). Cadmium phytoextraction rate for S. alfredii was also significantly and negatively correlated with n and logK (). Nevertheless, the maximum sorption capacity (Qmax) predicted by the Langmuir isotherm and the Kf constant from the Freundlich isotherm did not significantly correlate with Cd in maize, or with S. alfredii phytoextraction rate. Therefore, the effect of amendments on Cd uptake by plants seems related to the stability or energy constants of sorption, which provides a useful tool.
Figure 1 Correlations between Cd concentrations in maize and sorption stability constants generated from Freundlich (n) and Langmuir isotherms (K) for soil amendments (The horizontal lines represent the intervals of the mean Cd concentration of the control without amendment. One * and two ** indicate significant at probability 95% and 99% for the correlation).
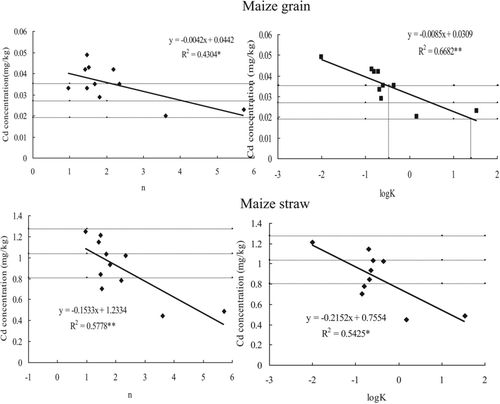
Figure 2 Correlations between the Cd phytoextraction rates by Sedum alfredii and sorption stability constants of soil amendments simulated by the Freundlich (n) and Langmuir (K) isotherm models (The horizontal lines represent the intervals of the mean Cd phytoextraction of the control without amendment. One * and two ** indicate significant at probability 95% and 99% for the correlation).
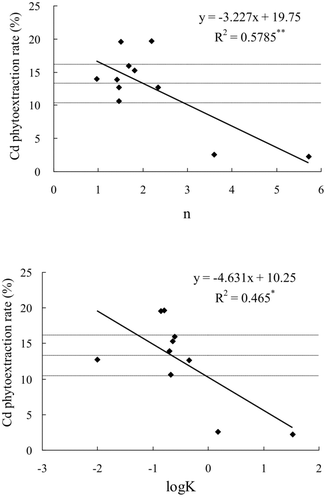
shows that there were four amendments that could significantly decrease Cd in maize straw, but only two amendments significantly decreased Cd phytoextraction by S. alfredii ( and ; alkaline mica and zeolite). Alkaline mica and zeolite produced an n ≥ 3.59 and K ≥ 1.49 L/mg (logK ≥ 0.17) (). Amendments that were amendments in adsorption effectiveness were mushroom manure and HAs (), with n ranging from 1.51 to 2.19, and K from 0.14 to 0.16 L/mg ().
DISCUSSION
In the sorption procedure, the Cd-amendment solutions were initially adjusted to pH 5, as suggested by Kooner et al. (Citation1992), but the solution pH was not maintained. The measured pH after the sorption equilibrium was 6.18–6.85 and 6.36–7.17 for mica and zeolite, respectively, which was similar to the amended soil (). This may also partly simulate the real effect of the amendment on soil pH and might be good for predicting the behavior of the amendment in soil. Under this condition, The degree of sorption was partly due to the precipitation of Cd. Therefore, the sorption constants obtained from the study were the result of precipitation and adsorption. Similar effects have been described in the literature, for example, Sastre et al. (Citation2006) pointed out that metal precipitation should not be disregarded in the processing of adsorption process.
The alkaline zeolite and mica were found to be effective for normal crops in this study since they were able to significantly decrease Cd phytoavailability in the studied acid soil. The other amendments including all organic amendments could be used for cropping the hyperaccumulator if necessary. In particular, soil amendments possessing Freundlich n values between 1.51 and 2.19 (0.14 ≤ K ≤ 0.16 L/mg) such as HAs and mushroom manure can reduce the Cd concentration in maize straw but not phytoextraction of Cd by S. alfredii, making them suitable for co-cropping. Chicken manure and diatomite are not suitable for cropping maize on Cd contaminated soils, because they may increase the uptake of Cd (). Diatomite had a very low stability constant (K = 0.01 L/mg), which explains the lack of ability to decrease Cd uptake. However, the increase in Cd uptake with amendment of chicken manure cannot be explained by a low sorption stability constant. The involved mechanisms need to be elucidated.
CONCLUSION
The maximum Cd sorption capacity for montmorillonite (40.82 mg/g) was the highest among all studied amendments, followed by pig manure (37.04 mg/g) and wormcast manure (36.10 mg/g). Mica had the lowest Cd sorption at 1.83 mg/g. The n value fitted by the Freundlich equation and the logarithm of equilibrium constant K fitted by the Langmuir equation presented a significant linear negative correlation with Cd uptake by plants, At an application rate of 10 g/kg in a co-cropping system, HAs and mushroom manure increased the Cd uptake in S. alfredii, but not in maize, thus are suitable for the co-crop system. Alkaline zeolite and mica had the highest fixation ability and decreased Cd uptake by both S. alfredii and maize and had a K value of ≥1.49 L/mg and n ≥3.59. These amendments are good for cropping maize, but not suitable for cropping the hyperaccumulator. These sorption indicators are helpful to choose the appropriate amendments for different crops in contaminated soils.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (grant 4107136) and Guangdong Province (S2011030002882) and the Science and Technology Project of Guangdong Province (grant 2010B03180006, 2012A030700003).
© Yan Sun, Qi-Tang Wu, Charles C.C. Lee, Baoqin Li, and Xinxian Long
REFERENCES
- Bailey , S E , Olin , T J , Bricka , R M and Adrian , D D . 1999 . A review of potentially low cost sorbents for heavy metals . Wat Res , 33 : 2469 – 2479 .
- Chen , H M , Zheng , C R , Tu , C and Shen , Z G . 2000 . Chemical methods and phytoremediation of soil contaminated with heavy metals . Chemosphere , 41 : 229 – 234 .
- Doong , R , Wu , Y W and Lei , W G . 1998 . Surfactant enhanced remediation of cadmium contaminated soils . Wat Sci Tech , 37 : 65 – 71 .
- Garćla-Sánchez , A , Alastuey , A and Querol , X . 1999 . Heavy metal adsorption by different minerals: application to the remediation of polluted soils . Sci Total Environ , 242 : 179 – 188 .
- Gupta , S S and Bhattacharyya , K G . 2006 . Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly(oxo zirconium) and tetrabutylammonium derivatives . J Hazard Mater , 128 ( 2–3 ) : 247 – 257 .
- Gworek , B. 1992a . Inactivation of cadmium in contaminated soils using synthetic zeolites. Environ Pollut . 75 : 269 – 271 .
- Gworek , B. 1992b . Use of synthetic zeolites of 3A and 5A type for lead immobilization in anthropogenic soils . Polish J Soil Sci , 25 : 35 – 39 .
- Haidouti , C. 1997 . Inactivation of mercury in contaminated soils using natural zeolites . Sci Total Environ , 208 : 105 – 109 .
- Herwijnen , R , Hutchings , T R , Al-Tabbaa , A , Moffat , A J , Johns , M L and Ouki , S K . 2007 . Remediation of metal contaminated soil with mineral-amended composts . Environ Pollut , 150 : 347 – 354 .
- Hong , N and Hou , J . 2004 . SAS for windows (V8) , Beijing , , China : Tsinghua University Press .
- Ibrahim , H S , Jamil , T S and Hegazy , E Z . 2010 . Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II. Isotherm models . J Hazard Mater , 182 : 842 – 847 .
- Kalmykova , Y , Strmvall , A and Steenari , B . 2008 . Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations . J Hazard Mater , 152 : 885 – 891 .
- Karapinar , N and Donat , R . 2009 . Sorption behaviour of Cu2+ and Cd2+ onto natural bentonite . Desalination , 249 : 123 – 129 .
- Kumpiene , J , Lagerkvist , A and Maurice , C . 2008 . Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments-A review . Waste Manage , 28 : 215 – 225 .
- Kooner , ZS. 1992 . Adsorption of copper onto goethite in aqueous systems . Environ Geol Wat Sci , 20 : 205 – 212 .
- Li , T Q , Di , Z Z , Yang , X E and Sparks , D L . 2011 . Effects of dissolved organic matter from the rhizosphere of the hyperaccumulator Sedum alfredii on sorption of zinc and cadmium by different soils . J Hazard Mater , 192 : 1616 – 1622 .
- Liu , A and Gonzalez , R D . 1999 . Adsorption/desorption in a system consisting of humic acid, heavy metals, and clay minerals . J Colloid Interf Sci , 218 : 225 – 232 .
- Liu , L N , Chen , H S , Cai , P , Liang , W and Huang , Q Y . 2009 . Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost . J Hazard Mater , 163 : 563 – 567 .
- Liu , P. 2007 . Polymer modified clay minerals: a review . Applied Clay Sci , 38 : 64 – 76 .
- Liu , X M , Wu , Q T and Banks , M K . 2005 . Effect of simultaneous establishment of Sedum alfredii and Zea mays on heavy metal accumulation in plants . Int J Phytoremed , 7 : 43 – 53 .
- Lu , RK. 2000 . Analytical Methods of Soil and Agricultural Chemistry , Beijing , , China : Agricultural Science and Technology Press .
- McGrath , S P , Zhao , F J and Lombi , E . 2002 . Phytoremediation of metals, metalloids, and radionuclides . Adv Agron , 75 : 1 – 56 .
- Mercier , L and Detellier , C . 1995 . Preparation, characterization, and applications as heavy metals sorbents of covalent grafted thiol functionalities on the interlamellar surface of montmorillonite . Environ Sci Technol , 29 : 1318 – 1323 .
- Missana , T , Gutierrez , M G and Alonso , U . 2008 . Sorption of strontium onto illite/smectite mixed clays . Phys Chem Earth , 33 ( S ) : 156 – 162 .
- Nissen , L R , Lepp , N W and Edwards , R . 2000 . Synthetic zeolites as amendments for sewage sludge—based compost . Chemosphere , 41 : 263 – 269 .
- Safa , M , Larouci , M , Meddah , B and Valemens , P . 2012 . The sorption of lead, cadmium, copper and zinc ions from aqueous solutions on a raw diatomite from Algeria . Wat Sci Technol , 65 : 1729 – 1737 .
- Sastre , J , Rauret , G and Vidal , M . 2006 . Effect of the cationic composition of sorption solution on the quantification of sorption-desorption parameters of heavy metals in soils . Environ Pollut , 140 : 322 – 339 .
- Sauvé , S , Turmel , S M , Roy , A G and Courchesne , F . 2003 . Solid solution partitioning of Cd, Cu, Ni, Pb, and Zn in the organic horizons of a forest soil . Environ Sci Technol , 37 : 5191 – 5196 .
- Tandy , S , Bossart , K , Mueller , R , Ritschel , J , Hauser , L , Schulin , R and Nowack , B . 2004 . Extraction of heavy metals from soils using biodegradable chelating agents . Environ Sci Technol , 38 : 937 – 944 .
- Walker , D J , Clemente , R , Roig , A and Bernal , M P . 2003 . The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils . Environ Pollut , 122 : 303 – 312 .
- Walker , D J , Clemente , R and Bernal , M P . 2004 . Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste . Chemosphere , 57 : 215 – 224 .
- Wang , G , Chen , J B , Gao , S , Xu , G M and Huang , G B . 1999 . Influence of application of rice straw and Chinese mile vetch on the species and ecological effect of added copper in two soils . Acta Ecol Sinica (in Chinese) , 19 : 551 – 556 .
- Wu , P X , Wu , W M , Li , S Z , Xing , N , Zhu , N W , Li , P , Wu , J H , Yang , C and Dang , Z . 2009 . Removal of Cd2+ from aqueous solution by adsorption using Fe-montmorillonite . J Hazard Mater , 169 : 824 – 830 .
- Wu , P X , Zhang , Q and Dai , Y P . 2011 . Adsorption of Cu(II),Cd(II) and Cr(III) ions from aqueous solutions on humic acid modified Ca-montmorillonite . Geoderma , 164 : 215 – 219 .
- Wu , Q T , Wei , Z B and Ouyang , Y . 2007 . Phytoextraction of metal contaminated soil by hyperaccumulator Sedum alfredii H: effects of chelator and co-planting . Wat Air Soil Pollut , 180 : 131 – 139 .
- Zhang , Q , Li , J M , Xu , M G , Song , Z G and Zhou , S W . 2006 . Effects of amendments on bioavailability of cadmium and zinc in compound contaminated red soil . J Agro-Environ Sci (in Chinese) , 25 : 861 – 865 .
- Zheng , M X , Feng , L , Liu , J , Li , L Q and Lin , A J . 2007 . Effects of chelators on species and bioavailability of cadmium in soil . Environ Chem , 26 : 606 – 609 .
- Zhu , W Q , Jia , X Y , Li , X M and Cheng , H X . 2008 . Adsorption behaviour of cadmium and lead in pig manure waste and its vermicompost . J Agro-Environ Sci (in Chinese) , 27 : 1796 – 1802 .
- Zorpas , A A , Constantinides , T , Vlyssides , A G , Haralambous , I and Loizidou , M . 2000 . Heavy metal uptake by natural zeolite and metals partitioning in sewage sludge compost . Bioresource Technol , 72 : 113 – 119 .
