?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In laboratory experiments, Lepidium sativum L. and Mentha spicata L. were grown in compost spiked with mercury. After cultivation for 20 and 68 days, respectively, translocation factors of 0.05 ≤ TF ≤ 0.2 (Lepidium sativum) and accumulation factors of 2.2 ≤ AF ≤ 12 (Mentha spicata) were recorded. Plants were then harvested and used as feedstock for bench-scale anaerobic digesters. The reactors operated in continuously-stirred batch mode for a period of ten days. Inhibition of anaerobic biogas production was apparent with one sample set evidencing mercury-induced bacteriostatic toxicity. Otherwise, ex-situ characterization of digestate showed that the reactors were within stable operating range. A canola oil-sulphide polymer derived from bio-waste was also used as an intermediary treatment stage to test its capacity for extracting mercury from half the samples prior to anaerobic digestion, and also from the post-experimentation reactor digestate. The polymer removed mercury from digestate with a 40–50% efficacy across all samples, suggesting its potential as a sludge clean-up option. Anaerobic digestion combined with staged polymer extraction offers a potential route for the disposal of phytoremediation crops and ultimately the recovery of mercury, coincident with the production of a bioenergy vector.
Introduction
In August 2017, the Minamata Convention entered into force, with a stated aim to protect human health and the environment from the adverse effects of mercury (Hg) (United Nations Environment Programme Citation2017). Colloquially known as “quicksilver,” mercury is a liquid metal at ambient temperatures and pressures; occurring naturally in elemental form, it also forms salts in two ionic states and various organometallic compounds (Beckers and Rinklebe Citation2017). Mercury is known to cause neurotoxicity, immunotoxicity, endocrine disruption, and embryotoxicity in living organisms; thus it represents a significant threat to health worldwide (Budnik and Casteleyn Citation2018). This threat is amplified, and remediation measures made more challenging, because Hg is highly mobile and persistent within the biosphere, accumulating in both terrestrial and aquatic ecosystems (Boening Citation2000; Obrist et al. Citation2017; Budnik and Casteleyn Citation2018). Currently, artisanal and large-scale gold mining are identified as the main anthropogenic Hg emission sources, but pollution also comes from various other activities such as waste incineration, smelting, aluminum production, and oil refining (Streets et al. Citation2017). These are supplemented by a substantial amount of re-emissions of Hg from historic anthropogenic pollution sites (UNEP Citation2013).
High costs are involved in implementing the conventional methods of Hg contaminated land remediation, making them prohibitive for many jurisdictions, particularly where artisanal gold mining is practiced (Esdaile and Chalker Citation2018). These methods involve excavation followed by second and usually third-stage washing or thermal treatment, and they are also known to have an accompanying risk of incidental Hg release (IPEN Citation2016). For these reasons alternative options are sought, with in-situ stabilisation/solidification considered best in terms of cost and hazard mitigation (Wang et al. Citation2012; Xu et al. Citation2015; Beckers and Rinklebe Citation2017). Suitable criteria for alternative options are that they must be inexpensive and scalable; and that they must be able to sequester mercury in a way that prevents it being emitted to the air, leaching into groundwater, or invading the food chain (Esdaile and Chalker Citation2018).
A wide range of flora have been reported as having the ability to extract Hg from soil and accumulate it within their tissues (Beauford et al. Citation1977; Leonard et al. Citation1998; Greger et al. Citation2005; Smolinska and Rowe Citation2015; Sasmaz et al. Citation2016; Mbanga et al. Citation2019). Applications are still at the trial stage, but one obvious problem is how to dispose of the Hg contaminated biomass post phytoextraction. Sas-Nowosielska et al. (Citation2004), and Kovacs and Szemmelveisz (Citation2017) suggested a range of options—composting, compaction and pyrolysis, followed by incineration, solidification and liquid extraction; but anaerobic digestion (AD), an established method for reducing the organics content of biomass and producing a useful energy vector, was not considered. Yet, recent studies have experimentally tested the premise of combining trace element phytoextraction with AD (Van Slycken et al. Citation2013; Willscher et al. Citation2013; Zhang et al. Citation2013; Cao et al. Citation2015; Tian and Zhang Citation2016; Lee et al. (Citation2018). But, no reports were found to have assessed the two processes in terms of Hg.
Considering the AD process only, attempts to understand the effects of Hg contamination on anaerobic reactors are very scarce (Paulo et al. Citation2015). Lingle and Hermann (Citation1975) spiked twelve 4 litre batch reactors with varying quantities of phenyl mercuric chloride and mercuric chloride and noted that in all cases ≥96% of Hg became bound to the sludge solids. Abdel-Shafy and Mansour (Citation2014) spiked sewage sludge with Hg at concentrations of ≤0.5 ppm and reported a decrease in AD efficiency over 300 days; although no control sample was used, and limited data was provided on the variability of the feedstock.
Recently a new polysulfide material has been created which can effectively capture Hg from various media (Crockett et al. Citation2016; Worthington et al. Citation2017). Synthesized from relatively cheap and abundant waste streams and industrial by-products, the polymer offers numerous new applications for environmental remediation (Crockett et al. Citation2016). It has previously been found as effective at removing Hg from soil and water, but has yet to be tested on plant matter in aqueous solution, AD digestate, or sludge.
The objectives of this study were threefold: (1) To extend the knowledge base with respect to the effects of Hg on the AD process; (2) To experimentally assess Hg phytoextraction crops as AD feedstock; and (3) To determine the efficacy of bio-waste polymer for abstracting Hg at different stages in the process, namely phytomass in aqueous solution and post-AD process digestate. Outcomes of the above objectives all contribute to the main research question: could the bio-waste polymer be used as a potential approach to mitigate the toxic Hg from crops and digestate? Results could help identify new approaches to the removal, containment, and recovery of Hg from contaminated land and sludge.
Materials and methods
Materials
Lepidium sativum was germinated from seed obtained from Thompson and Morgan (Ipswich, UK); while Mentha spicata was acquired as small plants (10 ≤ cm ≤ 12 high), purchased from a local horticulturalist (Leicester Rowena, UK). Both had previously been reported as Hg phytoextractors (Beauford et al. Citation1977; Smolinska and Leszczynska Citation2015). John Innes number 2 compost (Leicester Rowena, UK) was used as growth media for all cultivation experiments. This material has a standard composition of sterilized loam, peat, and grit-sand in the ratio 7:3:2, and a constrained pH of 6.5 (John Innes Manufacturer’s Association Citation2018).
For all dilutions Purite water (Reverse osmosis, de-ionised water from Suez Water, Bristol, UK) was used, and for filtration filters No1 by Whatman were used. Standard solution of 1000 ppm Hg in 1 M HNO3 (as H2HgN2O7 – J/8293/08) was supplied by Fisher Scientific, Loughborough, UK. For Hg analysis, laboratory grade O2 was supplied by BOC, UK.
The waste-based polymer used for Hg extraction has been synthesized and generously provided by the group of Justin Chalker from Flinders University, Australia. The polymer was a porous polysulfide formed by co-polymerisation of plant oil triglyceride with sulfur in excess of sodium chloride. Worthington et al. (Citation2017) successfully used this polymer for Hg capture.
Horticulture
From March to May 2018, two phytoextraction sample sets were cultured: Lepidium sativum L. (cress), and Mentha spicata L. (mint). In-house analytical tests were also completed (in triplicate) on the growth medium with results shown in . Values for total solids (TS) and volatile solids (VS) were determined using standard methods (APHA Citation1999). For Total Organic Carbon (TOC), 100 g of compost was mixed with 100 ml of Purite, stirred, then centrifuged at 10,000 rpm for 12 minutes. The supernatant was filtered and the filtrate analyzed in a DC-190 High temperature Total Organic Carbon Analyzer (Rosemount Analytical, St. Louis, US). Elemental analysis was completed using an Exeter Analytical CE440 (Coventry, UK), with 2.62 (± 0.9) mg of sample placed into a tin capsule, crimped, and then analyzed with helium as carrier gas. The instrument was calibrated at the beginning and end of each run using an acetanilide standard.
Table 1. Growth medium (John Innes No 2) background composition following drying for 48 hours at 20 °C. C, H, and N on a dry basis.
Growth media was first dried at 20 °C for 48 hours, then separated into eight 1 litre batches. Four of these 1 L batches were placed into clean individual 2 L capacity plants pots for Mentha spicata, and four into clean 1 L capacity trays for Lepidium sativum. One control sample (zero Hg) was created for each sample set. The remaining three containers for each sample set were impregnated with Hg, and for this a reagent was prepared by diluting a standard of 1000 ppm Hg in 1 M nitric acid into the following 100 mL concentrations using Purite water as solvent: 2.5, 5, 10 ppm. Each of these three 100 mL mixtures was added to an individual tray/pot in the sample set by gentle mixing. Concentrations were chosen based on prior literature where 5 ≤ ppm ≤ 10 ppm was identified as a possible toxic limit for Mentha spicata (Beauford et al. Citation1977).
For Lepidium sativum seed, a mass of 1.4 (± 0.05) g was planted into each sample tray. For Mentha spicata, a single cultivar was transplanted into each individual pot following gentle washing of roots and leaves using Purite water. All plant and seed samples were then grown on in a laboratory environment at a constant ambient temperature of 18 ± 2 °C, with artificial ∼ 5650 lux light provided by two Växer LED units (900 lm and 4200 K color temperature) by Ikea (Leiden, Netherlands). The lights were positioned 0.7 m from the base of the plant pots/trays, and activated on a timer switch set for a regular daily photoperiod of nine hours. Plants were watered simultaneously with Purite as required, then left to grow for 20 days (Lepidium sativum) and 68 days (Mentha spicata) respectively. This growth period was recommended as a minimum based on the findings of Suszcynsky and Shann (Citation1995), who reported that Hg moved to roots from day 10. Phytoextraction capacity was then measured in terms of Accumulations Factor (AF), Bioconcentration Factor (BF) and Translocation Factor (TF) (Smolinska and Leszczynska Citation2015), where:
(1)
(1)
(2)
(2)
(3)
(3)
Mercury analyses
Hg concentration within each sample was quantified in triplicate using a DMA-80 tri-cell atomic adsorption thermal decomposition analyzer by Milestone (Sorisole, Italy). Laboratory-grade O2 (by BOC, UK) was used as oxidant and carrier gas. Samples were weighed then loaded into nickel receptacles of ca. 0.3 mL for analyses. Calibration checks, with 10 ng Hg standards, were run prior to each experiment, and the system was purged automatically by running repeated blanks where high Hg values were obtained (Hg ≥1000 μg.kg-1). The instrument detection limit was 0.01 ng Hg, equivalent to ca. 100–200 ppt; with a precision of 1.5% Relative Standard Deviation (RSD) for liquid samples, and 5–7% RSD for solid samples.
Plant harvesting and preparation
Lepidium sativum germinated quickly and attained final height (ca. 3 cm) within 7 days. Both species maintained visible health throughout the growth period, with no discernible differences between Hg-spiked samples and the control. Following the cultivation period, all plant sample sets were gently separated from their compost, with stubborn particulates carefully rinsed from roots using Purite water. A small random section of each cultivar was removed for Hg analysis, with the remainder saved as AD feedstock. Growth media from each of the eight samples was also analyzed for Hg content after the plants had been extracted. The remaining phytomass from each sample set was then combined into one batch according to species, mixed, chopped, and submerged in ca. 200 mL Purite water which was subsequently made up to a total volume of 500 mL. From these stock samples, two identical 250 mL sub-sets were created—one was to be treated with polymer while the other remained untreated (see § 2.4). Following polymer treatment (or otherwise) these aqueous mixtures were then used as AD feedstock. Time between harvest and AD feeding was less than two days. Each of the samples were also characterized for TS and VS as previously described. Background values are shown in .
Table 2. Background parameters for the two sample sets (mean, n = 3, ± standard deviation).
Polymer treatment
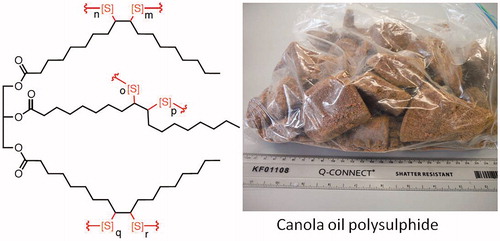
The polymer was supplied in “blocks” of varying sizes and irregular shapes (). Its composition was 50% sulfur and 50% canola oil by mass, excluding porogenic common salt (NaCl) which was ca. 70 wt% (Chalker Citation2018). Prior to use, NaCl was removed by submergence in Purite water for one hour, with four water changes. For more details on the polymer and washing methods see Worthington et al. (Citation2017).
Figure 1. Canola oil polysulfide in the form of blocks. Adapted from Worthington et al. Citation2017.
Prior to AD experimentation, samples 2 and 4 (c.f. § 2.5) were created by static immersion of 31.5 (± 0.5) g washed polymer in each of the respective 250 mL sub-sets. These mixtures were left to stand for 24 hours at room temperature in a beaker covered with Parafilm M (Bemis, USA). The mass of polymer used (in block form) was based on manufacturer recommendations where 2.00 g of sorbent was reported to remove 98% of Hg after 24 hours from a 10 mL solution containing 0.15 mg.mL-1 organomercury (Kerafast Citation2019). Polymer was then removed using forceps, prior to the whole sample being fed into the AD reactors.
At the end of the AD experiments, a further polymer treatment was made on the digestate removed from all reactors. This was to determine the efficacy of the polymer for abstracting Hg from this (different) medium only. The methods used were as described above.
Anaerobic digestion
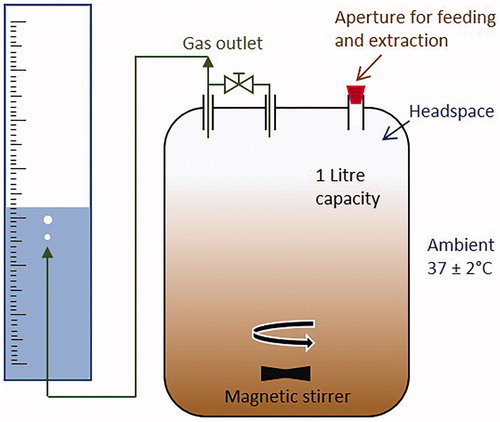
Five laboratory-scale AD reactors were assembled, each of 1 litre total capacity (). They were constructed from borosilicate glass vessels with compatible lids (Fisher Scientific – 11371995 and 11372025). Gas outlet tubes were connected to the lids, with a manually operable sampling port in-line. A Y-shaped connector in the gas line then fed both outlets into a calibrated water displacement cylinder situated in a confining reservoir. The reactors operated in batch mode, continuously stirred at ∼100RPM by a magnetic bar, in a room maintained at constant (mesophilic) temperature of 37 ± 2 °C. Biogas was measured at the experimental mid-point (115 hours) by tapping the reactor headspace with a handheld gas analyzer (Gas Data GFM-416, Coventry, UK) connected to both the inlet and outlet tubes.
Prior to experimentation, 900 mL of inoculum from a functional AD reactor at a local waste-water treatment plant (Wanlip, UK) was inserted into each vessel. They were then fed with 50 mL of sewage sludge (from the same treatment works) on alternate days over a one week period, simultaneous to the extraction of 50 mL of digestate. On day seven, 250 mL of digestate was removed and the five reactors were then fed as follows.
Control: 173g of sewage sludge made up to 250mL with final effluent (both Wanlip, UK).
250 mL Mentha spicata aqueous mixture after treatment with polymer.
250 mL Mentha spicata aqueous mixture (untreated).
250 mL Lepidium sativum aqueous mixture after treatment with polymer.
250 mL Lepidium sativum aqueous mixture (untreated).
The reactors were left to operate over a period of ten days. Upon cessation, the digestate was removed and characterized. Values of ammonium nitrogen (NH4–N) and Volatile Fatty Acids (VFA) were determined by UV spectroscopy (Hach Lange DR 3900, Germany) using chemical kits LCK 303 and LCK 365 respectively. Additionally, the Ripley ratio (alkalinity:acid balance) method was used to determine the ratio of VFA: TA (Total Alkalinity), with 0.1 M HCl used as titrant, and a Mettler Delta 340 ion probe to measure pH (Ripley et al. Citation1986).
Statistical treatment
Mean and standard deviation values were calculated for all in-triplicate measurements. Single factor analysis of variance (ANOVA) was determined using Excel software, with differences considered significant when probability was p < 0.05.
Results
Mercury phytoextraction
shows the Hg concentrations in both above and below ground plant tissue post horticulture experiments, along with phytoextraction capacity values. Statistically significant differences (p < 0.05) were detected for both above ground and root tissue Mentha spicata across the three sample sets, but there was no significant difference with comparative Lepidium sativum. High AF was observed for both species, with values above unity in all samples except for Lepidium sativum 2.5 ppm and 10 ppm (). The AF values for Mentha spicata were exceptionally high, due to the main hyperaccumulation of Hg being observed on the roots of these plants. This root-bound Hg did not however translocate into the stems and leaves, as evidenced by the low TF for this species. TF was slightly higher in Lepidium sativum and inversely proportional to the Hg concentration in the compost, a feature that was absent with Mentha spicata. Decreasing BF as a function of compost Hg concentration was also apparent for Lepidium sativum but again absent in Mentha spicata.
Table 3. Post phytoextraction Hg concentrations (with standard deviation) and phytoextraction factors measured on a wet plant mass basis (mean, n = 3, ± standard deviation).
Polymer treatment
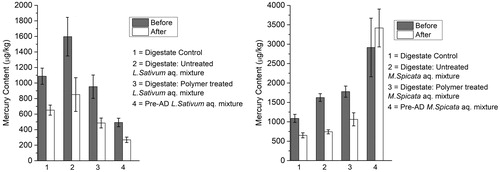
In seven out of eight samples tested after treatment with polymer, Hg concentration was significantly reduced. The polymer had abstracted 40–54% of the Hg from all samples, independent of initial concentration. These results are shown in along with the mean standard deviation. A similar extraction rate (40%) was recorded for the Lepidium sativum aqueous mixture (pre-AD) sample. The pre-AD Mentha spicata aqueous mixture was however anomalously high ( right, column 2), but with greater associated error.
Anaerobic digestion
Biogas production rates for all samples are shown in , with biogas composition measured at 115 hours displayed in . Both polymer-treated feedstocks initially produced more biogas than their untreated counterparts. This trend continued for Mentha spicata and by the tenth and final day of the experiments the polymer-treated feedstock had displaced 2.9 times more biogas volume. However, for the Lepidium sativum samples, both pretreated and untreated experiments ultimately ended up with comparable cumulative totals of biogas. During the first five days of experimentation, biogas production was inhibited with the untreated Lepidium sativum sample.
Figure 4. Biogas produced from batch anaerobic digesters fed with phytoextraction plants and a control sewage sludge sample, where P: feedstock pretreated with polymer for 24 hours; U: untreated feedstock. Reactors and treatments, n = 1; number of plant species per sample: N minus characterization subset (see §2.5).
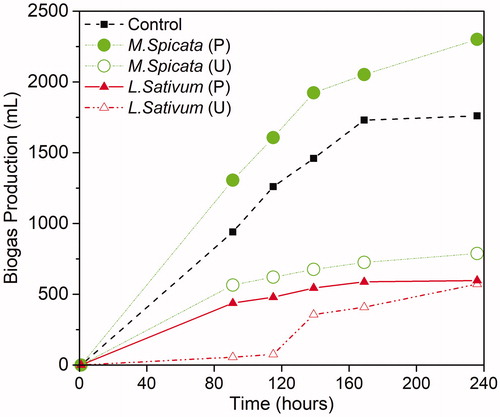
Table 4. Biogas species concentrations at 115 hours after commencement of experiments.
The results of digestate characterization revealed no apparent relation between concentrations of NH4–N and VFA or Ripley’s ratio and feedstock type. Both Lepidium sativum digestate samples had pH values which were 0.2 units higher than Control or Mentha spicata ().
Table 5. Results of L. sativum and M. spicata fed AD reactor digestate characterization (n = 1).
Discussion
High rates of phytoextraction have previously been reported for plants of the Family Brassicacae (Leonard et al. Citation1998; Smolinska and Szczodrowska Citation2017). In one previous study Lepidium sativum was found to have TF values of 0.07 and 0.8 when grown on soil concentrated with Hg in the range 10 ≤ mg.kg-1≤100 (Smolinska and Rowe Citation2015). This supports the trend observed here, where TF increased as an inverse function of Hg growth media concentration for this species. Other explanations for the high TF in Lepidium sativum compared to the samples analyzed by Smolinska and Rowe (Citation2015) are likely the longer growth period (20 days vs. 7) and the growth media (compost vs. soil). The addition of compost to Hg contaminated soil has previously been shown to increase rates of phytoextraction, likely due to increased bioavailability from humic acid-Hg complexes (Smolinska and Szczodrowska Citation2017).
Numerous physiological mechanisms have been suggested for how plants inhibit Hg transfer by accumulating, storing, and even detoxifying it at the root (phytostabilisation). These include entrapment in the apoplast of root cell walls and binding of Hg to lipidic substances and amino acids (cysteine) in the root epidermis (Rascio and Navari-Izzo Citation2011; Lomonte et al. Citation2014). It has also been proposed that the ability of some plants to accumulate high concentrations of heavy metals at the root is due to their active conditioning of the surrounding rhizosphere (Meagher and Heaton Citation2005). Pat-Espadas et al. (Citation2018) report that the presence of rhizobacteria, a plant growth promoting bacterium, enable growth of photosynthetic organisms and provide protection from environmental pollutants such as heavy metals. Other studies have identified the importance of environmental symbiosis between plants and mycorrhizal fungi (Cozzolino et al. Citation2016). This explains both the low TF and the overall high concentrations of Hg detected in Mentha spicata.
In an earlier research study with Mentha spicata as a phytoextractor, significant inhibition in plant growth was observed with soil Hg concentrations of between 5 and 10 ppm (Beauford et al. Citation1977). As Hg loading was comparable with this present work, the reason for why no visible evidence of phytotoxicity was observed could be due to Beauford et al. (Citation1977) planting their Mentha spicata as immature cuttings thus without a developed root structure to trap Hg. Another contributory factor could be the beneficial compost medium used here in contrast to Beauford et al. (Citation1977) “culture solution.”
Optimal phytoremediation plants are often defined as “hyperaccumulators.” However, specific criteria are given only for some metals, while elsewhere it is said that the shoots should have a higher concentration than the roots (Rascio and Navari-Izzo Citation2011). No plant species has been identified as a Hg hyperaccumulator (Smolinska and Leszczynska Citation2015). In this present study, both Mentha spicata and Lepidium sativum extracted large quantities of Hg from their growth media, but neither had a TF greater than unity.
Qualitatively, Lepidium sativum has a number of beneficial phytoremediation attributes: such as rapid growth rate (7–14 days), tolerance to a range of environmental stress (e.g. presence of heavy metals). (Smolinska and Leszczynska Citation2015). Negative aspects Disadvantages are its small size (low biomass production) and shallow root structure, although this is compensated for by ease of whole plant removal from the soil and its potential for repeated cropping throughout a long growing season. This property would also suit its use as a feedstock for anaerobic digestion where daily rather than batch feedings maintain stability. Mentha spicata is attractive for phytoremediation by its extremely high root uptake from a more extensive root structure combined with higher biogas production during AD.
Attempts to make direct comparison of TF, AF, and BF values with those from phytoremediation of contaminated soils should however be treated with some caution as the process of phytoextraction is a function of plant vitality and can be affected by many geochemical and environmental factors (Beckers and Rinklebe Citation2017). The applied Hg was retained by the compost was evident from results of growth media characterization (). In fact, rapid and highly nonlinear Hg adsorption has previously been reported when Hg(NO3)2 was applied to various soil types, with 93–99% of the added Hg being retained within 24 hours (Liao et al. Citation2009).
Two-way fluxes of Hg between plants, soils (particularly with high humus content) and surrounding air can be significant (Boening Citation2000; Ericksen and Gustin Citation2004; Xu et al. Citation2015). Although it is widely known that methylmercury, dimethylmercury and metallic Hg can evolve from anaerobic substrates, the processes are complex and determined by a range of factors. (Beckers and Rinklebe Citation2017). The high values of Hg found in the AD digestate can be explained by reference to previous research which showed that Hg tends to bind with sulfur and form stable cinnabar and metacinnabar in otherwise thermodynamically unfavorable conditions when catalyzed by mechanical agitation (Oji Citation1998; López et al. Citation2010).
The variation in results for pre- and post- polymer treatment of Mentha spicata aqueous mixture reflect the fact that mass transfer effects impaired Hg abstraction. Independent tests were done on separate batches of Lepidium sativum grown under different conditions then placed in Purite water (without polymer) and left for four days, over which time daily samples of supernatant were extracted and analyzed for Hg content. No significant difference was observed across the five days (p = 0.07), evidencing that Hg did not apparently leave the plant matter and enter into solution, rather it randomly attached itself in patches to the surface of the polymer. Any transfer from biomass to polymer at this stage of experimentation must therefore have been through solid to solid contact.
In the AD process, VFAs are intermediary biodegradation products and their presence at high concentrations in the digestate provides an indicator of incomplete feedstock degradation (Fernandes et al. Citation2009). In our experimental work the VFA values for all reactors (284–510 mg.L-1) were indicative of a relatively stable process. Similarly, values of NH4–N were outside of the range where inhibitory effects might be expected (Yenigün and Demirel Citation2013). However, the highest values for both VFA and NH4–N (data not shown) were detected in digestate from the higher Hg loaded Lepidium sativum sample. This sample also exhibited an initial stage of biogas inhibition.
Biogas production rates for Mentha spicata exhibited characteristic decelerated exponential growth, a function of early stage cellulose and hemicellulose digestion followed by degradation of the more recalcitrant lignin (Moestedt et al. Citation2015). In contrast, the biogas curves for Lepidium sativum provide an insight on the possible effects of Hg loading on the AD process. It has previously been reported that some trace elements can cause an inhibitory effect, but that the impact is only temporary and often followed by stimulated methanogenesis, i.e., the process is bacteriostatic and not bacteriocidal (Oleszkiewicz and Sharma Citation1990). This would explain the initial inhibition observed with the higher Hg loaded Lepidium sativum AD sample in comparison to the polymer treated (lower Hg-loaded) equivalent. Such a “lag phase” followed by stimulated methanogenesis has previously been reported in experiments using low-substrate concentrations for a number of heavy metals, Hg included (Capone et al. Citation1983).
For environmental reasons, many international drivers promote the removal of Hg from sewage sludge and the diversion of such wastes from landfill (European Union Citation1999). With its high nutrient content this sludge could be beneficial as a soil improver, but the Hg content precludes its use (Hossain et al. Citation2009). Polysulfide treatment of sludge or digestate may be a novel solution. The Hg-polymeric sulfur bond is extremely strong, forming nontoxic and insoluble metacinnabar, thus safely immobilizing Hg against secondary release (Worthington et al. Citation2017). However, removing Hg from the polymer is not easy and though no publication on this exists the manufacturers reportedly work with a local recycler who incinerates the polymer-Hg complex to recover metallic Hg by conventional retort methods (Chalker Citation2018).
Conclusions
Combining phytoextraction with anaerobic digestion has potential as an alternative route for the disposal of phytoremediation crops and ultimately the recovery of Hg, coincident with the production of a clean energy vector. In this study, we corroborate some earlier reports that Hg can cause merely temporary inhibition to the anaerobic digestion process followed by stimulated methanogenesis. The phenomenon merits further exploration, particularly with higher Hg-loading and to elucidate the underlying biochemical mechanisms involved. These results suggest that Hg contamination of anaerobic feedstocks in elevated concentrations need not be detrimental to biogas production and could be beneficial as a method of both treating waste and concentrating the Hg in chemical stable form.
Prior attempts to scientifically assess the premise of combined phytoextraction and anaerobic digestion do not appear to have extended to Hg. Here its merits are considered in laboratory trials only. These may not necessarily be representative of field trials, and to obtain a truer understanding of both phytoextraction capabilities and the feeding of post phytoextraction biomass into anaerobic digesters, experiments using crops grown on contaminated land are suggested.
The consistency of results reported here when extracting Hg from digestate with canola oil-sulphide polymer suggest wider applications, particularly for the wastewater treatment industry or with further studies on digestate from stand-alone biogas plants as a means of extracting Hg. This may facilitate the future use of dried sludge solids/digestate as a soil improvement medium and mitigate potential Hg emissions from thermal waste treatment processing. Studies on how to safely dispose of the polymer and/or extract the Hg post treatment are needed.
Acknowledgements
Thanks to Patricia Thornley (Supergen) for grant administration, Mr Geoffrey Russell (Loughborough University) for technical support, and Dr Justin Chalker (Flinders University) for polymer provision.
Disclosure statement
Authors 1, 2, and 6 declare no personal or financial interest. Authors 3 and 4 have a provisional patent, number GB1813068.2, with reference Phyto1. No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdel-Shafy HI, Mansour M. 2014. Biogas production as affected by heavy metals in the anaerobic digestion of sludge. Egypt J Petroleum. 23(4):409–417. doi:10.1016/j.ejpe.2014.09.009.
- APHA. 1999. Standard methods for the examination of water and wastewater. 20th ed. Washington DC: American Public Health Association, American Water Works Association, Water Environment Federation. Section 2540.
- Beauford W, Barber J, Barringe AR. 1977. Uptake and distribution of mercury within higher plants. Physiol Plant. 39(4):261–265. doi:10.1111/j.1399-3054.1977.tb01880.x.
- Beckers F, Rinklebe J. 2017. Cycling of mercury in the environment: Sources, fate, and human health implications: a review. Crit Rev Env Sci Tech. 47(9):693–794. doi:10.1080/10643389.2017.1326277.
- Boening DW. 2000. Ecological effects, transport, and fate of mercury: a general review. Chemosphere. 40(12):1335–1351. doi:10.1016/S0045-6535(99)00283-0.
- Budnik LT, Casteleyn L. 2018. Mercury pollution in modern times and its socio-medical consequences. Sci Total Environ. 654:720-734. doi:10.1016/j.scitotenv.2018.10.408.
- Cao Z, Wang S, Wang T, Chang Z, Shen Z, Chen Y. 2015. Using contaminated plants involved in phytoremediation for anaerobic digestion. Int J Phytoremediation. 17(1–6):201–207. doi:10.1080/15226514.2013.876967.
- Capone DG, Reese DD, Kiene RP. 1983. Effects of metals on methanogenesis, sulfate reduction, carbon dioxide evolution, and microbial biomas in anoxic salt marsh sediments. Appl Environ Microb. 45(5):1586–1591. doi:10.1128/AEM.45.5.1586-1591.1983.
- Chalker JM. Personal communication. 6th June 2018.
- Cozzolino V, De Martino A, Nebbioso A, Di Meo V, Salluzzo A, Piccolo A. 2016. Plant tolerance to mercury in a contaminated soil is enhanced by the combined effects of humic matter addition and inoculation with arbuscular mycorrhizal fungi. Environ Sci Pollut Res Int. 23(11):11312–11322. doi:10.1007/s11356-016-6337-6.
- Crockett MP, Evans AM, Worthington M, Albuquerque IS, Slattery AD, Gibson CT, Campbell JA, Lewis DA, Bernardes JL, Chalker JM. 2016. Sulfur-limonene polysulfide: a material synthesized entirely from industrial by-products and its use in removing toxic metals from water and soil. Angew Chem Int Ed Engl. 55(5):1714–1718. doi:10.1002/anie.201508708.
- Ericksen JA, Gustin MS. 2004. Foliar exchange of mercury as a function of soil and air mercury concentrations. Sci Total Environ. 324(1-3):271–279. doi:10.1016/j.scitotenv.2003.10.034.
- Esdaile LJ, Chalker JM. 2018. The mercury problem in artisanal and small-scale gold mining. Chemistry. 24(27):6905–6916. doi:10.1002/chem.201704840.
- European Union. 1999. Council directive 1999/31/EC of 26 April 1999 on the landfill of waste (online) [accessed 2019 Sep 27]. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31999L0031.
- Fernandes TV, Klaasse Bos GJ, Zeeman G, Sanders JPM, van Lier JB. 2009. Effects of thermo-chemical pre-treatment on anaerobic biodegradability and hydrolysis of lignocellulosic biomass. Bioresour Technol. 100(9):2575–2579. doi:10.1016/j.biortech.2008.12.012.
- Greger M, Wang Y, Neuschütz C. 2005. Absence of Hg transpiration by shoot after Hg uptake by roots of six terrestrial plant species. Environ Pollut. 134(2):201–208. doi:10.1016/j.envpol.2004.08.007.
- Hossain MK, Strezov V, Nelson PF. 2009. Thermal characterisation of the products of wastewater sludge pyrolysis. J Anal Appl Pyrol. 85(1–2):442–446. doi:10.1016/j.jaap.2008.09.010.
- IPEN. 2016. Guidance on the identification, management and remediation of mercury contaminated sites (online) [accessed 2019 Sep 27]. http://ipen.org/documents/ipen-guidance-identification-management-and-remediation-mercury-contaminated-sites.
- John Innes Manufacturer’s Association. 2018. Formulation (online) [accessed 2019 Sep 27]. https://johninnes.info/compost/formulation.html.
- Kerafast. 2019. Polysulfide mercury sorbent (microporous) (online) [accessed 2019 Sep 27]. https://www.kerafast.com/PDF/Polysulfide%20Polymer%20Mercury%20Sorbent%20(Porous).pdf.
- Kovacs H, Szemmelveisz K. 2017. Disposal options for polluted plants grown on heavy metal contaminated brownfield lands - a review. Chemosphere. 166:8–20. doi:10.1016/j.chemosphere.2016.09.076.
- Lee J, Park KY, Cho J, Kim JY. 2018. Releasing characteristics and fate of heavy metals from phytoremediation crop residues during anaerobic digestion. Chemosphere. 191:520–526. doi:10.1016/j.chemosphere.2017.10.072.
- Leonard TL, Taylor GE, Gustin MS, Fernandez G. 1998. Mercury and plants in contaminated soils: 1. Uptake, partitioning, and emission to the atmosphere. Environ Toxicol Chem. 17(10):2063–2071. doi:10.1002/etc.5620171024.
- Liao L, Selim HM, DeLaune RD. 2009. Mercury adsorption-desorption and transport in soils. J Environ Qual. 38(4):1608–1616. doi:10.2134/jeq2008.0343.
- Lingle JW, Hermann ER. 1975. Mercury in anaerobic sludge digestion. J Water Pollut Control Fed. 47(3 pt 1):466–471.
- Lomonte C, Wang Y, Doronila A, Gregory D, Baker AJM, Siegele R, Kolev S. 2014. Study of the spatial distribution of mercury in roots of vetiver grass (Chrysopogon zizanioides) by micro-pixe spectrometry. Int J Phytoremediation. 16(7–12):1170–1182. doi:10.1080/15226514.2013.821453.
- López FA, López-Delgado A, Padilla I, Tayibi H, Alguacil FJ. 2010. Formation of metacinnabar by milling of liquid mercury and elemental sulfur for long term mercury storage. Sci Total Environ. 408(20):4341–4345. doi:10.1016/j.scitotenv.2010.07.008.
- Mbanga O, Ncube S, Tutu H, Chimuka L, Cukrowska E. 2019. Mercury accumulation and biotransportation in wetland biota affected by gold mining. Environ Monit Assess. 191(3):186–198. doi:10.1007/s10661-019-7329-z.
- Meagher RB, Heaton A. 2005. Strategies for the engineered phytoremediation of toxic element pollution: mercury and arsenic. J Ind Microbiol Biotechnol. 32(11–12):502–513. doi:10.1007/s10295-005-0255-9.
- Moestedt J, Malmborg J, Nordell E. 2015. Determination of methane and carbon dioxide formation rate constants for semi-continuously fed anaerobic digesters. Energies. 8(1):645–655. doi:10.3390/en8010645.
- Obrist D, Agnan Y, Jiskra M, Olson CL, Colegrove DP, Hueber J, Moore CW, Sonke JE, Helmig D. 2017. Tundra uptake of atmospheric elemental mercury drives arctic mercury pollution. Nature. 547(7662):201–216. doi:10.1038/nature22997.
- Oji LN. 1998. Mercury disposal via sulfur reductions. J Env Eng. 124(10):945–952. doi:10.1061/(ASCE)0733-9372(1998)124:10(945).
- Oleszkiewicz JA, Sharma VK. 1990. Stimulation and inhibition of anaerobic processes by heavy metals - a review. Biol Waste. 31(1):45–67. doi:10.1016/0269-7483(90)90043-R.
- Paulo LM, Stams AJM, Sousa DZ. 2015. Methanogens, sulphate and heavy metals: a complex system. Rev Environ Sci Biotechnol. 14(4):537–553. doi:10.1007/s11157-015-9387-1.
- Pat-Espadas AM, Loredo Portales R, Amabilis-Sosa L, Gómez G, Vidal G. 2018. Review of constructed wetlands for acid mine drainage treatment. Water. 10(11):1685–1625. doi:10.3390/w10111685.
- Rascio N, Navari-Izzo F. 2011. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 180(2):169–181. doi:10.1016/j.plantsci.2010.08.016.
- Ripley LE, Boyle WC, Converse JC. 1986. Improved alkalimetric monitoring for anaerobic digestion of high strength wastes. Journal of the Water Pollution Control Federation. 58 (5):406–411.
- Sasmaz M, Akgül B, Yildirim D, Sasmaz A. 2016. Mercury uptake and phytotoxicity in terrestrial plants grown naturally in the Gumuskoy (Kutahya) mining area, Turkey. Int J Phytoremediation. 18(1):69–76. doi:10.1080/15226514.2015.1058334.
- Sas-Nowosielska A, Kucharski R, Małkowski E, Pogrzeba M, Kuperberg JM, Kryński K. 2004. Phytoextraction crop disposal-an unsolved problem. Environ Pollut. 128(3):373–379. doi:10.1016/j.envpol.2003.09.012.
- Smolinska B, Leszczynska J. 2015. Influence of combined use of iodide and compost on Hg accumulation by Lepidium sativum L. J Environ Manage. 150:499–507. doi:10.1016/j.jenvman.2014.12.043.
- Smolinska B, Rowe S. 2015. The potential of Lepidium sativum L. for phytoextraction of Hg-contaminated soil assisted by thiosulphate. J Soils Sediments. 15(2):393–400. doi:10.1007/s11368-014-0997-y.
- Smolinska B, Szczodrowska A. 2017. Antioxidative response of Lepidium sativum L. during assisted phytoremediation of Hg contaminated soil. N Biotechnol. 38(Pt B):74–83. doi:10.1016/j.nbt.2016.07.004.
- Streets DG, Horowitz HM, Jacob DJ, Lu Z, Levin L, ter Schure AFH, Sunderland EM. 2017. Total mercury released to the environment by human activities. Environ Sci Technol. 51(11):5969–5977. doi:10.1021/acs.est.7b00451.
- Suszcynsky EM, Shann JR. 1995. Phytotoxicity and accumulation of mercury subjected to different exposure routes. Environ Toxicol Chem. 14(1):61–67. doi:10.1002/etc.5620140108.
- Tian Y, Zhang H. 2016. Producing biogas from agricultural residues generated during phytoremediation process: possibility, threshold, and challenges. Intl J Green Energy. 13(15):1556–1563. doi:10.1080/15435075.2016.1206017.
- UNEP 2013. Global Mercury Assessment 2013: sources, emissions, releases and environmental transport (online). United Nations Environment Programme [accessed 2019 Sep 27]. http://wedocs.unep.org/handle/20.500.11822/7984?show=full.
- United Nations Environment Programme. 2017. Minamata convention on mercury, text and annexes. Nairobi: United Nations Environment Programme. p. 1–67.
- Van Slycken S, Witters N, Meers E, Peene A, Michels E, Adriaensen K, Ruttens A, Vangronsveld J, Du Laing G, Wierinck I, et al. 2013. Safe use of metal-contaminated agricultural land by cultivation of energy maize (Zea mays). Environ Pollut. 178:375–380. doi:10.1016/j.envpol.2013.03.032.
- Wang J, Feng X, Anderson CWN, Xing Y, Shang L. 2012. Remediation of mercury contaminated sites – a review. J Hazard Mater. 221–222:1–18. doi:10.1016/j.jhazmat.2012.04.035.
- Willscher S, Mirgorodsk D, Jablonski L, Ollivier D, Merten D, Büchel G, Wittig J, Werner P. 2013. Field scale phytoremediation experiments on a heavy metal and uranium contaminated site, and further utilization of the plant residues. Hydrometallurgy. 131-132:46–53. doi:10.1016/j.arabjc.2013.08.010.
- Worthington MJH, Kucera RL, Albuquerque IS, Gibson CT, Sibley A, Slattery AD, Campbell JA, Alboaiji SFK, Muller KA, Young J, Adamson N, et al. 2017. Laying waste to mercury: inexpensive sorbents made from sulfur and recycled cooking oils. Chemistry. 23(64):16219–16230. doi:10.1002/chem.201704108.
- Xu J, Garcia Bravo A, Lagerkvist A, Bertilsson S, Sjöblom R, Kumpiene J. 2015. Sources and remediation techniques for mercury contaminated soil. Environ Int. 74:42–53. doi:10.1016/j.envint.2014.09.007.
- Yenigün O, Demirel B. 2013. Ammonia inhibition in anaerobic digestion: a review. Process Biochem. 48(5–6):901–911. doi:10.1016/j.procbio.2013.04.012.
- Zhang H, Tian Y, Wang L, Zhang L, Dai L. 2013. Ecophysiological characteristics and biogas production of cadmium-contaminated crops. Bioresour Technol. 146:628–636. doi:10.1016/j.biortech.2013.07.148.