Abstract
Cadmium (Cd) is the main heavy metal pollutant in soil. The combination of genetic engineering technology and Rizobium rhizogenes mediated technology can effectively improve the enrichment efficiency of heavy metals in super accumulators and reduce soil heavy metal pollution. In this study, the transgenic hairy root system containing the IRT1 gene of Cd hyperaccumulator-Brassica campestris L. was successfully constructed by the R. rhizogenes mediated method (IRT1 gene come from Arabidopsis thaliana). The hairy roots of each subculture can grow stably within 6 weeks, and IRT1 gene will not be lost within 50 subcultures., which is detected using PCR method. The results of Cd enrichment experiments showed that after treatment with 100 µmol/L Cd for 14 days, the growth state of transgenic IRT1 hairy roots only showed slight browning. Also, the accumulation value of Cd reached 331.61 µg/g and the enrichment efficiency of transgenic IRT1 hairy roots was 13.8% higher than that of wild-type hairy roots. Western blotting results showed that the expression of IRT1 protein in transgenic hairy roots was significantly higher than that of wild-type hairy roots under Cd stress. The above results indicated that the overexpression of IRT1 gene can help B. campestris L. hairy roots to effectively cope with Cd stress and improve its ability to enrich Cd.
NOVELTY STATEMENT
In this study, the transgenic hairy root system containing the IRT1 gene of Cd hyperaccumulator-Brassica campestris L. was successfully constructed by the Rizobium rhizogenes mediated method. At the same time, the growth state and cadmium enrichment efficiency of transgenic hairy roots under different concentrations of Cd stress were studied. Overexpression of IRT1 gene can effectively improve the tolerance of hairy root to Cd. The enrichment efficiency of transgenic IRT1 hairy roots was 13.8% higher than that of wild-type hairy roots. The transgenic IRT1 hairy root system established in this study can be used as a reliable experimental model for the study of Cd adsorption mechanism, and can be further regenerated to obtain transgenic IRT1 B. campestris L. plants for the study of heavy metal Cd pollution remediation.
Introduction
Cadmium (Cd) is a non-threshold toxin that can indicate toxic effects at very low concentrations and is known as a toxic heavy metal (Rahman and Singh Citation2019). Cd can enter the air, soil, water and other environments in various ways, causing serious deterioration of microorganisms, plants and animals. More importantly, Cd, like other toxic metals, has a long half-life in the environment and biological systems and is difficult to be eliminated in soil. (Pehlivan et al. Citation2009; Wang and Demshar Citation1992). Therefore, it is of great significance to find a suitable method to eliminate Cd in the environment.
Phytoremediation has been considered as a cost-effective green alternative to traditional soil remediation technologies, hyperaccumulator plays an crucial role in this process. B. campestris L. is one of the ideal species used for studying the molecular mechanism of Cd tolerance in cruciferous plants and analyzing the important Cd tolerance gene. Effective use of B. campestris L. for the repairing Cd contaminated soil has been reported by many researchers (Sabir et al. Citation2020; Xu et al. Citation2018).
The enhancement of the enrichment ability of hyperaccumulator can be achieved by using transgenic methods. The accumulation and tolerance of plants to heavy metals can be greatly enhanced by overexpressing natural or modified genes involved in different molecular mechanisms, such as genes encoding antioxidant enzymes, in the biosynthesis of phytochelatins, ion transporters, etc (Mani and Kumar Citation2014). Zheng enhanced the tolerance of rice to salt stress, low temperature stress and heavy metals (CdCl2 and CuSO4) stress by overexpressing OsSMP1 (stress membrane protein) (Zheng et al. Citation2021). In addition, heavy metal ion transporters can directly control the absorption, distribution and accumulation of metals, and the transportation of toxic heavy metals from soil to roots and from roots to other parts of plants is similar to the process of ingesting essential trace elements. Therefore, it is possible to improve the ability of remediation of the heavy metal contaminated soil by improving the expression and activity of such genes (Sun et al. Citation2022). Iron-regulated transporter (IRT1) is the main transporter for plants to absorb Fe2+, which has low selectivity and can transport Cd and other toxic heavy metals (He et al. Citation2017). Zhu reduced the expression of IRT1 in Arabidopsis thaliana by adding tungstate to agar medium, and found that the concentration of Cd in the roots was reduced by about 50% (Zhu et al. Citation2020). Chen confirmed that when peanuts are exposed to Cd in an iron-deficient environment, IRT1 expression is up-regulated and participates in iron homeostasis, and it is responsible for Cd absorption, distribution and transport (Chen et al. Citation2019). It can be seen that IRT1 plays an indispensable role in the transportation of Cd. Therefore, in our study, IRT1 is selected as the exogenous target gene for transgenic hairy roots of B. campestris L.
Heavy metals are mainly taken into plants through selective uptake of plant roots (Seneviratne et al. Citation2019). Moreover, the root, as an organ in direct contact with heavy metals, has a very important response to heavy metals. Many studies have shown that the content of accumulated Cd in roots is greater than that in leaves (Huang et al. Citation2020; Zhang Citation2017, Citation2020). Therefore, using roots to further explore the restoration capacity of hyperaccumulator has good development prospects and practical significance. The hairy roots induced by R. rhizogenes can be propagated indefinitely, the genotype and phenotype are closer, and can be used as a reliable and repeatable experimental system to study hyperaccumulator (Li et al. Citation2022). The time required is often substantially reduced by using hairy roots for experimental investigation. Due to these characteristics, in the last few decades, hyperaccumulator hairy roots are often used to study the absorption and accumulation of heavy metals (Cd, Cu, Ni, Pb, and U), metal recovery in phytomining applications (Pérez-Palacios et al. Citation2017; Pandey et al. Citation2021; Zhou et al. Citation2018). In the field of environmental restoration, hairy roots can be used to screen genetic transformants and guide subsequent whole plant experiments (Kofroňová et al. Citation2019).
Above all, the study here aims to establish a transgenic IRT1 B. campestris L. hairy root system. Moreover, the system was used to investigate the efficiency of the transferred exogenous IRT1 gene for advancing the ability of Cd enrichment. This research idea can provide a reference model for the transgenic hairy roots of Cd hyperaccumulator in the future, and provide a new and effective way for study on the mechanism of plant Cd enrichment.
Materials and methods
Material and culture conditions
R. rhizogenes ATCC15834 was bought from Guangdong province microbial culture collection center. The purchased R. rhizogenes was subcultured twice, one single colony was selected and inoculated in 50 mL liquid medium, cultured at 28 °C and 220 rpm, and the shaking stopped when the OD 600 was 0.5 ∼ 0.6, added 20% glycerin and store in refrigerator at −20 °C (Ye et al. Citation2020).
The sterilized B. campestris L. seeds (Academy of Agricultural Sciences Seed Technology Co., Ltd, Beijing, China) were germinated on hormone-free MS culture medium (Murashige and Skoog Citation1962) containing 1% (w/v) agar and 3% sucrose. The seeds were cultured at 25 °C, 16 h light/8 h dark photoperiod for 10 days to obtain cotyledon explants.
Each experimental treatment was run in triplicate. The pH of all MS culture medium was adjusted to 5.8 ± 0.1 and then the media were autoclaved at 118 kPa atmospheric pressure at 120 °C for 20 min.
Target gene synthesis
The Coding sequence (CDS) of IRT1 was obtained from the NCBI database (Gene ID: 827713), totaling 1044 bp. Based on the multiple cloning site region (MCS) of plasmid pRI101 (purchased from Takara) and the IRT1 restriction site analyzed by DNAMAN, Sal I and BamH I restriction sites were selected, and primers were designed. The IRT1 sequence (1 056 bp) was synthesized by Sangon Biotech (Shanghai) Co., Ltd.
Construction of recombinant plasmid and recombinant rizobium
The binary vectors pRI101 and pUC57-IRT1 were transformed into DH5α to amplify the vector and target gene. In order to construct the pRI101-IRT1 recombinant plasmid, Sal I and BamH I restriction sites were used, and the IRT1 gene from pUC57 was cloned into pRI101 by T4 ligase.
The recombinant plasmid was transformed into R. rhizogenes (ATCC15834) by heat shock method. The composite system (1 ng recombinant plasmid pRI101-IRT1: 100 µL Rizobium competent cells) was treated with ice bath for 30 min, and then quickly transferred to 42 °C water bath for 60 s∼90 s, followed by quick ice bath of the cells for 2 ∼ 3 min. The cells were added 1 mL R. rhizogenes liquid medium(YEB) (1 L YEB liquid medium: MgSO4·H2O 0.5 g, yeast extract 1 g, beef Extract 5 g, sucrose 5 g, peptone 5 g, no resistance), shaked at 28 °C 100 rpm for 1 h, centrifuged at 3 000 rpm for 4 min, discard a portion of the supernatant, and applied 100 µL of bacterial solution to YEB liquid culture medium containing 50 µg/mL kanamycin. The plates were placed for about 30 min for drying out of water, and then incubated in the 28 °C incubator for 16 h to grow single colony. Single colony was cultured overnight in 3 mL YEB liquid medium.
Induction and identification of transgenic hairy roots and wild-type hairy roots
Under sterile conditions, the cotyledons were cut to a size of 0.5 cm2, 10 pieces were inoculated on pre-cultured MS medium containing 0.1 mg/L naphthylacetic acid (NAA, Chembase, Beijing, China), and pre-cultured at 25 °C for 2 days in the dark. Then, the non-transformed and the recombinant R. rhizogenes activated bacterial solution was used to infect the cotyledon explants for 20 min, respectively, and the infected explants were cultured in a dark environment at 25 °C. After hairy roots grew, the hairy roots were placed in MS solid medium containing 500 mg/L cefotaxime sodium for sterilization and cultured, and the medium is replaced once every 7 days.
Genomic DNA of wild-type and transgenic IRT1 hairy roots were extracted using Plant genomic DNA extraction kit (Tian gen Biotech Co., Ltd., Beijing, China), and PCR detection of rolB and IRT1 genes was performed. PCR products were electrophoresed on a 1% agarose gel, stained in ethidium bromide, and the DNA was visualized under ultraviolet light.The primer sequences were shown in . IRT1 PCR result is a part of IRT1, with a size of 471 bp. The PCR cycling conditions: Denaturation step, 94 °C, 10 min; Cycles, 35 cycles: 94 °C, 1 min; Annealing, 56 ̊°C, 1 min, and elongating, 72 ̊°C, 1 min; Elongation, 72 °C, 10 min.
Table 1. Primer sequences.
Experimental design
Weighed 0.22835 g of cadmium chloride (CdCl2•5/2H2O, M = 228.35) into 10 mL sterile water to prepare the final concentration of 100 µmol/mL mother liquor, filtered and sub packed in EP tubes for storage at − 20 °C.
The wild-type hairy roots and transgenic IRT1 hairy roots cultured for 30 days were selected and cut into 4 ∼ 5 cm root tissues with root tip. Put 0.1 g healthy tissues into bottle containing 20 mL solid MS medium (contains 0, 50 and 100 µmol/mL Cd) and treated for periods of 7 and 14 days, and cultured in the dark at 25 ∼ 27 °C.
Fresh and dry weights
For fresh weights calculation, transgenic and wild-type hairy roots exposed to Cd at different times and concentrations were weighed after their water was carefully removed with filter papers. These hairy roots were placed at 50 ∼ 60 °C until the weight was constant, and then the dry weights of the hairy roots were weighed.
Quantification of cadmium concentrations
After harvest, the content of Cd in hairy roots and medium was measured by atomic absorption spectrophotometer. Put the hairy roots and the remaining medium samples into the oven for drying (50 °C∼60 °C), and grind them into powder with liquid nitrogen after complete drying for determination. Weighed 0.5 g powder sample into a 100 mL erlenmeyer flask and added 15 mL mixed acid (VHNO3: VHClO4 = 4: 1) for digestion. Finally, heated and dried the sample, measured the volume with 1% nitric acid, and determined the content of Cd in hairy roots and culture medium under the measurement conditions of wavelength 228.8 nm, lamp current 7 mA, slit width 113 nm, drying temperature 110 °C, 30 s, and ashing temperature 500 °C.
Enrichment efficiency of hairy roots to Cd = (Cd content enriched by hairy roots/Cd content in initial culture medium) × 100%.
Western blotting analysis of IRT1 expressed in transgenic hairy roots
Total protein was extracted from hairy roots samples using a Plant Total Protein Extraction Kit (Sangon Biotech, Shanghai, China) and the protein concentration was determined by using a Pierce™ BCA Protein Assay Kit (Thermo Scientific, Shanghai, China). β-actin was used as a reference protein (Abbkine, United States). The protein samples were separated by SDS-PAGE and transferred to membranes. The membrane was washed and incubated with primary antibody (IRT1 antibody was purchased from Agrisera, Sweden) overnight at 4 °C. Subsequently, the membrane was washed and incubated with secondary antibody (HRP-labeled Goat Anti-Rabbit IgG and HRP-labeled Goat Anti-Mouse IgG were purchased from Solelybio, Beijing, China) at room temperature for 2 h, and then the protein band was visualized by using the ECL kit. In order to see the change of protein level more clearly and intuitively, Image J was used to further analyze the bands.
Statistical analysis
All measurements were plotted using Microsoft Office 2016 and Origin Pro 2021. The statistical analyses were performed using IBM SPSS Statistics 22, and the results were expressed as the means ± SD. One-way ANOVA was conducted, followed by the least significant difference (LSD) test. The correlations were assessed using Pearson product-moment correlation analysis. Regression analyses were conducted using the monadic linear regression method. p < 0.05 and p > 0.05 indicate significant and nonsignificant differences, respectively.
Results
Induction and identification of transgenic IRT1 hairy roots
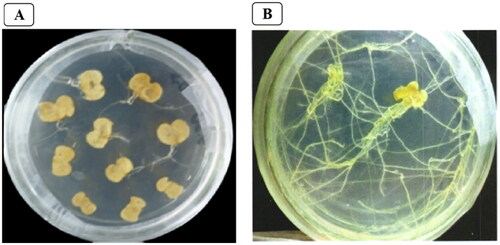
The sterile leaves were infected with the recombinant ATCC15834 containing pRI101-IRT1, and hairy roots grew up around 14 days (). The color of the leaves became yellow after induction. The linear tissue growing out of the wound was the hairy root, the main roots were long and the lateral roots were short. After 28 days of culture, the hairy roots that grow rapidly and in good condition were cut from the explants and placed on hormone-free MS medium for subculture. The hairy roots cultured in vitro sprouted into new hairy roots at the incision. The root tips and root hairs were obvious, with many branches and grow very quickly. DNA was extracted from the hairy roots of B. campestris L. for 30 days () and detected by PCR.
Figure 1. Growth of B. campestris L. explants infected with recombinant R. rhizogenes ATCC15834 for 14 days (A) and 30 days of B. campestris L. hairy root (B).
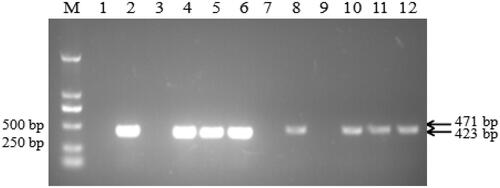
The results of DNA gel electrophoresis () showed that a specific DNA fragment of 423 bp, namely the rolB gene, was successfully amplified using the transgenic hairy root genome (lanes 4-6) . The rolB gene is related to the formation of hairy roots and is the most critical gene in the process of Ri plasmid transformation. The presence or absence of rolB can be used as a marker for the successful construction of hairy roots. Using the transgenic hairy root genome as template, it is amplified another specific DNA fragment of about 471 bp (lanes 10-12), namely the IRT1 gene, which was the same as the amplification result using the recombinant plasmid pRI101-IRT1 as a template. Using B. campestris L. genome as the negative control, no fragments (rol B and IRT1) were amplified (lanes 3 and 9). Therefore, the above experiments proved that the rolB and IRT1 genes were integrated into the genome of B. campestris L. hairy roots.
Figure 2. PCR detection of rolB and IRT1 genes of transgenic B. campestris L.hairy root. M: DL2000 DNA Marker; Lane 1-6: amplified rolB gene fragments; lane 7-12: amplified IRT1 gene ftragments. 1: blank control; 2: Ri plasmid; 3: Negative control; 4-6: transgenic IRT1 hairy roots; 7: blank control; 8: IRT1-pRI101 plasmid; 9: Negative control; 10-12: transgenic IRT1 hairy roots.
Growth of transgenic IRT1 hairy roots under cd stress
In order to further analyze whether IRT1 gene overexpression can effectively improve the tolerance of B. campestris L. hairy roots to Cd, Cd stress experiments on transgenic IRT1 hairy roots and wild-type hairy roots were performed. First, the biomass of the two hairy roots under Cd stress were compared. The fresh weights of hairy roots affected by Cd at different times and concentrations were shown in . In the absence of Cd, transgenic IRT1 and wild type hairy roots reached the maximum fresh weight level of 0.28 g and 0.24 g on the 14 d. When the hairy roots exposed to Cd (50 µmol/L), the fresh weight of transgenic IRT1 hairy roots and wild-type hairy roots increased slightly on the 7 d and 14 d compared with the 0 d. When the hairy roots exposed to increased concentration of Cd (100 µmol/L), the growth of transgenic IRT1 hairy roots and wild-type hairy roots was restricted, fresh weight showed a downward trend. The fresh weight of IRT1 transgenic hairy roots was 0.08 g and 0.07 g at 7 d and 14 d, while that of wild hairy roots was 0.06 g and 0.04 g. The results showed that the fresh weight of IRT1 transgenic hairy roots decreased more slowly than that of wild hairy roots. On the 14th day, with the increase of Cd concentration, the growth of hairy roots of transgenic IRT1 and wild-type hairy roots were inhibited, and the trend of fresh weight decline was obvious.
Figure 3. Effect of different concentrations of Cd (0、50、100µmol/L) on fresh weight (A) and dry weight (B) of B. campestris L. hairy roots. I: transgenic hairy root of IRT1 gene; W: wild-type hairy root. (The lowercase letters a-e represent the significant diffierence in the biomass of 7 d and 14 d under cadmium stress, different letters stands for statistically significant differences, same letters stands for no significant difference, p < 0.05)
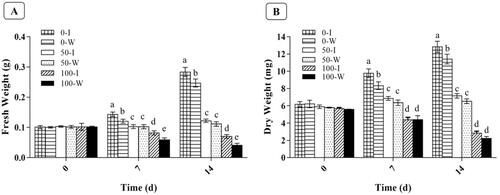
presented the effect of different concentrations of Cd on the dry weight of two kinds of hairy roots. Similar to the change trend of fresh weight, with the increased Cd concentration, the dry weight of the two hairy roots showed obvious downward trend, and the dry weight of wild-type hairy roots showed a more significant downward trend.
Cadmium accumulation in transgenic IRT1 hairy roots under cd stress
It can be seen from that the Cd uptake by transgenic IRT1 and wild-type hairy roots increased with the increased Cd concentration under three concentration gradients (0, 50, 100 µmol/L). At 7 d, in the 50, 100 µmol/L Cd culture environment, the amount of Cd adsorbed by the transgenic hairy root of IRT1 gene was 2.124 µg and 3.891 µg, and the amount of Cd enriched by the wild type hairy root was 1.685 µg and 1.899 µg. The content of Cd enriched by the transgenic hairy root of IRT1 was higher than that of the wild type hairy root. At 14 d, in the 50 µmol/L Cd, transgenic and wild type hairy roots are not good at Cd enrichment, reaching 2.317 µg and 2.284 µg; at 100 µmol/L Cd, the Cd contents in two hairy roots after 14 days treatment reached the maximum absorption value. The Cd content enriched by transgenic IRT1 hairy roots was 33.161 µg, 1.14 times that of wild-type hairy roots.
Table 2. Cd content (µg/100 mg) enriched by hairy roots cultured in different Cd concentrations.
Cd enrichment efficiency of transgenic IRT1 hairy roots under cd stress
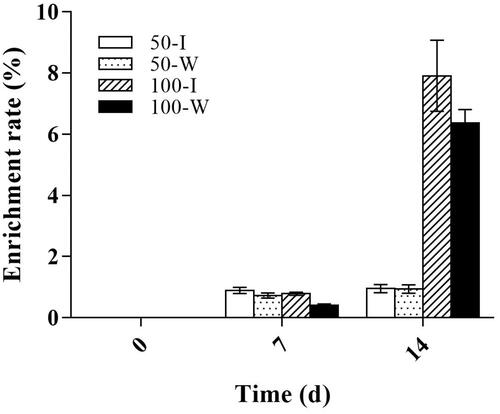
Enrichment efficiency of B. campestris L. hairy roots exposed to Cd was presented in . Under the condition of 50 µmol/L Cd stress, the Cd enrichment efficiency of transgenic IRT1 and wild-type hairy roots was 0.93% and 0.74% on the 7 d respectively, and the enrichment efficiency was only slightly increased on the 14 d. However, under 100 µmol/L Cd treatments, the enrichment efficiency of transgenic IRT1 and wild-type hairy roots reached 7.26% and 6.38% on the 14 d; Compared with the 7 d, the enrichment efficiency of Cd was improved by 8.5 times and 15.2 times, respectively. The above results indicated that in the environment of 50 µmol/L Cd or within a short cultivation period (less than 7 days), the Cd enrichment efficiency of transgenic IRT1 and wild-type hairy roots were not very high. Under the condition of 100 µmol/L Cd treatment, the enrichment efficiency of Cd in two types hairy roots was significantly improved. Furthermore, the enrichment efficiency of Cd in transgenic IRT1 hairy roots was 13.73% higher than that of wild-type.
Figure 4. Enrichment efficiency of B. campestris L. hairy roots at different Cd concentrations. I: transgenic IRT1 hairy root; W: wild-type hairy root; p < 0.05.
The residual Cd content in the culture medium was shown in . It can be seen, that Cd residual decreased with increasing exposure times and concentration. This result was consistent with the increase of Cd content enriched in hairy roots in . In the environment of 100 µmol/L Cd, the Cd content of transgenic IRT1 and wild type hairy roots decreased by 81.64% and 68.63% respectively on the 14 d.
Table 3. The residual Cd (µg/20 mL) in medium.
Expression of IRT1 gene in transgenic IRT1 hairy roots under cd stress
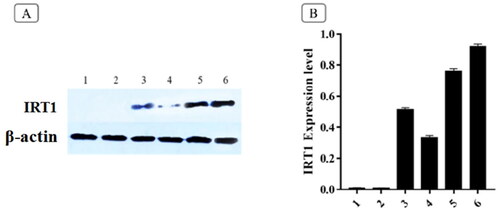
As a direct transporter of iron, IRT1 is also involved in the transport of other heavy metals; under external stress conditions, its transcription and translation levels are strictly regulated (Connolly et al. Citation2002). In this study, we introduced the IRT1 gene into the hairy roots of B. campestris L. to strengthen the enrichment of Cd. The previous results indicated that, compared with wild type, the hairy roots of B. campestris L. transfected with the IRT1 gene showed better growth status and higher Cd enrichment capacity under higher concentration of Cd (100 µmol/L) stress. In order to further verify whether the IRT1 gene is involved in the transport of Cd in the hairy roots, we analyzed the expression of the IRT1 protein by Western blotting, and the result was given in . The wild-type hairy roots hardly expressed IRT1 at low Cd concentration (50 µmol/L) and the expression level of IRT1 increased under higher concentration Cd (100 µmol/L). However, under normal growth conditions (without Cd), the transgenic IRT1 hairy roots had certain expression of IRT1; under Cd treatment, the expression of IRT1 was positively correlated with the concentration of Cd.
Figure 5. Expression of IRT1 (Western Blotting) in hairy roots under Cd stress (A) and IRT1 expression gray analysis (B). p < 0.05. 1, 2 and 3 are the IRT1 expression levels of wild-type hairy root when the Cd concentration is 0, 50, and 100 µmol/L, respectively; 4, 5 and 6 are the IRT1 expression levels of transgenic hairy root when the Cd concentration is 0, 50, and 100 µmol/L, respectively.
Discussion
Nowadays, the removal of environmental pollutants through hyperaccumulator plants and phytoremediation have been recognized as environmentally friendly and cost-effective sustainable technologies. However, hyperaccumulators often have defects such as low biomass, slow growth, strong regionality and long repair time (Feki et al. Citation2021). In order to accelerate the application of phytoremediation technology and understand the molecular mechanism of hyperaccumulator, We hope to transform hyperaccumulators by genetic manipulation. The uptake and translocation of non-essential heavy metals in plant are always through metal transporters for essential micronutrient transport, including NRAMP, ZIP (Zrt- and Irt-related protein), heavy metal P-type ATPases, and YSL (Yellow Stripe-Like) transporters (Wang et al. Citation2019). IRT1 is a member of the ZIP (zinc regulated transporter) protein family. Arabidopsis IRT1 was first discovered and cloned, and showed a diversity of metal ion absorption. Many researchers have proved that the IRT1 gene can alleviate metal toxicity through up-regulation under Cd stress condition according to comparative transcriptome analysis (Chen et al. Citation2019; Yu et al. Citation2018).
In our study, the hairy root of B. campestris L. was used as the research material, and the IRT1 gene was selected for stress resistance analysis. We successfully integrated the rolB and IRT1 genes into the genome of B. campestris L. root and were able to express it stably, the hairy root system can grow stably within 6 weeks and the IRT1 gene wouldn’t be lost within 50 subcultures. By analyzing the biomass, enrichment efficiency and IRT1 gene expression of transgenic IRT1 hairy roots under different Cd concentration conditions, it was shown that IRT1 gene can respond to Cd stress. With the increase of time and Cd concentration, the fresh weight and dry weight of the two hairy roots had a significant downward trend at 100 µmol/L on the 14 d, and the downward trend of the wild type hairy roots was more obvious. Possible causes of weight loss are with the increase of Cd concentration, the toxic effect of Cd is more serious. Under the action of high concentration of Cd, the metabolism of plant cells is inhibited, the chromosome aberration rate and cell mitosis number in cells are reduced, and the synthesis of soluble protein is blocked, leading to the stagnation or decline of biomass. The Cd uptake by transgenic IRT1 and wild-type hairy roots increased with the increase of Cd concentration under three concentration gradients (0, 50, 100 µmol/L). This may be due to the fact that the low concentration Cd may not cause obvious damage to plant tissues and plants will accumulate Cd and also promote the absorption of other trace elements. But as the concentration of Cd was increased and the cultivation time was prolonged, the expression of related ion transport proteins were also increased, so that the Cd content in hairy roots was significantly increased. Further, this fact could be related to the growth phase of hairy roots cultures. At 14 days, some species would be reaching stationary phase. The Cd enrichment efficiency in the transgenic hairy roots was increased by 13.73% compared with the wild-type. Therefore, the transgenic IRT1 hairy roots have better Cd enrichment characteristics and enrichment efficiency than that of wild-type hairy roots. It is indicated that the expression of IRT1 is beneficial to enhance the resistance and enrichment ability of hairy roots to heavy metals. At the same time, the expression level of the IRT1 gene is up-regulated under heavy metal stress and the expression increased with the increase of stress degree. It can be inferred that IRT1 plays an important role in the adaptability of B. campestris L. hairy roots to Cd stress in the environment. Chen compared and analyzed the Cd content and IRT1 expression of different amaranth varieties, and found that the varieties with high IRT1 expression had more Cd content in vivo (Chen et al. Citation2015). Zhang improved Cd accumulation efficiency of hairy roots by 19.03% by constructing transgenic hairy roots of Solanum nigrum (Zhang Citation2016). In Connolly’s research on transgenic IRT1 Arabidopsis thaliana, the transgenic plants had the high accumulation of Cd (200 µg/100 mg dry weight) and the enrichment effic iency increased by 60% (Connolly et al. Citation2002).
Hairy roots are often used to study root physiology, biosynthetic pathways and the production of secondary metabolites due to their advantages of being able to grow rapidly under sterile conditions without hormones. The development of the hairy root biotechnology platform has made hairy roots applied in the field of phytoremediation. The researchers have successively verified the response of hairy roots of Wedelia trilobata, Solanum nigrum L., Cucumis sativus, and Adenophora lobophylla to Cd (Shi et al. Citation2010, Citation2012; Wu et al. Citation2001; Zhang et al. Citation2009). It proves that hairy roots can be used as a sensitive tool for detecting and evaluating Cd pollution in the environment. However, the molecular mechanism study of single gene through transgenic hairy root has not yet been found. In our study, the IRT1 gene was transferred into B. campestris L. by using hairy root as a transition system, which shortened the time for constructing the transgenic plant, and the subsequent culture method was simple. The transgenic IRT1 B. campestris L. hairy roots system provides an easy-to-operate platform for the future study of the role of IRT1. Afterwards, we will optimize the hairy root culture conditions to improve the Cd enrichment ability of the transgenic IRT1 B. campestris L. hairy roots.
Phytoremediation of pollutants by genetically modified hairy roots can be further developed by means of absorption/stress tolerance mechanisms and basic metabolic information, but hairy roots have limited application in the field of phytoremediation, so artifical wetlands can be used to apply hairy root technology good choice (Rai et al. Citation2020). At the same time, the transgenic IRT1 hairy root material obtained in this study can be further transferred into transgenic IRT1 plants in the future, which can improve the success rate of constructing transgenic plants, shorten the cultivation cycle and increase the biomass of hyperaccumulators. Transgenic IRT1 B. campestris L. plants can be better used for the remediation of Cd contaminated soil, which has broad development and application prospects.
Conclusion
In this study, stable transgenic hairy root system containing the IRT1 gene of Cd hyperaccumulator-B. campestris L. was successfully constructed by the R. rhizogenes mediated method. By analyzing the biomass, enrichment efficiency and IRT1 gene expression of transgenic IRT1 hairy roots under different Cd concentration conditions, it was shown that IRT1 gene can respond to Cd stress. The overexpression of IRT1 gene can help B. campestris L. hairy roots to effectively cope with Cd stress and improve its ability to enrich Cd. The transgenic IRT1 hairy roots have better Cd enrichment characteristics and enrichment efficiency than that of wild-type hairy roots. The transgenic IRT1 hairy root system established in this study can be used as a reliable experimental model for the study of Cd adsorption mechanism, and can be further regenerated to obtain transgenic IRT1 B. campestris L. plants for the study of heavy metal Cd pollution remediation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Chen C, Cao Q, Jiang Q, Li J, Yu R, Shi G. 2019. Comparative transcriptome analysis reveals gene network regulating cadmium uptake and translocation in peanut roots under iron deficiency. BMC Plant Biol. 19(1):35. doi:10.1186/s12870-019-1654-9.
- Chen Y, Li Q, He B, Mei X, Lei Y, Xu Z, Zhou L. 2015. Metal Accumulation and Expressions of Roots Two Key Transporter Genes, HMA4 and IRT1, of High/Low Cd-Accumulating Amaranth Cultivars. J Agro Environ Sci. 34:1041–1046. doi:10.11654/jaes.2015.06.004.
- Connolly E, Fett J, Guerinot M. 2002. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 14(6):1347–1357. doi:10.1105/tpc.001263.
- Feki K, Tounsi S, Mrabet M, Mhadhbi H, Brini F. 2021. Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ Sci Pollut Res Int. 28(46):64967–64986. doi:10.1007/s11356-021-16805-y.
- He X, Fan S, Zhu J, Guan M, Liu X, Zhang Y, Jin C. 2017. Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil. 416(1-2):453–462. doi:10.1007/s11104-017-3232-y.
- Huang Y, Zu L, Zhang M, Yang T, Zhou M, Shi C, Shi F, Zhang W. 2020. Tolerance and distribution of cadmium in an ornamental species Althaea rosea Cavan. Int J Phytoremediation. 22(7):713–724. doi:10.1080/15226514.2019.1707771.
- Kofroňová M, Hrdinová A, Mašková P, Soudek P, Tremlová J, Pinkas D, Lipavská H. 2019. Strong antioxidant capacity of horseradish hairy root cultures under arsenic stress indicates the possible use of Armoracia rusticana plants for phytoremediation. Ecotoxicol Environ Saf. 174:295–304. doi:10.1016/j.ecoenv.2019.02.028.
- Li J-W, Zeng T, Xu Z-Z, Li J-J, Hu H, Yu Q, Zhou L, Zheng R-R, Luo J, Wang C-Y. 2022. Ribozyme-mediated CRISPR/Cas9 gene editing in pyrethrum (Tanacetum cinerariifolium) hairy roots using a RNA polymerase II-dependent promoter. Plant Methods. 18(1):32. doi:10.1186/s13007-022-00863-5.
- Mani D, Kumar C. 2014. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. Int J Environ Sci Technol. 11(3):843–872. doi:10.1007/s13762-013-0299-8.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 15(3):473–497. doi:10.1111/j.1399-3054.1962.tb08052.x.
- Pandey N, Rai K, Rai S, Pandey-Rai S. 2021. Heterologous expression of cyanobacterial PCS confers augmented arsenic and cadmium stress tolerance and higher artemisinin in Artemisia annua hairy roots. Plant Biotechnol Rep. 15(3):317–334. doi:10.1007/s11816-021-00682-5.
- Pehlivan E, Özkan A, Dinç S, Parlayici S. 2009. Adsorption of Cu2+ and Pb2+ ion on dolomite powder. J Hazard Mater. 167(1-3):1044–1049. doi:10.1016/j.jhazmat.2009.01.096.
- Pérez-Palacios P, Agostini E, Ibáñez SG, Talano MA, Rodríguez-Llorente ID, Caviedes MA, Pajuelo E. 2017. Removal of copper from aqueous solutions by rhizofiltration using genetically modified hairy roots expressing a bacterial Cu-binding protein. Environ Technol. 38(22):2877–2888. doi:10.1080/09593330.2017.1281350.
- Rahman Z, Singh VP. 2019. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess. 191(7):419. doi:10.1007/s10661-019-7528-7.
- Rai P, Kim K, Lee S, Lee J. 2020. Molecular mechanisms in phytoremediation of environmental contaminants and prospects of engineered transgenic plants/microbes. Sci Total Environ. 705:135858. doi:10.1016/j.scitotenv.2019.135858.
- Sabir A, Naveed M, Bashir MA, Hussain A, Mustafa A, Zahir ZA, Kamran M, Ditta A, Núñez-Delgado A, Saeed Q, et al. 2020. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J Environ Manage. 265:110522. doi:10.1016/j.jenvman.2020.110522.
- Seneviratne M, Rajakaruna N, Rizwan M, Madawala H, Ok YS, Vithanage M. 2019. Heavy metal-induced oxidative stress on seed germination and seedling development: a critical review. Environ Geochem Health. 41(4):1813–1831. doi:10.1007/s10653-017-0005-8.
- Shi H, Tsang E, Wang Y, Chan A. 2010. Effect of cadmium, alone or in combination with CaCl2, on the growth, antioxidative enzyme activity and cadmium absorption of Solanum nigrum L. var pauciflorum hairy roots. Sheng wu Gong Cheng Xue Bao. 26:147–158.
- Shi H, Wang Y, Tsang P, Chan L. 2012. Alleviated affect of exogenous CaCl2 on the growth, antioxidative enzyme activities and cadmium absorption efficiency of Wedelia trilobata hairy roots under cadmium stress. Sheng Wu Gong Cheng Xue Bao. 28(6):747–762.
- Sun F, Chen Z, Zhang Q, Wan Y, Hu R, Shen S, Chen S, Yin N, Tang Y, Liang Y, et al. 2022. Genome-Wide Identification of the TIFY gene family in Brassiceae and its potential association with heavy metal stress in rapeseed. Plants (Basel). 11(5):667. doi:10.3390/plants11050667.
- Wang C, Chen X, Yao Q, Long D, Fan X, Kang H, Zeng J, Sha L, Zhang H, Zhou Y, et al. 2019. Overexpression of TtNRAMP6 enhances the accumulation of Cd in Arabidopsis. Gene. 696:225–232. doi:10.1016/j.gene.2019.02.008.
- Wang S, Demshar H. 1992. Determination of blood lead in dried blood-spot specimens by Zeeman-effect background corrected atomic absorption spectrometry. Analyst. 117(6):959–961. doi:10.1039/an9921700959.
- Wu S, Zu Y, Wu M. 2001. Cadmium response of the hairy root culture of the endangered species Adenophora lobophylla. Plant Sci. 160(3):551–562. doi:10.1016/s0168-9452(00)00429-5.
- Xu Z, Mei X, Tan L, Li Q, Wang L, He B, Guo S, Zhou C, Ye H. 2018. Low root/shoot (R/S) biomass ratio can be an indicator of low cadmium accumulation in the shoot of Chinese flowering cabbage (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee) cultivars. Environ Sci Pollut Res Int. 25(36):36328–36340. doi:10.1007/s11356-018-3566-x.
- Ye P, Wang M, Zhang T, Liu X, Jiang H, Sun Y, Cheng X, Yan Q. 2020. Enhanced cadmium accumulation and tolerance in transgenic hairy roots of Solanum nigrum L. Expressing Iron-Regulated Transporter Gene IRT1. Life (Basel). 10(12):324. doi:10.3390/life10120324.
- Yu R, Ma Y, Li Y, Li X, Liu C, Du X, Shi G. 2018. Comparative transcriptome analysis revealed key factors for differential cadmium transport and retention in roots of two contrasting peanut cultivars. BMC Genomics. 19(1):938. doi:10.1186/s12864-018-5304-7.
- Zhang P, Huang H, Liu W, Zhang C. 2017. Physiological mechanisms of a wetland plant (Echinodorus osiris Rataj) to cadmium detoxification. Environ Sci Pollut Res Int. 24(27):21859–21866. doi:10.1007/s11356-017-9744-4.
- Zhang P, Li Q, Zhang C. 2020. Root adaptation in echinodorus osiris rataj plant under cadmium stress. Int J Phytoremediation. 22(5):534–539. doi:10.1080/15226514.2019.1686605.
- Zhang T. 2016. Establishment of IRT1 transgenic hairy roots line of Solanum nigrun L. and preliminary study on its response to cadmium stress[dissertation]. Beijing Jiaotong University, Beijing, China.
- Zhang Y, Shi HP, Po E, Tsang K. 2009. Influences of heavy metal cadmium alone and in combination with zinc on the growth and activities of antioxidant enzymes of Cucumis sativus hairy roots. Sheng Wu Gong Cheng Xue Bao. 25(1):60–68.
- Zheng S, Liu S, Feng J, Wang W, Wang Y, Yu Q, Liao Y, Mo Y, Xu Z, Li L, et al. 2021. Overexpression of a stress response membrane protein gene OsSMP1 enhances rice tolerance to salt, cold and heavy metal stress. Environ Exp Bot. 182:104327. doi:10.1016/j.envexpbot.2020.104327.
- Zhou Y, Yang Z, Gong L, Liu R, Sun H, You J. 2018. Molecular characterization of GmSTOP1 homologs in soybean under Al and proton stress. Plant Soil. 427(1-2):213–230. doi:10.1007/s11104-018-3645-2.
- Zhu Y, Du W, Fang X, Zhang L, Jin C. 2020. Knockdown of BTS may provide a new strategy to improve cadmium-phytoremediation efficiency by improving iron status in plants. J Hazard Mater. 384:121473. doi:10.1016/j.jhazmat.2019.121473.
