?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The purpose of this study was to evaluate the potential of microbial-enhanced Brassica oleracea for the phytoremediation of seleniferous soils. The effect of selenite (Se(IV)) and selenate (Se(VI)) on B. oleracea (1–100 mg.L−1) was examined through germination (7 d) and pot (30 d) trials. Microbial analysis was conducted to verify the toxic effect of various Se concentrations (1–500 mg.L−1) on Rhodococcus opacus PD360, and to determine if it exhibits plant growth promoter traits. R. opacus PD630 was found to tolerate high concentrations of both Se(IV) and Se(VI), above 100 mg.L−1. R. opacus PD630 reduced Se(IV) and Se(VI) over 7 days, with a Se conversion efficiency between 60 and 80%. Germination results indicated lower concentrations (0–10 mg.L−1) of Se(IV) and Se(VI) gave a higher shoot length (> 4 cm). B. oleracea accumulated 600–1,000 mg.kg−1 dry weight (DW) of Se(IV) and Se(VI), making it a secondary accumulator of Se. Moreover, seeds inoculated with R. opacus PD360 showed increased Se uptake (up to 1,200 mg Se.kg−1 DW). In addition, bioconcentration and translocation factors were greater than one. The results indicate a synergistic effect between R. opacus PD630 and B. oleracea for Se phytoextraction from polluted soils.
NOVELTY STATEMENT
This article examines how Brassica oleracea may be used to improve seleniferous soils and how Rhodococcus opacus can be added to increase biofortification. The research shows great potential for combining Brassica species with bacterial isolates to remove selenium from heavily contaminated soils.
Introduction
Selenium (Se) is an important micronutrient, essential for humans and animals but can be harmful at high concentrations. Se is essential for mammals and bacteria, but views vary for plants (White Citation2016, Citation2018). Although Se levels in soils are generally low, elevated levels of Se (>5 mg.kg−1) up to and exceeding 100 mg.kg−1 may occur in some areas (Schiavon and Pilon-Smits Citation2017; White Citation2018). High levels of Se in soils are usually found in low-lying, poorly drained alkaline soils. Besides, due to uncontrolled anthropogenic activities, such as oil exploitation and agriculture, elevated levels of Se may occur (Brown and Shrift Citation1982; White Citation2017). The latter is a concern, as it can have a negative effect on plant development, and induce selenosis if livestock grazes on pastures with high Se concentrations (Brown and Shrift Citation1982; White Citation2017, Citation2018).
Plants take up Se in the form of selenite (SeO32−, Se (IV)) and selenate (SeO42−, Se (VI)) via the roots. However, no Se-specific transporter is used: selenate is taken up via the sulfate transporters, while Se (IV) via phosphate transporters and aquaporins (White Citation2016; Schiavon and Pilon-Smits Citation2017; White Citation2018). Plants are divided into three groups depending on their ability to grow on seleniferous soils and accumulate Se in their tissues (Brown and Shrift Citation1982; White Citation2016; Citation2017). Non-accumulators of Se do not colonize Se-rich soils and typically contain less than 100 mg Se.kg−1 dry weight (DW) within plant tissues. Secondary Se accumulators can tolerate Se within soils, and there is a direct correlation between the Se concentration in soil and plant tissues, which generally contain 100–1,000 mg.kg−1 DW. Such plants belong to the Amaranthaceae, Fabaceae, and Orobanchaceae families (Rosenfeld and Beath Citation1965; Brown and Shrift Citation1982). Se hyperaccumulators contain more than 1,000 mg.kg−1 DW Se within the plant tissue, consisting of plants such as Stanleya pinnata and Astragalus bisulcatus (Freeman et al. Citation2012).
The majority of Se accumulator species reside in hot arid climates. Hyperaccumulating plant species do not thrive in countries such as Ireland. Kavanagh et al. (Citation2018) explored the ability of a wide variety of species to be used for soil lithium remediation, suggesting that in the Irish climate, plant species such as Helianthus annuus and crops, such as Lactuca sativa and Solanum lycopersicum, have the potential to perform well as lithium hyperaccumulators. Plants from the genus Brassica can accumulate high metal concentrations, such as cadmium and nickel (Kavanagh et al. Citation2018). Yasin et al. (Citation2014) and White (Citation2016) reported that Brassica species are good hyperaccumulators of Se, due to their high affinity for sulfur. Likewise, Brassica species assimilate Se(VI) and Se(VI) to selenocysteine (SeCys) and selenomethionine (SeMet) after plant uptake (Schiavon and Pilon-Smits Citation2017; Trippe and Pilon-Smits Citation2020). In particular, Brassica oleracea (cabbage) has been shown to have phytoremediation capacity for metals, such as zinc, cadmium, and nickel (Peyravi et al. Citation2016; Natasha et al. Citation2020; Navarro-León et al. Citation2020). Furthermore, the B. oleracea growth cycle suits the mild Irish climate, with crops being grown successfully from autumn to mid-winter.
Rhizosphere microbes play an important role in promoting the accumulation of compounds, such as heavy metals, and promoting plant growth within plants (Durán et al. Citation2013; Debnath et al. Citation2016). Rhodococcus sp. are non-sporulating actinomycetes that degrade a range of different contaminants, and possess a broad metabolic spectrum (Yasin et al. Citation2015; Pilon-Smits et al. Citation2017). Rhodococcus is a metabolically diverse species and has the ability to degrade and accumulate a large number of organic compounds, including recalcitrant and toxic compounds (Eswayah et al. Citation2016; Di Canito et al. Citation2018). Rhodococcus opacus PD630 has been extensively studied for its ability to accumulate triacylglycerol and to act as a prokaryote model in relation to lipid biosynthesis and accumulation (Zhu et al. Citation2009).
The use of plant-microbe associations for the remediation of contaminated soils is a promising approach to enhance the efficiency and rate of phytoremediation. Rhizosphere microbes have been shown to contribute to plant Se uptake. Brassica juncea L. contained higher Se concentrations in its roots and had higher Se volatilization rates when inoculated with rhizosphere bacteria (De Souza et al. Citation1999). The capacity of Symphyotrichum ericoides to hyperaccumulate Se was benefited by rhizosphere microbes, with higher shoot and root Se concentrations (El Mehdawi et al. Citation2015). The mechanisms involved could be increased root surface area or root hair architecture, enhanced metal bioavailability, or increased metal translocation from roots to shoots (Mandal et al. Citation2017).
This study is driven by a twofold objective. Firstly, it seeks to delve into the remediation potential of R. opacus PD630 in environments rich in Se. This entails an in-depth exploration of R. opacus PD630’s capacity to mitigate Se levels within Se-rich soil, coupled with an examination of its repercussions on the growth and development of B. oleracea plants. The study scrutinizes the intricate interplay between this microbial agent and plant growth, aiming to elucidate whether the presence of R. opacus PD630 has an augmentative or inhibitory effect. Secondly, the research endeavors to probe the phytoremediation capabilities of B. oleracea in the context of Se accumulation from soils intentionally spiked with Se. This facet of the study seeks to ascertain whether B. oleracea can be categorized as a secondary Se accumulator. In its entirety, this investigation promises to furnish a nuanced understanding of the multifaceted dynamics involved in Se soil management and remediation, shedding light on the potential synergy between microbial and plant-based strategies in addressing Se-related environmental challenges.
Materials and methods
Microbiological analysis of R. opacus PD630
Determination of minimum inhibitory concentration and minimum bactericidal concentration
The Minimum Inhibitory Concentration (MIC) is defined as the lowest concentration of an inhibitor (Se) that prevents the growth of the selected microorganism, i.e., R. opacus PD630 (Presentato et al. Citation2016). In this study, MIC was determined in Luria broth (LB) medium (Oxiod, Thermo Fisher Scientific, Dublin, Ireland) with concentrations of Na2SeO4 and Na2SeO3 in the range of 0–500 mg.L−1, as described by Presentato et al. (Citation2016). Inoculation was performed using 100 μL of fresh and pre-grown R. opacus PD 630. After incubation at 30 °C for 48 h at 150 rpm, the optical density (OD) of the suspension was measured at 600 nm. Each test tube with no growth was used for Minimum Bactericidal Concentration (MBC) determination. For this purpose, 50 μL of the sample was streaked onto the LB medium without any Se according to Das et al. (Citation2014) and Parvekar et al. (Citation2020). These plates were incubated overnight at 30 °C.
Toxic effects of Se(IV) and Se(VI)
The disk diffusion method (Yaghoobizadeh et al. Citation2017) was selected for analysis of the toxic effect of Se(IV) and Se(VI) on R. opacus P630. 10 μL of the previous isolate grown on LB medium was inoculated onto LB agar plates by lawn culture. The disks were impregnated with different concentrations of Na2SeO4 or Na2SeO3 (in the range of 0–100 mg.L−1) applied to the surface of the medium and incubated at 30 °C for 24 h. The diameter of the inhibition zone was measured and reported in mm.
Growth profiles
Growth studies were performed in falcon tubes containing 50 mL LB medium in triplicate as described by Yaghoobizadeh et al. (Citation2017). Two subsets of growth studies were performed: supplemented with or without 100 and 500 mg.L−1 Na2SeO4 and Na2SeO3. 1 mL of overnight culture was inoculated in each flask, and it was incubated at 30 °C for 124 h. At predefined intervals, growth was monitored by total viable counts (TVC) on plate count agar (incubated at 30 °C for 24 h), with results reported as Log CFU.mL−1 (EquationEquation 1(1)
(1) ). The final growth curve was based on the average of three replicates.
(1)
(1)
Removal efficiency
The ability of R. opacus PD630 to remove selenate and selenite from the solution was examined by inoculating R. opacus PD630 into half-strength LB and minimal media broths that contained varying concentrations (10–500 mg.L−1) of Se(IV) and Se(VI). This was run over 7 days with samples taken on days 1, 3, 5, and 7. Se(IV) and Se(VI) were analyzed by ion chromatography (IC), whereas total Se by inductively coupled plasma – optimal emission spectrometry (ICP-OES) (see Analysis of Selenium section). The percentage removal was calculated using EquationEquation (2)(2)
(2) :
(2)
(2)
Where Cinf equals the initial concentration and Ceff is the final concentration after treatment.
Plant growth-promoting traits
R. opacus PD630 was assayed for plant growth-promoting (PGP) traits. 3 indole acetic acid (3-IAA) production, phosphate solubilization, nitrogen fixation activity on nitrogen-free media, and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity were evaluated using the methods described by Gupta and Pandey (Citation2019).
Bioinformatics analyses of selenium and PGP traits within the R. opacus PD630 genome
Gene mining of the R. opacus PD630 genome (NCBI:txid543736) was carried out to determine if the required genes for Se metabolism and PGP traits were present. This was done by utilizing the eggNOG genome annotation tools (v. 5) to determine what genes within the selenocompound metabolism (KEGG pathway: map 00450) were present. Furthermore, PGP traits and genes as shown in Table S1 were searched into the genome using the NCBI gene database and BLASTn as described by Lin et al. (Citation2015).
Germination and plant trials
Seed sterilization
Seeds were sterilized before the germination trials. Firstly, seeds were shaken in 70% ethanol for three minutes, then washed three times with sterile deionized H2O for 1 min. They were further washed with 25% sodium hypochlorite and 0.1% Tween20 solution for 15 min and finally washed with sterile deionized H2O for one minute. This procedure was repeated eight times. After washing, seeds were dried inside a laminar airflow on sterile filter paper for 2 h (Matilla et al. Citation2007).
Seed inoculation
After sterilization, seeds were inoculated with R. opacus PD630 following Matilla et al. (Citation2007) recommendations. This was carried out by growing a pure culture in tryptone soy broth (TSB; prepared according to the manufacturer’s instructions) at 30 °C for 24 h. A seed coating was performed on two batches of seeds: one with fresh R. opacus PD630 and the other with a killed control. For the killed control, the broth containing R. opacus PD630 was incubated at 100 °C for 6 h, prior to inoculation of the seeds. The seed coating was prepared by centrifuging 5 mL of R. opacus PD630 broth at 10,000 rpm for 5 min. The pellet was then resuspended in 45 mL of sterile PBS (phosphate buffer saline), creating one in ten dilutions. Pre-sterilized seeds were inoculated with bacteria by submersion in the coating solution for 30 min while shaking at 200 rpm. The coating solution was then decanted and seeds were air-dried in a laminar airflow on sterile filter paper for two hours, after which TVC was performed (incubation details in the Growth profiles section), to calculate the concentration of bacteria on the seed surface according to EquationEquation (1)(1)
(1) (Kim et al. Citation2013).
Germination trials
Seed germination trials were carried out as described by Kavanagh et al. (Citation2018) to assess the total germination percentage of B. oleracea in the presence of Se(IV) or Se(VI). These trials were carried out over 7 days to investigate the effects of varying concentrations of Se(IV) and Se(VI) on B. oleracea germination. Test groups contained 5 replicates of 4 seeds (N = 20 per Se type) for each concentration of Se(IV) and Se(VI). Concentrations ranged from 0 mg.L−1 (Control) to 100 mg.L−1. Seeds ((a) seeds without R. opacus PD630, (b) seeds with R. opacus PD630, and (c) seeds with killed control) were placed on a filter paper (Whatman No. 1) in a 90 mm petri dish, with 3 mL of a specific concentration of Se(IV) or Se(VI). Samples were incubated at 20 (± 1) °C for 7 days. After incubation, seedlings with a 5 mm radial length (Finnegan et al. Citation2017) were separated and counted for germination % (EquationEquation 3(3)
(3) ) and their shoot length was recorded.
(3)
(3)
Pot trials
All soil used in the experiments was topsoil, consisting of a sieved dark brown/black rich clay loam soil with high-humus content (1.6%) sampled in Sligo (Ireland). The soil was thoroughly mixed to ensure uniformity and passed through a 4 mm sieve. About 1 kg of oven-dry soil was thoroughly mixed with 1 L of deionized water containing dissolved Na2SeO4 and Na2SeO3 at appropriate concentrations (0, 1, 2, 3, 5, 10, 50 and 100 mg.kg−1). Approximately 1 kg of soil was transferred to polyethylene pots. Seeds (n = 3) were added into each pot at 5 mm below the surface. Three seed types were used: seeds that underwent surface sterilization, seeds inoculated with R. opacus PD630, and seeds inoculated with killed R. opacus PD630. In total, there were 144 pots, half (n = 72) for Se (IV) and the other half for Se(VI). These were each separated into three for each seed type (n = 24). Each treatment was carried out in triplicate. Shoot lengths were recorded at day 30 and were harvested when plants reached full growth at 120 days. Plants were removed from the soil, and above-ground biomass and roots were separated before drying at 100 °C for 24 h to record dry weights.
Analysis of Selenium
Microbial samples
Microbial samples were prepared by centrifuging 1,000 µl of broth at 5,000 g for 10 min. The pellets were resuspended in 200 µl concentrated HNO3 (65%) and then, transferred to a 15 ml tube containing 10 ml of HNO3 (65%) for digestion (2 h) at room temperature. The Se concentration of the digested samples was measured (Se oxyanions and Total Se) as described in the Determining Se concentration section.
Digestion of plant and soil biomass
10 mL of HNO3 65% was added to Teflon microwave tubes with ∼ 0.4 g of above-ground biomass sample. After being incubated for one hour at room temperature, the samples were digested in closed vessels using a microwave MARS 6™ (CEM Corporation, Matthews, USA) digestion system. This assay was carried out at 1,030 – 1,800 W, 200 °C, 20 – 25 min of ramp time, and 10 min of hold time. After cooling down, the content of each Teflon tube was poured into new plastic tubes and adjusted to 50 mL with Milli-Q water.
Determining Se concentration
The total Se concentration of the digested samples was determined by ICP-OES (Agilent Technologies, model 5110, Santa Clara, USA) after acidification with 5% HNO3 as described by Sinharoy and Lens (Citation2022). The ICP-OES measurement conditions were: read time 5 s, nebulizer argon flow 0.7 L.min−1, radiofrequency power 1.2 kW, plasma flow 12 L.min−1, stabilization time 15 s, and auxiliary flow 1 L.min−1. Se was read on axial mode at 196.026 nm.
Se(IV) and Se(VI) concentrations of the digested samples were determined using an IC equipped with a suppressed conductivity detector (DionexTM AquionTM, Thermo Scientific, Waltham, USA) as described by Mal et al. (Citation2021). AG14A 4 × 50 mm and AS14A 4 × 250 mm columns were used to achieve separation. A mixture of carbonate (3.03 mM) and bicarbonate (0.97 mM) was used as a mobile phase at a flow rate of 1 ml.min−1. Se recovery was evaluated using the certified reference material BCR402 White Clover (Sigma Aldrich, San Luis, USA)
Data analysis
Statistical analysis was carried out using SPSS® (Statistical Package for the Social Sciences, version 26.0, IBM Corp, Armonk, NY, USA), Microsoft Excel® (Professional Plus 2016, Redmond, WA, USA), and GraphPad Prism 8 (San Diego, CA, USA). A comparison of means (Tukey’s test) was conducted where a significant effect was found. A p-value of lower than 0.05 was considered significant.
Results and discussion
Microbial analysis
Microbial analysis was carried out on R. opacus PD630 to assess its ability to tolerate Se oxyanions and if it can be used in Se remediation. PGP studies were also conducted to assess if R. opacus PD630 impacts either B. oleracea germination or growth (pot trials) in the presence of Se oxyanions.
Selenium toxicity
shows MIC and MBC results for R. opacus PD630 exposed to various Se concentrations. R. opacus PD630 can indeed stand high concentrations of Se (Se (IV) and Se (VI)), up to 500 mg.L−1, because no total inhibition was observed. Moreover, R. opacus PD630 growth was not affected (p > 0.05) by concentrations of Se in the range of 1 and 3 mg.L−1. However, growth decreased with Se concentrations exceeding 5 mg.L−1. This was observed for both Se oxyanion species. Zone of inhibition results () confirmed that the higher the Se concentration, the less tolerable R. opacus PD630 is to Se oxyanions. Previously, Rhodococcus aetherivorans BCP1 was also shown to tolerate high concentrations of Se(IV) with a MIC of 500 mM (Presentato et al. Citation2018). This author associated the capacity of Actinobacteria to tolerate high Se(IV) concentrations with the presence of redox buffering molecules such as mycothiol in their cells.
Table 1. Minimum inhibition concentration, minimum bactericidal concentration, and zone of inhibition for R. opacus PD630 exposed to different Se concentrations.
Growth profiles of R. opacus PD630
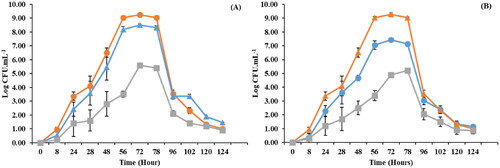
presents growth profiles of R. opacus PD630 grown in 0, 100, and 500 mg.L−1 Se(IV) and Se(VI) containing media. Initially, high growth rates were observed in the control (0 mg.L−1). The stationary phase occurred after 56 h, with a growth of about 9 Log CFU.mL−1, this phase lasted just 24 h, after which a decline (decay phase) occurred. R. opacus PD630 grown in 100 mg Se(IV). L−1 showed a similar result to the control with the stationary phase microbial count at 8 Log CFU.mL−1. However, 500 mg.L−1 Se(IV) and Se(VI) impacted on the growth with a 3 Log reduction compared with the control, and longer lag/log phases were observed. Nevertheless, R. opacus PD630’s growth of 1.52 and 1.35 Log CFU.mL−1 indicates the potential for R. opacus PD630 to survive at elevated Se concentrations. Growth profiles also revealed that Se(IV) is a more favorable speciation of Se. This can be explained by the fact Se(IV) is more easily reduced to elemental Se by bacteria than Se(VI) (Dungan et al. Citation2003).
Selenium removal efficiency using R. opacus PD630
Se(IV) shows removal efficiencies of 20–70% after day one, with the lower removal efficiency correlating with the lower Se concentration (). After seven days, the removal efficiency for all concentrations was > 60%, with the 50–500 mg.L−1 incubations exhibiting removal efficiencies > 90%. Unlike Se(IV), Se(VI) exhibits a lower removal efficiency. The Se (IV) removal efficiency was below 70% in the initial few days, and on day 7, the Se(VI) removal efficiencies were between 50–90%. This indicated that R. opacus PD630 is more effective with Se(IV). Furthermore, the reduction of Se(IV) and Se(VI) happened within the initial 3 days ().
Figure 2. Percentage removal of (A) Se(IV) and (B) Se(VI) in half-strength LB broth over seven days: day 1(■), day 2 (■), day 3 (■) and day 7 (■).
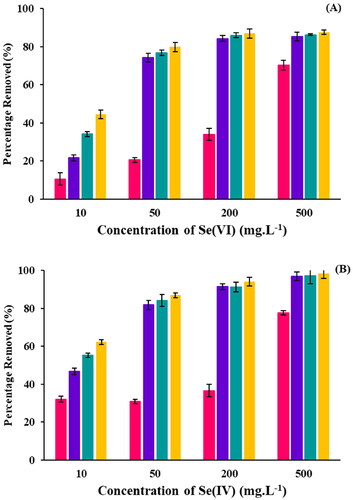
Figure 3. Reduction of (A) Se(IV) and (B) Se(VI) to total selenium over 7 days, and its percentage conversion to Total Selenium. Where: both 500 mg.L−1 (●) and 200 mg.L−1 (▲) are related to the reduction of Se, while 500 mg.L−1 (■) and 200 mg.L−1 (♦) are related to the percentage conversion of Se to Total Se.
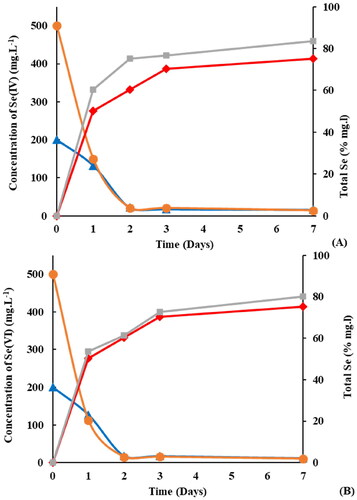
The ability of R. opacus PD630 to reduce both types of Se oxyanions shows R. opacus PD630’s potential to be used in the remediation of Se from contaminated soils and water bodies. Typically, the reduction of Se(VI) and Se(VI) is mediated by various microorganisms under both reductive and oxidative conditions. Several sulfate-reducing bacteria and phototrophic bacteria can reduce Se(VI) under anaerobic conditions (Nancharaiah and Lens Citation2015). On the other hand, some aerobic bacteria have been also pointed out to be able to reduce Se oxyanions as well (Durán et al. Citation2013). R. opacus PD630 is of interest as it is an aerobic microbe capable of reducing Se (IV) and Se(VI). Further research is required to investigate the metabolic pathways within this microbe and the genes and proteins involved in the Se bioconversion processes.
PGP traits
PGP traits were examined in R. opacus PD630 to determine if B. oleracea would have any impact on plant growth and could support the phytoremediation of Se from soils using B. oleracea. The 3-IAA assay and phosphate solubilization yielded positive results, while the ACC and nitrogen fixation assay yielded negative results. It is important to note for a microbe to be classified as PGP, it needs to have exhibited some of these traits (Cueva-Yesquén et al. Citation2020), but full confirmation is examined in both germination and pot trial studies when the effects of R. opacus PD630 on plant growth are determined.
Computational analysis for gene mining showed the presence of some of the genes related to these PGP traits (Table S1 of the Supplementary Material). R. opacus PD630 does contain genes concerning phosphate solubilization through inorganic P, i.e., ppqE and gdh genes. Furthermore, it was shown that R. opacus PD630 contains the necessary genes for phytohormone production (IAA production, abscisicn acid precursor, cytokinesis gibberellins and salicylic acid (degradation)), antimicrobial compound production (2,4-diacetyl phloroglucinol production), induced systemic resistance (Acetoin and 2,3-butanediol production), iron acquisition, siderophore production and phosphate solubilization. This indicates that this species might play a role in PGP. Due to this, it was decided to examine the effect of inoculating seeds with R. opacus PD630 prior to both germination and growth to screen potential PGP effects, which can support B. oleracea growth on seleniferous soils.
Germination study
The objectives of the plant studies were to (1) evaluate the effect that Se has on the germination of B. oleracea seeds, (2) determine if B. oleracea are accumulators of Se and (3) verify R. opacus PD630 impact on plant growth and the uptake of Se in B. oleracea.
Total viable counts on B. oleracea seeds
Seeds without any R. opacus PD630 and killed R. opacus PD630 show TVCs between 0.01–0.7 Log CFU.mL−1 (). This indicated that little to no microbes were present after surface sterilization and inoculation with the killed control. Seeds that contained “live” R. opacus PD630 showed TVCs in the range of 5.2–5.3 Log CFU.mL−1.
Table 2. Total viable counts on seed used in both germination and pot trials. Results are the average (n = 3).
Seed germination and seedling shoot length
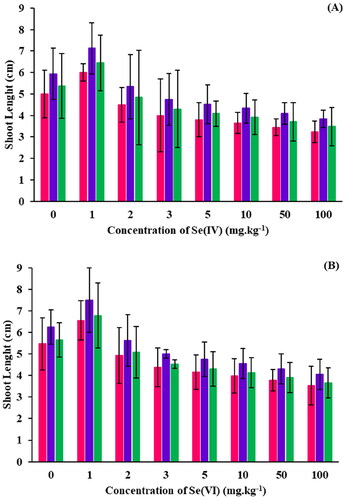
Germination studies showed that all B. oleracea plants at all treatments successfully germinated at the Se(IV) and Se(VI) concentrations investigated (Table S2 of the Supplementary Material), confirming that Se oxyanions are not toxic to that crop. Regarding seedling shoot lengths ( and Table S3), an increase was observed for 1 mg.L−1 Se(IV) compared to the control (0 mg.L−1 Se(IV), after which a decrease in shoot length was observed upon increasing the Se(IV) concentration. A reduction in shoot length between 45–90% for seeds germinated in concentrations ≥ 5 mg.L−1 Se(IV) was observed (p < 0.05). On the other hand, although germination occurred at all Se(VI) concentrations tested, shoot lengths differed. Initially, both 1 and 2 mg.L−1 Se(VI) had shoot lengths of 7.2 and 5.58 cm, compared to 4.85 cm for the control (0 mg.L−1). This confirms that a small quantity of Se is advantageous for crop development and increases plant growth (Pilon-Smits et al. Citation2009; Zhu et al. Citation2009; Schiavon and Pilon-Smits Citation2017). In contrast, concentrations higher than 3 mg.L−1 resulted in decreasing shoot lengths as the Se(VI) concentration increased (p < 0.05).
Figure 4. Shoot lengths for seeds germinated in (A) Se(IV) and (B) Se(VI): surface sterilized seeds (■), seeds inoculated with R. opacus PD630 (■) and seeds inoculated with killed R. opacus PD630 (■).
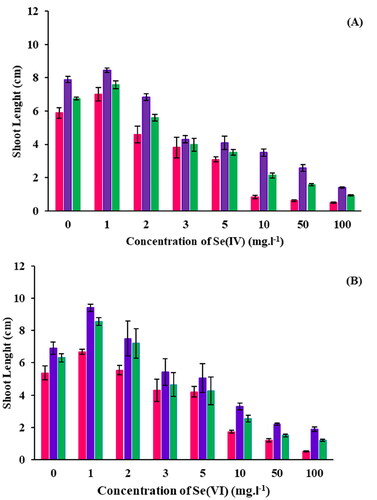
The effects of seed inoculation were also examined (). Seeds that were surface sterilized showed shorter shoot lengths than those that were inoculated with either R. opacus PD630 or killed R. opacus PD630 (p < 0.05) (Table S2). Moreover, there was a 5–25% increase in the shoot length across all Se(IV) concentrations when compared to the seeds that underwent surface sterilization. In most cases, both seeds with R. opacus PD630 and killed R. opacus PD630 performed better than seeds that were just surface sterilized, showing R. opacus PD630 has indeed PGP traits (see the PGP traits section).
Pot trials
Shoot lengths
Like in the germination studies, all seeds treated with R. opacus PD630 showed an increased shoot length when compared to seeds without inoculation (p < 0.05) ( and Table S3 of the Supplementary Material). This further confirms that R. opacus PD630 has PGP traits and impacts B. oleracea seed growth. Statistical analysis showed that seeds that were grown up to 3 mg.kg−1 compared to the control showed no significant differences (p > 0.05), confirming that a small amount of Se within the soil does not have any negative impact on the crop growth by acting as a nutrient to the crop (Yasin et al. Citation2014). Seed grown at 5–100 mg.kg−1 did show a significant difference (p < 0.05) when compared to the control, which indicates that as Se levels in soil increase, Se becomes more toxic to plants, and could potentially cause Se toxicity (Yasin et al. Citation2014).
Dry weight of roots and shoots
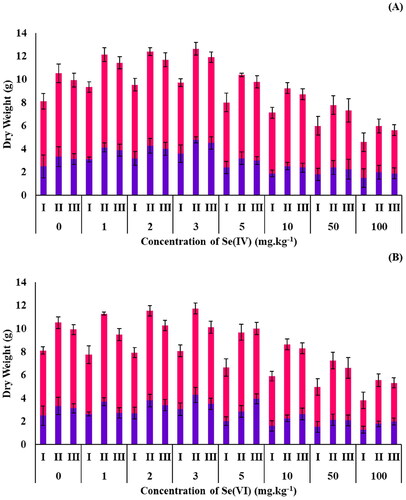
Regarding DW results, seeds that were inoculated with R. opacus PD630 showed high DW biomass yields compared to seeds without R. opacus PD630 ( and Table S4 of the Supplementary Material). Moreover, seeds inoculated with R. opacus PD630 (live and killed) had a greater DW than seeds that were only surface sterilized for all concentrations tested (p < 0.05). This suggests that growing B. oleracea with R. opacus PD630 would lead to higher yields and could be of benefit if they were to be used in phytomining (Kavanagh et al. Citation2018), or as biomass for biofuels (Holder et al. Citation2011). The DW of B. oleracea plants grown at the lower Se(IV) and Se(VI) concentrations (1–5 mg.kg−1) showed no significant difference compared to the control (p > 0.05), indicating these Se concentrations have no impact on B. oleracea growth. In contrast, a higher Se concentration showed a significant difference (p < 0.05).
Se uptake
To determine if B. oleracea is an accumulator of Se, the levels of Se in the plant tissue (root and shoot) were analyzed. shows Se accumulated in both roots and shoots of B. oleracea in all treatments and concentrations of Se(IV). Se concentrations in the shoots were higher than in the roots in all treatments. The greater the initial Se concentration, the higher the Se uptake by the plant (). The control samples accumulated 600–1,100 mg.kg1 DW Se(IV) in both root and shoot combined, suggesting that they are secondary and in some instances hyperaccumulators of Se(IV). Likewise for Se(VI) spiked soil, Se(VI) accumulation in control samples ranged from 500–1,000 mg.kg−1 DW in combined plant tissues, making them secondary accumulators for Se(VI). These results are explained by the difference between the uptake mechanisms for each Se speciation. Se (VI) can enter the root cells via sulfate transporters, whereas Se(VI) uptake involves phosphate transporters (Lima et al. Citation2018). Despite this, B. oleracea is a good candidate for the remediation of Se from contaminated soils. Other studies have shown the ability of other Brassica species to remediate Se from soils (Esringü and Turan Citation2012). In particular, one study looked at B. juncea and found a total Se accumulation in plant tissues higher than 1,300 mg.kg−1 DW (Yasin et al. Citation2014). This is greater than what is reported in this study using B. oleracea, and further supports that B. oleracea has the potential for Se accumulation.
Figure 7. Se content in roots (■) and shoots (■) of B. oleracea plants grown in spiked soils using (A) Se(IV) and (B) Se(VI) at varying concentrations of Se: surface sterilized seeds (I), seeds inoculated with R. opacus PD630 (II) and seeds inoculated with killed R. opacus PD630 (III).
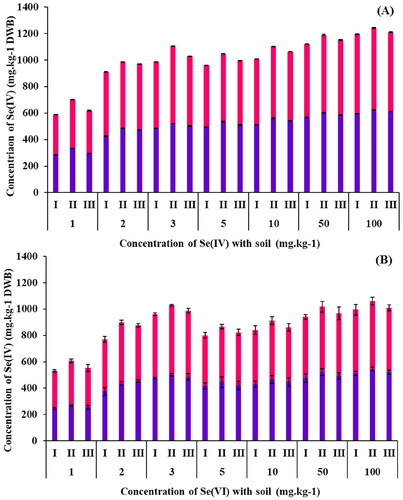
On the other hand, uptake by seeds inoculated with R. opacus PD630 showed a higher Se uptake than surface-sterilized seeds or killed control seeds. Significant differences (p < 0.05) were observed between all concentrations tested when surface sterilized seeds were compared to the other two treatments (Table S5). The concentration of Se(IV) and Se(VI) ranged from 600–1,200 mg.kg1 DW in these plants, indicating this microbe had an impact on Se uptake, alongside plant growth. The addition of R. opacus PD630 onto the seeds prior to growth has allowed a higher accumulation of Se(IV) and Se(VI) in both roots and shoots of B. oleracea (). This is consistent with Durán et al. (Citation2013), who evaluated the Se uptake by wheat grains inoculated with selenobacteria strains (Stenotrophomonas sp. B19, Enterobacter sp. B16, and Pseudomonas sp. R8). The grains of the inoculated plants had a higher Se content in comparison with non-inoculated controls.
Bioconcentration and translocation factor
The bioconcentration factor (BCF) was determined based on the soil to root ratio, as well as the soil to shoot ratio (). The BCF was greater than one in all treatments investigated, suggesting the potential for phytoremediation using B. oleracea. Moreover, the BCF of shoots:soil ratio was greater than the BCF of the roots:soil ratio, suggesting that B. oleracea accumulated Se(IV) and Se (VI) within the leaves. These results suggest that B. oleracea can be used for phytoextraction of Se-contaminated soils.
Table 3. Bioconcentration and translocation factor of Se uptake within B. oleracea plants at the end of the trial period (3 months).
The translocation factor (TF) was greater than one for all the treatments investigated. In some cases, even secondary accumulation or hyperaccumulation of Se was observed (, ). Hyperaccumulation was observed in B. oleracea plants grown in soil with Se(IV) concentrations exceeding 5 mg.kg−1. Se(IV) accumulation ranged from 285 to 610 mg.kg−1 in both roots and shoots, with a cumulative accumulation of 590–1210 mg.kg−1. The BCF and TF give information concerning a plant’s ability to hyperaccumulate a substance, while also indicating if they show any potential to be used in phytoremediation. The capacity of a crop to be used for phytoremediation is defined by a BCF greater than one. At the same time, the TF explores the ability of plants to transfer compounds from the roots to the leaves, and it is defined that any species with a TF greater than 1 is an accumulator of that compound (Kafle et al. Citation2022; Zand and Mühling Citation2022; Zhu et al. Citation2022). The TF values obtained for B. oleracea () show a better performance in comparison to Chi et al. (Citation2018), who found TF values lower than 1 for up to 5 mg.kg−1 of Se for Brassica rapa ssp pekinensis L. and Brassica oleracea var. capitata L.
Furthermore, the BCF and TF are higher in seeds treated with R. opacus PD630 when compared to seeds with no R. opacus PD630 (), indicating that they have additional potential for increasing Se accumulation in plant tissues. According to Lima et al. (Citation2018), one of the mechanisms for Se tolerance in hyperaccumulators is the ability to have an upregulated antioxidant mechanism (antioxidant enzymes and defense phytohormones) to cope with the oxidative stress caused by the excess of Se in the cellular environment. In this sense, R. opacus PD630 contains the necessary genes for phytohormone production (See PGP traits section) and redox buffering molecules (Presentato et al. Citation2018). Not only does this indicate that R. opacus PD630 has an impact on B. oleracea growth, but also enhances the plant’s Se uptake capacity. This confirms the efficiency of this plant-microbe combination for the remediation of Se-contaminated soil and opens perspectives for their further potential use for crop Se-biofortification (Bañuelos et al. Citation2015; Li et al. Citation2021) or for Se bio-ore production in phytomining or agromining (van der Ent et al. Citation2015; Kotamraju et al. Citation2023).
Conclusion
The ability of Rhodococcus opacus PD630 and Brassica oleracea for the remediation of Se from spiked dark brown/black rich clay loam soils was assessed. R. opacus PD630 showed great potential in reducing Se to the less toxic elemental Se and it was able to withstand high levels (500 mg.L−1) of Se(IV) and Se(VI). Moreover, R. opacus PD630 significantly enhanced B. oleracea growth and Se uptake, likely due to its PGP traits (IAA production and phosphate solubilization). Se translocation from the roots to the shoots was also observed. More work is required to understand the full mechanisms of Se extraction by this plant-microbe combination and to examine their potential for a full-scale application in the treatment of Se-contaminated soils.
No potential conflict of interest was reported by the author(s).
Supplemental Material
Download MS Word (37.2 KB)Acknowledgments
The authors thank Borja Khatabi Soliman Tamayo, Leah Egan, and Manuel Suarez (National University of Ireland, Galway) for their technical support.
Additional information
Funding
References
- Bañuelos GS, Arroyo I, Pickering IJ, Yang SI, Freeman JL. 2015. Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem. 166:603–608. doi: 10.1016/J.FOODCHEM.2014.06.071.
- Brown TA, Shrift A. 1982. Selenium: toxicity and tolerance in higher plants. Biol Rev. 57(1):59–84. doi: 10.1111/j.1469-185X.1982.tb00364.x.
- Di Canito A, Zampolli J, Orro A, D’Ursi P, Milanesi L, Sello G, Steinbüchel A, Di Gennaro P. 2018. Genome-based analysis for the identification of genes involved in o-xylene degradation in Rhodococcus opacus R7. BMC Genomics. 19(1):587. doi: 10.1186/S12864-018-4965-6
- Chi S, Xie W, Xu W, Zhou X, Chai Y, Zhao W, Li T, Li Y, Zhang, C, Yang. 2018. Differences in Selenium uptake and transport, and related gene expression in three Brassica vegetables. Appl Ecol Env Res. 16(3):2781–2793. doi: 10.15666/aeer/1603_27812793.
- Cueva-Yesquén LG, Goulart MC, Attili de Angelis D, Nopper Alves M, Fantinatti-Garboggini F. 2020. Multiple plant growth-promotion traits in endophytic bacteria retrieved in the vegetative stage from passionflower. Front Plant Sci. 11:621740. doi: 10.3389/FPLS.2020.621740.
- Das P, Sinha S, Mukherjee SK. 2014. Nickel Bioremediation Potential of Bacillus thuringiensis KUNi1 and Some Environmental Factors in Nickel Removal. Biorem J. 18(2):169–177. doi: 10.1080/108898682014889071.
- Debnath R, Yadav A, Gupta VK, Singh BP, Handique PJ, Saikia R. 2016. Rhizospheric bacterial community of endemic Rhododendron arboreum Sm. Ssp. delavayi along Eastern Himalayan Slope in Tawang. Front Plant Sci. 7:1345. doi: 10.3389/FPLS.2016.01345/BIBTEX.
- Dungan RS, Yates SR, Frankenberger WT. 2003. Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ Microbiol. 5(4):287–295. doi: 10.1046/J.1462-2920.2003.00410.X.
- Durán P, Acuña JJ, Jorquera MA, Azcón R, Borie F, Cornejo P, Mora ML. 2013. Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: a preliminary study as a potential Se biofortification strategy. J Cereal Sci. 57(3):275–280. doi: 10.1016/j.jcs.2012.11.012.
- van der Ent A, Baker AJM, Reeves RD, Chaney RL, Anderson CWN, Meech JA, Erskine PD, Simonnot M-O, Vaughan J, Morel JL, et al. 2015. Agromining: farming for metals in the future? Environ Sci Technol. 49(8):4773–4780. doi: 10.1021/es506031u.
- Esringü A, Turan M. 2012. The roles of diethylenetriamine pentaacetate (DTPA) and ethylenediamine disuccinate (EDDS) in remediation of selenium from contaminated soil by brussels sprouts (Brassica oleracea var. Gemmifera). Water Air Soil Pollut. 223(1):351–362. doi: 10.1007/S11270-011-0863-0/TABLES/2.
- Eswayah AS, Smith TJ, Gardiner PHE. 2016. Microbial transformations of selenium species of relevance to bioremediation. Appl Environ Microbiol. 82(16):4848–4859. doi: 10.1128/AEM.00877-16.
- Finnegan C, Ryan D, Enright A-M, Garcia-Cabellos G. 2017. Developing microbial Inocula to support biofuel crop cultivation on tributyltin contaminated marine sediments. JAEM. 5(2):47–56. doi: 10.12691/jaem-5-2-1.
- Freeman JL, Marcus MA, Fakra SC, Devonshire J, McGrath SP, Quinn CF, Pilon-Smits EAH. 2012. Selenium hyperaccumulator plants Stanleya pinnata and Astragalus bisulcatus are colonized by se-resistant, se-excluding wasp and beetle seed herbivores. PLOS One. 7(12):e50516. doi: 10.1371/journal.pone.0050516.
- Gupta S, Pandey S. 2019. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) plants. Front Microbiol. 10:1506. doi: 10.3389/fmicb.2019.01506.
- Holder JW, Ulrich JC, DeBono AC, Godfrey PA, Desjardins CA, Zucker J, Zeng Q, Leach ALB, Ghiviriga I, Dancel C, et al. 2011. Comparative and functional genomics of Rhodococcus opacus PD630 for biofuels development. PLOS Genet. 7(9):e1002219. doi: 10.1371/JOURNAL.PGEN.1002219.
- Kafle A, Timilsina A, Gautam A, Adhikari K, Bhattarai A, Aryal N. 2022. Phytoremediation: mechanisms, plant selection and enhancement by natural and synthetic agents. Environ Adv. 8:100203. doi: 10.1016/j.envadv.2022.100203.
- Kavanagh L, Keohane J, Cabellos GG, Lloyd A, Cleary J. 2018. Induced plant accumulation of lithium. Geosci. 8(2):56. doi: 10.3390/geosciences8020056.
- Kim SA, Kim OM, Rhee MS. 2013. Changes in microbial contamination levels and prevalence of foodborne pathogens in alfalfa (Medicago sativa) and rapeseed (Brassica napus) during sprout production in manufacturing plants. Lett Appl Microbiol. 56(1):30–36. doi: 10.1111/lam.12009.
- Kotamraju A, Logan M, Lens PNL. 2023. Integrated bioprocess for Se(VI) remediation using duckweed: coupling selenate removal to biogas production. J Hazard Mater. 459:132134. doi: 10.1016/J.JHAZMAT.2023.132134.
- Li J, Otero-Gonzalez L, Parao A, Tack P, Folens K, Ferrer I, Lens PNL, Du Laing G. 2021. Valorization of selenium-enriched sludge and duckweed generated from wastewater as micronutrient biofertilizer. Chemosphere. 281:130767. doi: 10.1016/J.CHEMOSPHERE.2021.130767.
- Lima LW, Pilon-Smits EAH, Schiavon M. 2018. Mechanisms of selenium hyperaccumulation in plants: a survey of molecular, biochemical and ecological cues. Biochim Biophys Acta Gen Subj. 1862(11):2343–2353. doi: 10.1016/J.BBAGEN.2018.03.028.
- Lin J, Peng T, Jiang L, Ni JZ, Liu Q, Chen L, Zhang Y. 2015. Comparative genomics reveals new candidate genes involved in selenium metabolism in prokaryotes. Genome Biol Evol. 7(3):664–676. doi: 10.1093/GBE/EVV022.
- Mal J, Sinharoy A, Lens PNL. 2021. Simultaneous removal of lead and selenium through biomineralization as lead selenide by anaerobic granular sludge. J Hazard Mater. 420:126663. doi: 10.1016/J.JHAZMAT.2021.126663.
- Mandal A, Thakur JK, Sahu A, Bhattacharjya S, Manna MC, Patra AK. 2017. Plant-microbe interaction for the removal of heavy metal from contaminated site. In: Choudhary, D, Varma, A, Tuteja, N., editors. Plant-Microbe Interaction: An Approach to Sustain Agriculture. Singapore: Springer. p. 227–247. doi: 10.1007/978-981-10-2854-0_11
- Matilla MA, Espinosa-Urgel M, Rodríguez-Herva JJ, Ramos JL, Ramos-González MI. 2007. Genomic analysis reveals the major driving forces of bacterial life in the rhizosphere. Genome Biol. 8(9):R179. doi: 10.1186/gb-2007-8-9-r179.
- El Mehdawi AF, Paschke MW, Pilon-Smits EAH. 2015. Symphyotrichum ericoides populations from seleniferous and nonseleniferous soil display striking variation in selenium accumulation. New Phytol. 206(1):231–242. doi: 10.1111/NPH.13164.
- Nancharaiah YV, Lens PNL. 2015. Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev. 79(1):61–80. doi: 10.1128/MMBR.00037-14.
- Natasha N, Shahid M, Saleem M, Anwar H, Khalid S, Tariq TZ, Murtaza B, Amjad M, Naeem MA. 2020. A multivariate analysis of comparative effects of heavy metals on cellular biomarkers of phytoremediation using Brassica oleracea. Int J Phytoremediation. 22(6):617–627. doi: 10.1080/15226514.2019.1701980.
- Navarro-León E, López-Moreno FJ, Rios JJ, Blasco B, Ruiz JM. 2020. Assaying the use of sodium thiosulphate as a biostimulant and its effect on cadmium accumulation and tolerance in Brassica oleracea plants. Ecotoxicol Environ Saf. 200:110760. doi: 10.1016/j.ecoenv.2020.110760.
- Parvekar P, Palaskar J, Metgud S, Maria R, Dutta S. 2020. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent. 7(1):105–109. doi: 10.1080/26415275.2020.1796674.
- Peyravi M, Jahanshahi M, Alimoradi M, Ganjian E. 2016. Old landfill leachate treatment through multistage process: membrane adsorption bioreactor and nanofitration. Bioprocess Biosyst Eng. 39(12):1803–1816. doi: 10.1007/s00449-016-1655-0.
- Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M. 2009. Physiological functions of beneficial elements. Curr Opin Plant Biol. 12(3):267–274. doi: 10.1016/j.pbi.2009.04.009.
- Pilon-Smits EAH, Winkel LHE, Lin Z-Q. 2017. Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects. Cham: Springer. doi: 10.1007/978-3-319-08807-5_4.
- Presentato A, Piacenza E, Anikovskiy M, Cappelletti M, Zannoni D, Turner RJ. 2016. Rhodococcus aetherivorans BCP1 as cell factory for the production of intracellular tellurium nanorods under aerobic conditions. Microb Cell Fact. 15(1):204. doi: 10.1186/S12934-016-0602-8
- Presentato A, Piacenza E, Anikovskiy M, Cappelletti M, Zannoni D, Turner RJ. 2018. Biosynthesis of selenium-nanoparticles and -nanorods as a product of selenite bioconversion by the aerobic bacterium Rhodococcus aetherivorans BCP1. N Biotechnol. 41:1–8. doi: 10.1016/J.NBT.2017.11.002.
- Rosenfeld I, Beath OA. 1964. Selenium: Geobotany. Biochemistry, Toxicity, and Nutrition. New York: Academic Press.
- Schiavon M, Pilon-Smits EAH. 2017. The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology. New Phytol. 213(4):1582–1596. doi: 10.1111/nph.14378.
- Sinharoy A, Lens PNL. 2022. Biological selenate and selenite reduction by waste activated sludge using hydrogen as electron donor. J Environ Manage. 319:115745. doi: 10.1016/J.JENVMAN.2022.115745.
- De Souza MP, Chu D, Zhao M, Zayed AM, Ruzin SE, Schichnes D, Terry N. 1999. Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian Mustard. Plant Physiol. 119(2):565–574. doi: 10.1104/PP.119.2.565.
- Trippe RC, Pilon-Smits EAH. 2020. Selenium transport and metabolism in plants: phytoremediation and biofortification implications. J Hazard Mater. 404(Pt B):124178. doi: 10.1016/j.jhazmat.2020.124178.
- White PJ. 2016. Selenium accumulation by plants. Ann Bot. 117(2):217–235. doi: 10.1093/aob/mcv180.
- White PJ. 2017. The genetics of selenium accumulation by plants. In: Pilon-Smith E, Winkel L, Lin ZQ, editors. Selenium in Plants. Plant Ecophysiology. Vol 11. Cham: Springer. p. 143–163. doi: 10.1007/978-3-319-56249-0_9.
- White PJ. 2018. Selenium metabolism in plants. Biochim Biophys Acta Gen Subj. 1862(11):2333–2342. doi: 10.1016/j.bbagen.2018.05.006.
- Yaghoobizadeh F, Ardakani M, Zolgharnein H. 2017. Isolation, screening and identification of the best selenium- reducing bacteria and study on the sorption mechanism. EEB. 15:161–171. doi: 10.22364/eeb.15.15.
- Yasin M, El-Mehdawi AF, Anwar A, Pilon-Smits EAH, Faisal M. 2015. Microbial-enhanced selenium and iron biofortification of Wheat (Triticum aestivum L.) - applications in phytoremediation and biofortification. Int J Phytoremediation. 17(1–6):341–347. doi: 10.1080/15226514.2014.922920.
- Yasin M, El Mehdawi AF, Jahn CE, Anwar A, Turner MFS, Faisal M, Pilon-Smits EAH. 2014. Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant Soil. 386(1–2):385–394. doi: 10.1007/s11104-014-2270-y.
- Zand AD, Mühling KH. 2022. Phytoremediation capability and copper uptake of Maize (Zea mays L.) in copper contaminated soils. Pollut. 2(1):53–65. doi: 10.3390/pollutants2010007.
- Zhu W, Zhu D, He J, Lian X, Chang Z, Guo R, Li X, Wang Y. 2022. Phytoremediation of soil co-contaminated with heavy metals (HMs) and tetracyclines: effect of the co-contamination and HM bioavailability analysis. J Soils Sediments. 22(7):2036–2047. doi: 10.1007/S11368-022-03206-Y
- Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA. 2009. Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 14(8):436–442. doi: 10.1016/j.tplants.2009.06.006.