Abstract
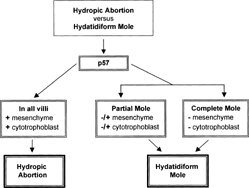
Classification of molar gestations into complete and partial and their differentiation from hydropic abortions traditionally are accomplished by morphology alone. The process sometimes may be inaccurate or inconclusive. With the availability of p57 immunostaining it may be possible to objectively classify these lesions. We used p57 for the differential diagnosis of hydropic abortions and molar gestations and correlated the findings with the clinical outcome of patients in each category. First, 86 cases were originally classified by histomorphology into hydropic abortion (42) and molar gestations (23 complete and 21 partial). Based on the pattern of p57 staining the cases were reclassified into 45 hydropic abortions, 15 partial moles and 26 complete moles (3 cases with previous diagnosis of complete mole based on morphology were reclassified as hydropic abortion). Clinical follow-ups ranged from 6–24 months and showed persistent trophoblastic disease in 8 cases (31%) of complete moles and 3 cases (20%) of partial moles (p = 0.47). No hydropic abortion cases demonstrated persistent trophoblastic disease. One patient with partial mole developed choriocarcinoma. This study confirms that p57 objectively distinguishes hydropic abortions from molar gestations (partial and complete moles). This differentiation is clinically relevant since patients with hydropic abortions do not need to be followed while patients with molar gestations do.
Molar pregnancy is a relatively common complication of gestation, occurring in approximately 1 in every 1000 to 2000 pregnancies in the United States. It is much more common in the Far East (10 in 1000 in Indonesia). Since women with molar pregnancies have increased risk of developing persistent trophoblastic disease and choriocarcinoma, the histologic differentiation between a molar gestation and a hydropic abortion is critical. These patients are usually followed postevacuation by beta-human chorionic gonadotrophin (βhCG) serum levels [Citation[1]]. Conversely, hydropic abortions carry no increased risk for neoplasia and do not require the same close follow-up. Although in most cases the diagnosis of hydropic abortion versus partial and complete hydatidiform mole can be made based on morphologic findings, there are histological overlaps and the correct classification of trophoblastic diseases carries considerable inter- and intraobserver variability [Citation[2]].
The first clinical manifestation of a complete mole is vaginal bleeding that can be accompanied by passage of vesicles. Associated signs and symptoms may include uterine enlargement beyond what is expected for the gestational age, extremely high βhCG levels with secondary hyperemesis, pre-eclampsia, and ovarian theca-lutein cysts. Elevated βhCG after 9 weeks, a uterus larger than expected for date, and presence of theca lutein cysts are all signs of developing persistent trophoblastic disease [Citation[3–5]]. With the advent of ultrasonography, absence of fetal heart movements and the presence of high βhCG levels in the first trimester, early diagnosis of molar gestation can be made with nearly 90% accuracy [Citation[6]]. Generalized hydrops and large vesicles resembling “bunches of grapes,” the most reliable features of molar gestation, are only seen in the second trimester. When complete moles are evacuated between 6–10 weeks of gestation, as is commonly practiced today, hydropic changes may not be apparent, even under the microscope [Citation[7]].
DNA analysis by flow cytometry or image analysis and clinicopathologic correlation have been applied to the study of complete moles (CM), partial moles (PM), and spontaneous abortions (SA) [Citation[8–11]]. DNA ploidy is useful in distinguishing complete from partial moles. It is critical however to exclude decidua that will be diploid since it is of maternal origin and to exclude necrotic tissue that will impair ploidy analysis adequacy [Citation[12]]. Proliferative markers (Ki-67, proliferating cell nuclear antigen, nucleolar organizer region, and p53) all have been used to aid in the diagnosis of gestational trophoblastic diseases (GTD) [Citation[13]], but some of these tests are technically demanding and tedious.
Recently, immunohistochemical demonstration of p57, a maternally expressed cell cycle inhibitor and tumor suppressor gene [Citation[14]], has been reported to be helpful in differentiating between trophoblastic disease and hydropic abortions [Citation[15], Citation[16]].
In our study we performed p57 immunostaining in selected examples of CM, PM, and hydropic abortions (HA). Original histologic diagnosis was revised based on the results of p57 staining and correlated with the patients' clinical information at the time of dilatation and curettage as well as with their subsequent follow-ups.
MATERIALS AND METHODS
Surgical pathology database files (1995–2001) at the University of Miami/Jackson Memorial Medical Center were searched for hydropic abortions and trophoblastic disease with Institutional Review Board approval. Only cases with available paraffin blocks and medical records were selected. Patients' medical records were reviewed for: age, race, gestational age, ultrasound findings, clinical diagnosis, treatment, and βhCG levels at presentation and after treatment: All patients had clinical follow-ups ranging from 6 to 24 months. The histology and p57 immunostains of all cases were reviewed without the knowledge of clinical information.
Macroscopic description was examined taking into account the amount of tissue obtained by curettage, presence or absence of grape-like structures, and presence of fetal parts. All available slides were studied and special attention was given to villous size, hydropic swelling, presence of cisterns, villous fibrosis, scalloping of the villous outline, presence of trophoblastic villous inclusions, trophoblastic proliferation, trophoblastic atypia, presence or absence of necrosis, presence of nucleated red blood cells (NRBC), or other fetal parts (i.e., fetal membranes).
A representative slide from each case was selected and p57kip2 (mitotic inhibitor/suppressor protein) immunohistochemistry was performed using mouse monoclonal antibody (Clone 57P06 #MS-1062-P, Neomarkers, Fremont, CA, USA) at a dilution of 1:200. A standard avidin-biotin-peroxidase technique was utilized after antigen retrieval by steamer in citric buffer (DAKO, Carpinteria, CA, USA). Diaminobenzidine was used as the chromogen. Nuclear positivity for p57 was intensified by a 1% cupric sulfate. Fast green was used as a cytoplasmic counterstain.
The slides were reviewed and classified as positive if the majority of the cells had dark-stained brown to black nuclei within the villous stroma and cytotrophoblast. They were considered as focally positive if less than 50% of the nuclei stained dark brown to black, and negative if no nuclei stained. The decidua always was positive and served as an internal positive control.
For DNA analysis of the partial mole that developed choriocarcinoma, the block of tissue was dissected to contain only lesional tissue. Several 50-micron sections were cut, deparaffinized, and rehydrated. After pepsin digestion and permeabilization, a single cell suspension was stained to saturation with propidium iodide. Flow cytometric data were collected on a Beckman-Coulter XL using singlet gating, and the data were analyzed with Multicycle.
RESULTS
The medical records showed that 62% of the patients were white, 36% black, and 2% Asian. This racial distribution remained within the same range in each group (HA, CM, PM). Average gestational age at presentation was 11.2 weeks, by last menstrual period, and 12.4 weeks by ultrasound, with a mean of 11 weeks in all groups. The mean maternal age also was similar among all groups (27–29 years). As expected, the mean uterine size was found to be larger in the cases of molar pregnancy (13–14 weeks) when compared with the hydropic abortions (11 weeks). The clinical information provided in the surgical pathology requisitions is summarized in .
TABLE 1. Summary of Clinical Information and Pathologic Diagnoses Based on ConventionalHistology and Immunohistochemistry
Based on the light microscopic examination of hematoxylin-and-eosin-stained slides, 42 cases (49%) were diagnosed as hydropic abortions, 21 (24%) were partial moles, and 23 (27%) were complete moles.
Immunohistochemical reaction for p57 was seen as dark brown to black nuclear staining. The reaction for p57 was strongly and diffusely positive in the cells of villous mesenchyme and cytotrophoblast of the HA, focally positive in PM, and negative in CM (). These illustrations reflect unusual cases in which immunohistochemistry helps to differentiate among them. In 81 (94%) of the cases p57 staining confirmed the morphologic diagnosis (45 [52%] hydropic abortions and 41 [48%] hydatidiform moles: 15 [37%] partial and 26 [63%] complete). This yielded a sensitivity of 61% with a specificity of 95%. The positive predictive value was 93%. Two cases, originally diagnosed as hydropic abortions by morphology, stained as molar gestations and behaved as such clinically, with marked increase in βhCG.
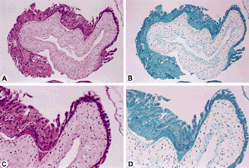
Figure 1. Very early hydropic abortion at 10 × lens (A and B) and 20 × lens (C and D) hematoxylin and eosin stain (A and C) and positive staining for p57 (B and D) in the cytotrophoblast, mesenchymal cells, and intermediate trophoblast.
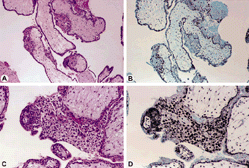
Figure 2. Partial mole at 10 × lens (A and B) and 20 × lens (C and D) show the hematoxylin and eosin stain (A and C) and focal positivity for p57 (B and D) in the cytotrophoblast but not the mesenchyme. The intermediate trophoblast is focally positive.
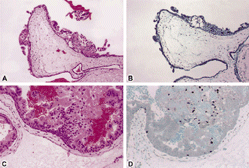
Figure 3. Complete mole is seen at 10 × lens (A and B) and 20 × lens (C and D) hematoxylin and eosin stain (A and C). There is no p57 staining (B and D) in the cytotrophoblast or in the intermediate trophoblast.
Only one patient diagnosed as a hydropic abortion had a detectable βhCG past the 10th week postevacuation. Thirty-five (41%) of the cases had a clinical diagnosis of abortion, 46 (53%) were diagnosed as hydatidiform moles, whereas five (6%) could not be clinically determined as an abortion or a molar pregnancy (). Of the cases clinically determined to be molar, 44 (51%) had a βhCG level of more than 120,000 mIU/mL at the time of presentation.
Persistent trophoblastic disease (PTD) was diagnosed if the patient had elevated βhCG past the 10th week postevacuation in absence of choriocarcinoma. If a sharp rise of βhCG was detected, dilatation and curettage was performed. Patients were followed for up to 48 weeks after clinical management and treatment. Of the 86 cases studied, 11 had persistent trophoblastic disease. Based on classification by p57 staining, 8 (31%) complete moles and 3 (20%) partial moles evolved into PTD (p = 0.47) (). One of the patients had a diagnosis of partial mole with a fetus. Flow cytometry performed on paraffin-embedded tissue from this suction dilatation and curettage demonstrated a DNA index of 1.82 (aneuploidy) confirming the diagnosis of partial mole.
TABLE 2. Diagnosis and Clinical Outcome
This patient had a βhCG of 4,000 I/U and increased fundal tissue detected by ultrasound study 1 month after uterine evacuation. Therefore, she underwent a uterine scraping that showed the presence of a choriocarcinoma. Immunohistochemistry for p57 demonstrated positive tumor cells.
DISCUSSION
Although morphologic features are often adequate to differentiate hydropic abortions from molar gestations, early hydropic abortions may exhibit atypical trophoblast proliferation and pronounced hydropic swelling that can be confusing, occasionally leading to an erroneous diagnosis of hydatidiform mole. Furthermore, the most reliable feature of molar gestation, the presence of macroscopically identifiable large hydropic vesicles, is only seen in the second trimester of gestation. The recent trend toward ultrasound study in early pregnancy has resulted in evacuation of hydatidiform moles at earlier stages before the development of the usually recognized diagnostic criteria; i.e., trophoblastic hyperplasia and cistern formation.
In our study we demonstrated that p57 is a useful marker to distinguish hydropic abortions from molar gestations, particularly in early gestation when the histologic findings can overlap. The degree of trophoblastic atypia contributes no information as to the prognosis of a complete mole [Citation[17]].
In reviewing the literature we have found some confusion regarding the parental origin of the human cyclin-dependent kinase inhibitor p57KIP2. This gene product is paternally imprinted but maternally expressed [Citation[16], Citation[17]].
The rationale behind the pattern of the staining in trophoblastic disease and hydropic abortions is the following: since p57 is a maternally expressed gene, the antigen-antibody reaction will take place between tissues containing maternal genetic material. Hydropic abortions contain 50% of genetic material from the mother and the staining will be strong in the cytotrophoblast, mesenchymal cells, and intermediate trophoblast. Partial mole is triploid and contains approximately 66% of androgenic genetic material and 33% will be maternally derived.
Therefore, the pattern of staining by p57 usually is a focal positivity in the cytotrophoblast but not in the mesenchyme; the intermediate trophoblast also is focally positive. On the other hand, complete mole is diploid but 100% androgenic because it arises from fertilization of an empty ovum by two sperms. As a consequence, there is no p57 staining in the cytotrophoblast or in the intermediate trophoblast. The advantages of p57 staining include applicability to archival paraffin sections, which allows for morphologic analysis of the positive cells instead of the blinded test offered by flow cytometry.
Although clinical features such as a highly elevated βhCG of a molar gestation, the routine histologic diagnosis of such a lesion during early pregnancy presents a challenge. Given the implications of the correct diagnosis of hydropic abortion versus a hydatidiform mole, we recommend submission of one block per cm2 of tissue for histological analysis and immunohistochemistry for p57 performed on one selected block. This approach may allow for a higher diagnostic accuracy (). Analysis of products of conception by flow cytometry and cytogenetic karyotyping also can be helpful but are more cumbersome, time-consuming, expensive, and usually require fresh tissue [Citation[19]]. In addition, these techniques are not generally available except in large medical centers. In our experience, the gynecologist's follow-up of a patient with a diagnosis of a molar pregnancy whether partial or complete is the same (βhCG monitoring).
Moreover, the WHO classification of gestational neoplasms encompasses both entities under the umbrella of hydatidiform mole [Citation[20]]. There are several cases of well-documented partial mole developing into choriocarcinomas [Citation[21]]. In our series of 15 partial moles one of our cases also transformed to a choriocarcinoma (6.6%), and the preceding condition was a partial mole confirmed by DNA analysis.
On the other hand, one should morphologically, immunohistochemically, and clinically continue to separate hydropic abortion from hydatidiform moles, as they represent completely different disease entities. In histologic sections, p57 may be a helpful marker for the surgical pathologist to distinguish hydropic abortions from molar pregnancies.
REFERENCES
- Soper JT, Lewis JL, Hammond CB. Gestational trophoblastic disease. Hoskins WJ, Perez GA, Young RC. Principles and Practice of Gynecologic Oncology. Philadelphia, Lippincott-Raven. 1997; 1039
- Szulman AE. Trophoblastic diseases: Complete and partial hydatidiform moles. Lewis SH, Perrin E. Contemporary Issues in Surgical Pathology. Pathology of the Placenta. New York, Churchill Livingstone. 1999; 259
- Kohorn EI. Hydatidiform mole and gestational trophoblastic disease in Southern Connecticut. Obstet Gynecol. 1982; 59: 78, [PUBMED], [INFOTRIEVE]
- Morrow CP, Kletzky OA, Disaia PJ, Townsend DE, Mishell DR, Nakamura RM. Clinical and laboratory correlates of molar pregnancy and trophoblastic disease. Am J Obstet Gynecol. 1977; 128: 424, [PUBMED], [INFOTRIEVE]
- Schlaerth JB, Morrow CP, Kletzky OA, Nalick RH, D'Ablaing GA. Prognostic characteristics of serum human chorionic gonadotrophin titer regression following molar pregnancy. Obstet Gynecol. 1981; 58: 478, [PUBMED], [INFOTRIEVE]
- Romero R, Horgan JG, Kohorn EI, Kadar N, Taylor KJ, Hobbins JC. New criteria for the diagnosis of gestational trophoblastic disease. Obstet Gynecol. 1985; 66: 553, [PUBMED], [INFOTRIEVE]
- Newlands ES, Paradinas FJ, Fisher RA. Recent advances in gestational trophoblastic disease. Hematol Oncol Clin North Am. 1999; 13: 225, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Barclay ID, Dabbagh L, Babiak J, Poppema S. DNA analysis (ploidy) of molar pregnancies with image analysis on paraffin tissue sections. Am J Clin Pathol. 1985; 100: 451
- Kunze WP. Cytophotometric DNA-measurements in abortion. Pathol Res Pract. 1978; 162: 253, [PUBMED], [INFOTRIEVE]
- Lage JM, Bagg A. Hydatidiform moles: DNA flow cytometry, image analysis and selected topics in molecular biology. Histopathology. 1996; 28: 379, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Williams RA, Charlton IG, Howat AJ. Image analysis DNA densitometry measurements on complete and partial hydatidiform mole and nonmolar products of conception. Int J Gynecol Pathol. 1995; 14: 300, [PUBMED], [INFOTRIEVE], [CSA]
- Fukunaga M. Flow cytometric and clinicopathologic study of complete hydatidiform moles with special reference to the significance of cytometric aneuploidy. Gyn Onc. 2001; 81: 67, [CSA], [CROSSREF]
- Kale A, Soylemez F, Ensari A. Expressions of proliferation markers (Ki-67, proliferating cell nuclear antigen, and silver-staining nucleolar organizer regions) and of p53 tumor protein in gestational trophoblastic disease. Am J Obstet Gynecol. 2001; 184: 567, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Matsuoka S, Thompson JS, Edwards MC, Barletta JM, Grundy P, Kalikin LM. Imprinting of the gene encoding a human cyclin-dependent kinase inhibitor, p57KIP2, on chromosome 11p15. Proc Natl Acad Sci USA. 1996; 93: 3026, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chilosi M, Piazzola E, Lestani M, et al, . Differential expression of p57kip2, a maternally imprinted cdk inhibitor, in normal human placenta and gestational trophoblastic disease. Lab Invest. 1998; 78: 269, [PUBMED], [INFOTRIEVE], [CSA]
- Castrillon DH, Sun D, Weremowicz S, Rosemary AF, Crum CP, Genest DR. Discrimination of complete hydatidiform mole from its mimics by immunohistochemistry of the paternally imprinted gene product p57 kip2. Am J Surg Path. 2001; 25: 1225, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Genest DR, Dorfman DM, Castrillon DH. Ploidy and imprinting in hydatidiform moles. Complementary use of flow cytometry and immunohistochemistry of the imprinted gene product p57kip2 to assist molar classification. J Reprod Med. 2002; 47: 342, [PUBMED], [INFOTRIEVE], [CSA]
- Genest DR, Laborde O, Berkowitz RS, Goldstein DP, Bernstein MR, Lage J. A clinicopathologic study of 153 cases of complete hydatidiform mole (1980–1990): Histologic grade lacks prognostic significance. Obstet Gynecol. 1991; 78: 402, [PUBMED], [INFOTRIEVE]
- Mazur MT, Kurman RJ. Gestational trophoblastic disease. Mazur MT, Kurman RJ. Diagnosis of Endometrial Biopsies and Curettings. A Practical Approach. New York, Springer. 1995; 70
- World Health Organization Scientific Group. Tumors of the uterine corpus and gestational trophoblastic disease, Technical Report Series 692. Geneva, World Health Organization. 1983; 1
- Seckl MJ, Fisher RA, Salerno G, Rees H, Paradinas FJ, Foskett M, et al, Choriocarcinoma and partial hydatidiform moles. Lancet. 2000; 356: 36, [PUBMED], [INFOTRIEVE], [CROSSREF]