?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
An influx of terrestrial dissolved organic carbon (DOC) into freshwater habitats can regulate a range of ecosystem characteristics, from water clarity to productivity. To understand the extent to which DOC can regulate ecosystem functioning, we conducted a survey to determine the source of DOC in low Arctic ponds close to the Arctic Circle (Kangerlussuaq, Greenland), including its role in food web dynamics. We used a multiple element (carbon, nitrogen, and hydrogen) stable isotope approach to examine the proportional contribution of different sources to aquatic consumers in nine arctic ponds that spanned a broad gradient of DOC (6.6–60.1 mgL-1). Our results show that benthic and pelagic primary production decreased along a gradient of increasing DOC content. Additionally, the changes in the organic matter pool with increasing DOC translated into changes in consumer resource use. We found significant differences in resource use between species. All consumers relied on benthic autotrophic material when DOC was low; but when DOC was high some consumers changed their diet. Collectively, our findings demonstrate how the concentration of DOC influences aquatic production and our study can be used as a baseline to predict how the aquatic food web may respond to regionally changing DOC concentrations.
Introduction
Dissolved organic carbon (DOC) is a fundamental parameter that regulates freshwater ecosystem functioning (Solomon et al. Citation2015). It can affect the light environment (Anderson and Stedmon Citation2007; Forsström et al. Citation2015), UV exposure (Schindler et al. Citation1996; Williamson et al. Citation2015), primary productivity (Karlsson et al. Citation2009), heterotrophic productivity (Forsström, Roiha, and Rautio Citation2013; Roiha et al. Citation2016), and subsequently food-web dynamics in freshwaters. Most of the existing research on this topic has mainly focused on the quantification of DOC. Typically, the concentration of DOC directly contributes to the color of the water; for example, dark, humic lake water has high DOC concentrations. However, this general pattern does not always stand, because comparable DOC concentrations can range between clear to nearly opaque water in northern-latitude freshwaters (Anderson and Stedmon Citation2007; Forsström et al. Citation2015). The quality of DOC can also relate to the DOC source, with phytoplankton-based carbon sources (autochthonous) being less colored, more biolabile, and, hence, of higher quality compared with terrestrially derived (allochthonous) carbon (Anderson and Stedmon Citation2007; Berggren et al. Citation2010). Terrestrial-derived carbon is more heterogeneous, containing both biologically labile and recalcitrant carbon, and typically more colored. Although recalcitrant carbon is often considered to be of lower quality and, hence, less biolabile than compounds originating from aquatic primary production, several recent studies have shown that some terrestrially derived carbon is accessible for microbial productivity (Biasi, Rusalimova, and Meyer Citation2005; Burpee et al. Citation2016; Guillemette, Mccallister, and del Giorgio Citation2013). Generally, there is a loss of primary production as a result of an increase in color DOC and reduction in light (Karlsson et al. Citation2009), creating conditions that favor heterotrophic production of allochthonous carbon (Roiha et al. Citation2016) and alter the importance of this energy source for higher trophic levels.
Research aimed at determining the relative proportion of different carbon sources to higher trophic levels in freshwater ecosystems has been a central topic in limnology throughout the past decade. The sources can be divided into those with a terrestrial origin and those aquatic sources that are derived from phytoplankton-, benthic algae- or macrophyte-originating carbon. Since the work of Grey and Jones (Citation2001) and Pace et al. (Citation2004), there has been an increased focus on determining the proportional contribution of the terrestrial carbon to freshwater food webs (Berggren et al. Citation2014; Rautio, Mariash, and Forsström Citation2011a; Solomon et al. Citation2011). In some catchments, terrestrial carbon has been shown to contribute as much as 60 percent of some aquatic consumers’ diets (Wilkinson, Carpenter, and Cole Citation2013). However, there can be wide variation between water bodies, depending on their size, morphology, catchment characteristics, and consumer composition (Berggren et al. Citation2014) and even location within the same lake (Grosbois, del Giorgio, and Rautio Citation2017). There tends to be a higher influx of terrestrial carbon into water bodies in forested and wetland regions as opposed to waters surrounded by tundra, which can have little surrounding vegetation (Rantala et al. Citation2016) and limited inflow or outflow (Johansson et al. Citation2015), as is the case in parts of west Greenland. Some of the lowest terrestrial carbon contributions in aquatic consumers have been measured in a range of ologotrophic water bodies (Anderson and Stedmon Citation2007; Pace et al. Citation2007) that are also common in the Arctic. Increased warming in the northern regions is causing rapid thawing of permafrost, glaciers, and ice sheets, and in combination with changes to precipitation patterns and biogeochemistry (Saros et al. Citation2015), these processes can result in increased terrestrial carbon inputs into Arctic freshwaters (AMAP Citation2011; Johansson et al. Citation2015; Vonk et al. Citation2015). These new terrestrial carbon inputs cause changes in the concentration and liability of DOC entering the water bodies and has important implications for the metabolism of organisms exposed to this additional allochthonous carbon source (Burpee et al. Citation2016; Roiha, Laurion, and Rautio Citation2015). Shallow waters (<3 m), such as ponds, typically have high light penetration and higher turnover of nutrients from runoff, which promotes high productivity in benthic algae (Rautio et al. Citation2011b; Vincent and Laybourn-Parry Citation2008). In some instances, benthic primary productivity can surpass pelagic primary productivity (Vadeboncoeur et al. Citation2006) and be a high-quality food source for aquatic consumers (Cazzanelli et al. Citation2012; Mariash et al. Citation2014; Rautio and Vincent Citation2007). Macrophytes can also play a role in contributing to the carbon pool, and can outcompete benthic and pelagic algae for space and light while providing important refugia for zooplankton (Grosbois, del Giorgio, and Rautio Citation2017). In Arctic freshwaters, ice scouring and a short growing season keep macrophytes low; however, zooplankton have been shown to feed on periphyton growing on macrophytes, such as mosses (Riis, Christoffersen, and Baattrup-Pedersen Citation2015).
In this study, we focused on how the quality and quantity of DOC modifies the sources and transfer of carbon in Arctic food webs. However, the challenge of many sources contributing to the carbon pool—such as terrestrial, benthic algae; macrophytes; and phytoplankton—poses a problem for stable isotope food-web studies, where the number of sources are constrained by the number of elements to analyze for isotopic composition. We addressed this challenge by first estimating the contribution of allochthony in the DOM, and then using DOM and the primary autochthonous sources, phytoplankton and benthic algae, to estimate the proportional contribution of these carbon sources to aquatic consumer diets. While there has been great advancements in using stable isotope analysis to discern diet composition, trophic-level estimation, and food-web dynamics (see Middelburg Citation2014), most of the research has focused primarily in boreal, mid-latitude regions (Berggren et al. Citation2014). This study is the first to investigate how DOC modifies the resource use and diet composition of aquatic consumers, and it is the first to use both hydrogen and carbon isotopes to better understand the carbon composition of the DOM in low Arctic ponds. We hypothesize that ponds with higher DOC concentrations should have lower primary production (PP), higher bacterial production (BP), and allochthonous or microbial-supported food webs. Alternatively, lower DOC waters should have higher PP, lower BP, and a predominantly autochthonous-based food web. Our study investigated these food-web dynamics in shallow, low Arctic ponds in the Kangerlussuaq area, west Greenland, spanning an almost tenfold broad DOC gradient (6.6–60.1 mg L−1). In the Kangerlussuaq region, shallow ponds are common across the landscape, provide a freshwater resource and habitat for wildlife, and play key role in energy transfer across the landscape (Rautio et al. Citation2011b). Because previous work in the region has shown that that high-DOC waters can also be colorless (Anderson and Stedmon Citation2007), we made sure to analyze both the light attenuation and the optical properties of colored dissolved organic matter (CDOM), which can also identify the origin and quality of DOM in these ponds. Primary and bacterial productivity were measured to track changes in pond productivity across the DOC gradient. We further hypothesized that the difference in carbon sources will be transferred to the diets of consumers. To test this, we examined the carbon, nitrogen, and hydrogen stable isotopes for all basal organic-matter sources in the food web, including DOM, particulate organic matter (POM), aquatic plants, periphyton that grew on the aquatic plants, benthic algae, and terrestrial plants. We used the stable isotope data in Bayesian mixing models to investigate the diet contribution of the carbon sources to higher trophic levels, including zooplankton, predatory Coleoptera, and dipteran Chironomidae larvae.
Methods
Study sites
We studied nine water bodies in Kangerlussuaq (between 66° 56ʹ and 67° 9ʹ N, 50° 04ʹ, and 50° 44ʹ W), west Greenland, in July 2010. The water bodies were less than 3 m in depth, had a surface area of less than 1 × 104 m2, were fishless, and fell under the definition of “ponds” according to Rautio et al. (Citation2011b). The ponds spanned a large DOC gradient (6.6–60.1 mg L−1), ranged in altitude from 75 to 634 m a.s.l., were situated within 30 km from the ice-sheet margins, and did not dry in summer. The ice-free period lasts about 4 months, with ice melt usually occurring in early June. Most pond sites were situated in tussock tundra habitat, composed of dwarf shrubs (Betula nana, Salix glauca, Rhododendron tomentosum), sedges (Carex spp., Eriophorum spp.), and, especially at high elevations (ponds 8–9), mosses (Sphagnum spp.) and lichens (Alectoria spp., Peltigera spp.) dominated. Pond sites 1 and 2 were particularly rocky sites. The areal coverage of vegetation around the ponds was visually estimated as percent area of plant cover. The dominant macrophytes were Hippuris vulgaris, Menyanthes trifoliata (both species grew only in the shallow areas of less than 1 m),Potamogeton gramineus, P. filiformis, Myriophyllum alterniflorum, and aquatic mosses only at higher elevations (ponds 8 and 9). The abundance of macrophytes was recorded as percent cover of water-body area. The zooplankton communities were composed of cladocerans (Daphnia pulex, D. middendorffiana, and Eurycercus glacialis; a few other species occurred in small numbers), copepods (Leptodiaptomus minutus), fairy shrimp (Branchinecta paludosa), and, only in one pond, the tadpole shrimp (Lepidurus arcticus). Other important consumers were dipteran Chironomidae larvae (collectors, the Orthocladiinae and Chironominae subfamilies; predators, the Tanypodinae subfamily), aquatic snails (Lymnaea spp.) and predatory Coleoptera (Colymbetes dolabratus and Gyrinus opacus).
Field collection and analyses
We collected water samples from the upper meter of each pond by wading to the middle of the ponds, or as far as possible from the shore. Care was taken not to resuspend the sediment material into the water column where the samples were taken. Bulk sample water was retained for total phosphorous (TP), total nitrogen (TN), dissolved inorganic carbon (DIC), and particulate and dissolved organic matter (POM and DOM). For POM, first we filtered the bulk water through a 50 µm mesh net to remove large particles and organisms, then as much as 2 L of each water sample was passed through precombusted and preweighed GF/F filters (50 µm > POM > 0.7 µm), while the filtrate was retained as the DOM samples. For TN, DOC, and CDOM, bulk water was filtered through prerinsed 0.22 µm cellulose acetate filters, and the filtrate was stored in acid-rinsed and combusted 100 mL amber glass bottles at 4°C until analysis. DOC and TN were measured with a Shimadzu VCPG (Kyoto, Japan). TP (40 mL bulk water pre-acidified with H2SO4) samples were measured on an autoanalyzer Lachat (Loveland, CO, USA). We measured absorption of CDOM on a Cary 300 UV-Vis spectrophotometer (Varian Inc., Walnut Creek, CA, USA), using the method described by Cazzanelli et al. (Citation2012). Specific ultraviolet absorbance (SUVA) at 254 nm corresponds to the absorbance at 254 nm divided by the DOC concentration and indicates the level of aromaticity and, therefore, a proxy for the terrestrial origin of organic matter in the system (Weishaar et al. Citation2003). CDOM concentration, measured by the light absorption at 440 nm (a440), is an index of water clarity. Since a440 is strongly correlated to light extinction (Ask et al. Citation2009a; Forsström et al. Citation2015; Karlsson et al. Citation2009), we used it to calculate the diffuse attenuation coefficient Kd for PAR and the percentage of light at the bottom of the ponds using the equation:
where Ez and E(0) are the values of downward irradiance at depth z and just below the surface. We set E(0) as 100 percent to calculate how much PAR is left on the bottom of the pond.
Benthic and pelagic primary production was measured using the Rae-box technique (Rae and Vincent Citation1998). The system consisted of a white plastic box holding pairs of inverted glass scintillation vials in separate compartments. Irradiance was reduced over the vials with screens to give light transmission series of 0, 0.2, 1.5, 5, 60, and 100 percent. For seston, each glass vial was filled with 20 mL of pond water and subsequently spiked with 14C-bicarbonate (specific activity 80 µC mL−1). Similar incubations were done for benthic algae, except the benthic algae (collected as described further on) were suspended with filtered (<0.7 µm) pond water (20 mL). This resuspension may change the orientation of the benthic algae, as the cells are specific to a given depth within the mat; however, the use of a light gradient from 0 to 100 percent of ambient light ensures that correct maximum potential photosynthetic rate (Pmax) for a given community is obtained. After one hour of incubation, the production was terminated by filtering the samples onto fine glass fiber filters (GF/F) and then frozen. Radioactivity was later measured with a liquid scintillation counter (PerkinElmer Tri-Carb 2910TR), following the protocol by Rautio and Vincent (Citation2006). We performed DIC titrations with 0.1 mol L−1 HCl to determine carbon availability for photosynthesis in each pond. The photosynthesis versus irradiance curves were fitted with the equation of Platt, Gallegos, and Harrison (Citation1980), using an iterative nonlinear regression (R version 2.14) to be able to calculate the Pmax of the given algal community. Whole-pond primary production (PP) was extrapolated from the Pmax following the equation (Jassby and Platt Citation1976):
where I0 is the surface light (daily average for the open-water season), Ik is the light intensity at onset of saturation (180 and 300 µmol m−2 s−1, respectively, for phytoplankton and benthic algae; Vadeboncoeur et al. Citation2008), and Z represents the average vertical distribution of benthic algae and phytoplankton, which corresponds to the mean water depth. Kd is the diffuse attenuation coefficient, calculated as described previously.
Heterotrophic bacteria productivity was determined by 3-hour in situ incubations using the 3H-leucine protocol (Knap et al. Citation1994). Samples were run in triplicates and with trichloroacetic acid (TCA) added after incubation to stop bacterial production. Duplicate time zero controls had TCA added at the beginning of the incubation period. Storage and analysis of bacteria productivity were run according to Roiha et al. (Citation2012).
Pearson correlation analyses of water chemistry parameters were analysed with with R (R Core Team Citation2014; version 3.1.1), and were plotted in Prism GraphPad (version 5.0). All environmental variables were tested for normality with the Shapiro-Wilk test; conductivity, POM, CDOM, Kd, aquatic plant cover, and pelagic, benthic, and bacterial productivity were logarithmically transformed before analysis. For the mixing model analysis, we incorporated DOC as a grouping factor, using DOC ponds less than 20 mg L−1 as low-DOC ponds and high-DOC ponds were more than 20 mg L−1. This grouping was validated using Welch’s two-sample independent t test, where high- and low- DOC ponds were significantly (p < .05) different in terms of DOC, CDOM, TN, logPPpelagic, logPPbenthic, logPOM, logBacteria productivity, logKd, and altitude. By grouping our pond sites by DOC, we could compare food webs from functionally different freshwater ecosystems.
Preparation of stable isotope samples
Baseline environmental hydrogen (ω) for each pond was taken using 20 mL of pond water taken at 0.5 m depth and analyzed for δ2H. For δ13C and δ15N analyses of POM, we scraped the top layer of the filter (with the material retained) into tin cups. For hydrogen stable isotope analysis (SIA) of POM, we sonicated the filters in Milli-Q water for 30 minutes, the material released from filters was freeze-dried and collected for analysis. This extra step was preformed to ensure that the glass-fiber material from the filters would not interfere with the hydrogen SIA. For DOM, we retained the GF/F filtrate into 100 mL Nalgene bottles, which was then freeze-dried, and the residue material left in the bottles was collected for SIA. We gathered samples for SIA (benthic productivity) of benthic algae from soft sediment from three locations in each pond at 0.5–1 m depth, depending on the distribution of each substrate (ponds 1, 3, 4, 7, 8, and 9). Benthic cores were taken using a cylinder (5 cm diameter) that was pushed into the sediment and closed with airtight caps before lifting the undisturbed core. We subsampled the top approximately 3 mm of each benthic core using a cutoff syringe (8 mm diameter). Where significant amounts of benthic algae on rocks occurred, we scraped about 1 cm2 of algae using a scalpel (ponds 2, 5, and 8). As a potential autochthonous carbon source, we took clippings of the epiphytic algae (different taxa later pooled in analysis). When there were a lot of algae observed growing on the aquatic plants, this algal material was collected by rinsing the aquatic plants in a small amount of pond water and concentrating this algal water onto GF/F by vacuum filtration. Herein, we refer to these samples as aquatic plants and periphyton, respectively. From the catchment (within 5 m from the water edge of ponds), we gathered terrestrial plants for SIA, which were used as the baseline of terrestrial carbon signal. Zooplankton, water beetles, Lepidurus, and chironomids were collected using a 200 µm hand-net swept through open water and benthic habitats. All animals were kept overnight in GF/F filtered water to allow gut evacuation. Animals were then hand-sorted, and three replicates of the most abundant species from each pond were dried for SIA.
We performed all SIA on dried, homogenized samples—except the δ2H of environmental water was liquid—at the Alaska Stable Isotope Facility. Prior to carbon and nitrogen SIA, benthic mats, POM and DOM, and invertebrate samples were acid-fumed (with concentrated HCl under vacuum) to remove any inorganic carbon, and lipids were extracted from animal samples as described in Cazzanelli et al. (Citation2012). For hydrogen SIA, samples were lipid extracted but not acidified. All stable isotope data are reported in standard δ notation as parts per thousand (‰) relative to international standards (Vienna Pee Dee Belemnite for carbon, atmospheric air for nitrogen, and Vienna Standard Mean Ocean Water for hydrogen). Carbon and nitrogen stable isotopic compositions were determined by combusting the samples in a Costech ESC 4010 elemental analyzer interfaced via a ThermoConflo III to a Thermo Delta+XP isotope ratio mass spectrometer (IRMS). Samples for hydrogen stable isotope (δ2H) were analyzed using a Thermo Thermal Conversion Elemental Analyzer (TC-EA) attached via a Conflo III to a Thermo Delta V Plus IRMS (O’Brien and Wooller Citation2007). To negate the effect of exchangeable hydrogen on δ2H values, all samples and calibration standards were equilibrated with local water vapor (Wassenaar and Hobson Citation2003). Analytical precisions (defined here as one standard deviation around the mean of multiple analyses of a standard) were less than 0.3‰ for carbon and nitrogen (based on multiple analyses of a homogenous peptone standard) and less than 2‰ for hydrogen (based on analyses of benzoic acid, Fisher Scientific, Lot No. 947459).
Bayesian mixing models
Mixing models were used to calculate the proportional contribution of potential diet items used by aquatic consumers in high- and low-DOC ponds. Since we had more potential food sources than the mixing model allowed, we first used a four-source (aquatic plants, benthic algae, periphyton, and terrestrial plants), two-isotope (δ13C and δ2H) model to find the carbon source contributing most to the DOM isotope signal using the SIAR package (Parnell and Jackson Citation2013) in R (R Core Team R, Citation2014), version 3.1.1. Next, we used a three-source (DOM, phytoplankton, and benthic algae), two-isotope (δ13C and δ2H) Bayesian mixing model adapted from Wilkinson, Carpenter, and Cole (Citation2013) and Grosbois, del Giorgio, and Rautio (Citation2017). Both the trophic enrichment of consumers (δ15N) and the enrichment of environmental water (ω) were incorporated into the model, as calculated in Grosbrois et al. (Citation2016). The data were visualized with the package ggplot2, version 1.0.1 (Wickham Citation2009).
Phytoplankton end member delta values were calculated using a Bayesian model for estimating phytoplankton end member of δ2H values (environmental water) and δ13C values (Cpom) for each pond from Solomon et al. (Citation2011) and Wilkinson, Carpenter, and Cole (Citation2013; ). The epsilon (εH) was set at −130, 0.034, which was the lowest limit in Yang et al. (Citation2014). Periphyton δ2H values were generated from the mean −160 ± 20 (Grosbois, del Giorgio, and Rautio Citation2017). All other end member values were measured values. summarizes all the isotopic values used in the mixing models. Because there was no significant difference in δ15N values between predatory and collector chironomids, they were pooled together for the mixing model and referred to as Chironomidae.
Table 1. Summary of the range of phytoplankton end member data and its determination in previously published stable isotope mixing models
Table 2. Location, physicochemical properties, productivity, and vegetation cover of the study sites. The ponds are sorted by increasing DOC content. Bold values are mean ± standard deviation (SD) of the ponds in each category
Table 3. Summary of the averaged δ13C, δ15N, and δ2H values and standard deviation from the sources and aquatic consumers used for the mixing models. The δ2H values of consumers are adjusted accounting for dietary water in their body tissue
Results
Environmental parameters
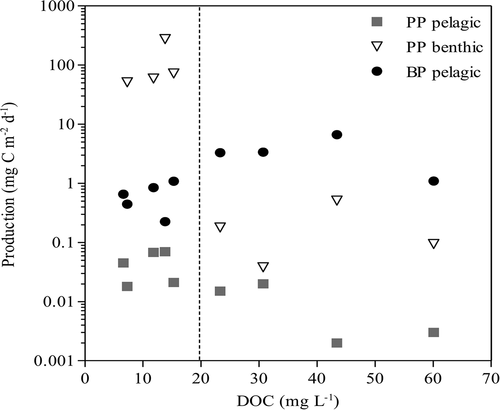
The content of DOC in the ponds varied almost tenfold, from 6.6 to 60.1 mg L−1 (; ). There was a strong positive correlation between DOC and light limitation in the water column, both with CDOM (r = 0.90, p = < .001) and Kd (r = 0.81, p = .008) (SI. 1). CDOM, related to light attenuation and water color, was an average of 10.2 m−1 in high-DOC ponds, compared to 1.5 m−1 in low-DOC ponds (). Primary production was most affected by DOC concentration and light (). Both benthic and pelagic primary production rates declined significantly with increasing DOC (pelagic primary production, r = −0.74, p = .02; benthic primary production, r = −0.78, p = .01) and with light limitation (increasing CDOM values; pelagic, r = −0.80, p < .001; benthic, r = −0.89, p < .001) (SI 1). While pelagic bacterial production was positively correlated to increasing DOC (r = 0.77, p < .01) and light limitation (CDOM: r = 0.79, p = .01; Kd: r = 0.77, p = .01), ranging between 0.22 and 4.44 µg C L−1 d−1 (0.22–6.66 mg C m−2 d−1 on an areal basis). TP and TN concentrations had a strong positive correlation with DOC (TP: r = 0.81, p < .001; TN: r = 0.93, p < .001) (SI 1). DOC was negatively correlated to altitude, r = −0.70, p = .04. There were no correlations between DOC and other environmental variables, such as DIC, temperature, conductivity, POM, and percent cover of catchment and aquatic plants; raw data are listed in .
Stable isotopes of basal carbon sources
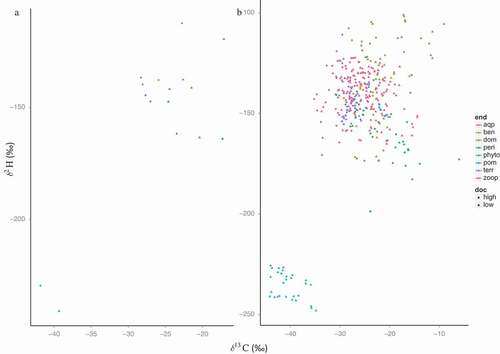
There was a wide overlap in the stable isotopic composition of the potential food sources, and only phytoplankton separated from the main cluster in both low- and high-DOC ponds (). The mean δ13C isotopic values for all basal carbon sources were more depleted in high-DOC ponds, but the δ2H values were more positive than in low-DOC ponds (). Consumers overlaid with the main cluster of food sources (). The DOM δ13C values were negatively correlated along the DOC gradient (r = −0.45, p = .02), meaning that with higher DOC concentrations, 13C becomes enriched. For δ2H of DOM, values positively correlate with DOM with increasing DOC (r = .39, p = 0.05). Mean average isotope values of basal food sources were significantly different between high- and low-DOC ponds (, ). Phytoplankton and DOM were the only food sources that significantly separated between high- and low-DOC ponds using both carbon (t20.6 = −2.7, p = .01; t24.5 = −3.5, p = .002, respectively) and hydrogen isotopes (Phytoplankton: t24 = 8.4, p < .001; DOM: t24.8 = 2.0, p = .05). Because DOM’s carbon and hydrogen were significantly different between high- and low-DOC ponds, we looked closer into its composition. According to the Bayesian model, the composition of the DOM in high-DOC ponds was 40 percent terrestrial carbon, 20–50 percent aquatic plants, and less than 20 percent benthic algae and periphyton. In the low-DOC ponds, the aquatic plants, benthic, periphyton, and terrestrial carbon sources contributed equally to the DOM pool (all 20–30 percent contribution).
Figure 2. The distribution of δ2H and δ13C (‰) values by (A) mean of end to better show the separation by DOC concentration and (B) full spread of data with consumers and potential food sources. High-DOC ponds are marked with a dot, and low-DOC ponds are marked with a triangle. Aqp = aquatic plants, ben = benthic algae, dom = dissolved organic matter, peri = periphyton, phyto = phytoplankton, pom = particulate organic matter, terr = terrestrial, zoop = all aquatic invertebrates collected
Consumer isotope mixing models
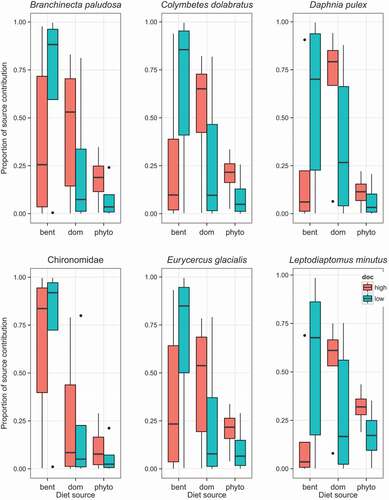
DOM was included in the consumer mixing models as an end-member to be able to better estimate the food-web changes between low- and high-DOC ponds. Based on the DOM isotopic composition we argue that DOM, in addition to indicating the trophic enrichment from microbial diet, also was a good indicator for the terrestrial organic-matter consumption. The other two end-members selected for the models were phytoplankton and benthic algae. All of the aquatic invertebrates assimilated all three resources, but in different proportions (), and their diets responded to changes in DOC. The taxa-specific response varied in different DOC levels; hence, taxa were responding to the different DOM pools. In low-DOC ponds, benthic source made up more than 65 percent of consumer diets, while it made up less than 25 percent of their diets in high-DOC ponds, with the exception of Chironomidae, whose diet was more than 75 percent benthic in both high- and low-DOC ponds (). Chironomids were the only consumer whose overall diet did not change in response to changes in DOC. Chironomidae had a diet of roughly 80 percent benthic, 10 percent DOM, and 10 percent phytoplankton sources in both pond types. B. paludosa, C. dolabratus, E. glacialis, D. pulex, and L. minutus all went from a predominantly benthic diet to a DOM-fueled diet when DOC was high (>20 mg L−1; ). Among zooplankton, D. pulex and L. minutus both had similar diets of 65:25:10 (% benthic:DOM:phytoplankton) in low-DOC ponds. However, in high-DOC ponds, D. pulex’s diet was 75 percent fueled by DOM and 25 percent autochthonous carbon, while L. minutus relied more on phytoplankton (30%) and DOM (60%). Phytoplankton made the lowest contribution to consumers’ diet, with less than 30 percent, and its contribution to diets was slightly higher in the high-DOC ponds. Among zooplankton, D. pulex had the highest reliance on DOM, 25 ± 25 percent in low-DOC ponds and 80 ± 17 percent in high-DOC ponds (). Given that in high-DOC ponds DOM was mostly terrestrial, our results indicate that D. pulex could opportunistically feed on terrestrial organic matter, and in low-DOC ponds used more by benthic sources. Similarly, E. glacialis had a diet reliant on benthic sources in low-DOC ponds, but in high-DOC ponds, DOM-sourced organic matter contributed most to its diet.
Figure 3. The contributions of benthic algae, dissolved organic matter (DOM), and phytoplankton-based carbon sources for the diets of the six most common aquatic invertebrates in high- and low-DOC ponds using a Bayesian mixing model, using δ13C, δ15N, and δ2H values. Whisker plots indicate the 95 percent Bayesian probability intervals
Discussion
Our results demonstrate the key role of DOC in pond-ecosystem functioning. Increasing DOC correlated with increased color (CDOM) and reduced light (Kd). Benthic and pelagic primary production decreased along a gradient of increasing CDOM and DOC content, indicating that algal production, in the water column and at the sediment surface, was light-limited. As autotrophic production was suppressed, increasing DOC stimulated pelagic bacterial production, resulting in a higher degree of heterotrophy in these Arctic ponds. Our findings agree with previous studies that have shown that total primary production declined while bacterial production increased along a DOC gradient (Ask et al. Citation2009b; Forsström, Roiha, and Rautio Citation2013; Karlsson et al. Citation2009). As we hypothesized, the changes in DOC content and related ecosystem changes, including light limitation and productivity change, led to significant changes in the basal carbon pool and consumer resource use.
In addition to the DOC quantity, we measured a set of quality indicators as well. We measured both light attenuation and composition of the DOC pool. DOC and CDOM were positively correlated, meaning that as DOC increased the darker the water color became in the ponds. Thus, we did not find evidence of photo bleaching of DOC, which can render high DOC ponds to be colorless, as has been reported in a few lakes in the Kangerlussuaq region (Anderson and Stedmon Citation2007). With SUVA, an indicator of greater contribution of terrestrially derived carbon, there was some indication of higher terrestrial carbon contribution to the carbon pool with higher DOC concentration; however, the results showed wider variability in SUVA values in the low range of DOC concentrations (<20 μg L−1). Another way we used to indicate the composition of the carbon pool was through the isotopic composition of DOM. In high-DOC ponds the DOM was 40 percent terrestrial carbon, while in low-DOC ponds the DOM was composed of a mixture of allochthonous and autochthonous sources. These results correspond to patterns seen from the SUVA results. Overall, our results show that quantity of DOC is not directly related to quality, and therefore a multi-proxy approach is recommended when assessing the ecological implications of DOC.
Changes to basal carbon sources based on stable isotopes
Our results show that the isotopic signatures of the basal diet sources changed with DOC concentration, and that this change was best traced by the δ13C values of the organic matter sources. Phytoplankton and DOM had significantly more depleted carbon values and less depleted hydrogen signatures in high-DOC ponds. The higher δH2 values indicate higher terrestrial organic source, and this organic matter can provide a substrate for bacterial metabolism (Solomon et al. Citation2015). Because both allochthonous and autochthonous sources contribute to POM and DOM pools, we first partitioned the DOM pool using a multielement stable isotope-mixing model to determine its proportional composition. As expected, there was more terrestrial organic matter in the DOM in high-DOC ponds. In low-DOC ponds, the DOM was primarily autochthonous sourced, with periphyton, benthic algae, and aquatic plants cumulatively contributing more than the terrestrial source.
While increasing terrestrial carbon is expected to enter freshwater systems as permafrost, glacier, and ice sheets continue to thaw in Artic regions, regional characteristics will ultimately determine the quantity and quality of DOC in freshwaters. In Kangerlussuaq, there has been a decline in DOC in lakes because of hydrology characteristics in the region (Saros et al. Citation2015). In addition, hydrological characteristics associated with altitude can also contribute to variation in DOC concentrations, because high altitude water bodies generally have smaller catchment areas and tend to have cooler temperatures, less runoff, and a reduced open-water season (Schindler Citation2009), which all can contribute to reduced allochthonous DOC inputs, as our results confirmed. Although the fate of carbon entering the freshwater system can be variable, from changes in precipitation, temperature, vegetation, and photooxidation (Cory et al. Citation2014), there are common biogeochemical and ecological consequences, such as reduced light, UV exposure, and increased heterotrophy in waters with increased DOC (Roiha et al. Citation2016; Williamson et al. Citation2015). The scale of change to ecosystem functioning is also a function of water-body size. Ponds, by definition, have a high surface-area-to-value ratio, and may show a higher response to changes in permafrost thaw, temperature, and light, similar to in the littoral zone in lakes. We have shown that changes associated to the DOC pool are reflected in the food web. First, we showed that both primary and bacteria productivity responded to the DOC pool; bacteria production increased with increasing DOC. Second, primary consumers’ diets opportunistically shifted from being fueled by benthic carbon to terrestrial-fueled diets at higher concentrations of DOC. Therefore, our study can be used as a baseline to predict how the aquatic food web may respond to regionally changing DOC levels.
Consumer resource use based on stable isotopes
Pelagic primary production represented a minor resource for consumers in both low- and high-DOC ponds (<20%). Our estimates of low phytoplankton diet by consumers correspond with the observed low rates of pelagic primary production in subarctic and Arctic ponds (Mariash et al. Citation2014; Rautio et al. Citation2011b; Rautio and Vincent Citation2007). According to our results, the lack of phytoplankton is compensated by higher proportional contributions from benthic and terrestrial resources to consumer diets. Among all six consumers included in our three-source mixing model, the benthic diet contributed more than 60 percent in low-DOC ponds, and this contribution could be higher considering that the DOM was mostly autochthonous in low-DOC ponds. Although we assume that benthic algae is mostly an autochthonous source, our sampling methods did not differentiate between the autotrophic or heterotrophic components of the complex biofilm we call benthic algae. There is a level of uncertainty associated with the phytoplankton isotope values, because they are derived from the proportion of direct water use in the diet and trophic fractionation. But by using the Bayesian isotope analysis, we improve the determination of resource use by including these types of uncertainties.
Taxa-specific resource use
Since we were able to include the majority of the pelagic-benthic food web, we could cover several different feeding strategies to estimate how changes in DOC modify their diets. While all consumers’ diets were dominated by autochthonous sources in low-DOC ponds, our results provide insight into taxa-specific use of resources with changing availability of different organic matter. Among zooplankton, the cladoceran D. pulex showed the highest reliance on an allochthonous source of organic matter, with as much as 80 percent in high-DOC ponds. Cladocerans can graze efficiently on bacteria (e.g., Demott Citation1990; Rautio and Vincent Citation2006) and may therefore access larger amounts of terrestrial organic matter via the microbial loop. Another cladoceran, E. glacialis, fed predominantly on benthic algae in low-DOC ponds, but proportionally more DOM-sourced diet in high-DOC ponds. B. paludosa, known to be a benthic and pelagic feeder (Mariash et al. Citation2014), showed wider foraging in high-DOC ponds. B. paludosa diets were a mix of benthic and DOM dominated when DOC was high. For the pelagic species, L. minutus had a diet restricted to DOM in high-DOC ponds. It is important to note that although it is possible that consumers could be feeding directly on the macromolecules of the DOM components, it is more likely that the consumers are feeding on the bacteria and heterotrophs that have metabolized organic-matter sources from the DOM (Jansson et al. Citation2007). It has been shown that heterotophic nanoflagellates and ciliates can take up macromolecules and make those available to larger grazers (Christoffersen, Bernard, and Ekebom Citation1996; Christoffersen and González Citation2003).
Among the benthic invertebrates sampled, C. dolabratus is the most abundant top predator in these ponds, mostly feeding on mosquitoe larvae (Culler, Ayres, and Virginia Citation2015). However, because we sampled later in the late summer period, no mosquito larvae were found in our nets, only exuvia, so we could not get an isotope signature for mosquitos. In any case, our analysis of C. dolabratus indicates that their diet changed to a proportionally higher reliance on DOM sources in high-DOC ponds. Since chironomids can have different feeding strategies (Berg Citation1995), we originally separated the chironomids into predators and grazers, but later pooled them for the SIA analysis. Our results showed that the chironomids fed predominantly on benthic autotrophs (mean 80%), with terrestrial and phytoplankton sources contributing less than 20 percent of their diet in both high- and low-DOC ponds. Chironomidae were the only taxa studied that did not change their diet, but this result could be caused by the taxonomic resolution, as we combined different species and functional groups. Reuss et al. (Citation2013) showed that even in a relatively simple habitat of Arctic lakes, chironomids occupy multiple trophic levels.
Overall, the consumers that had the largest diet response to changes in the DOC pool, were D. pulex, L. minutus, and C. dolabratus. These consumers had proportionally higher allochthonous diets in high-DOC ponds. L. arcticus, known to feed on benthic as well as pelagic food resources (Christoffersen Citation2001) was only found in the low-DOC ponds, which might suggest sensitivity to high DOC concentration that can potentially cause fluctuating oxygen levels.
Conclusions
Our results confirm that DOC plays an important role in controling the relative contribution between pelagic and benthic primary production and pelagic bacterial production, and that these basal organic-matter sources provide energy to aquatic consumers. Our novel multi-stable isotope approach is the first to partition the DOM pool to better define the basal resources for consumers in these types of low Arctic ponds. Our findings show that the DOM is a combination of autochthonous and allochthonous sources, but had higher allochthony and heterotrophy when DOC was high. When DOC was low, benthic sources provided a significant proportion of consumer diets in oligotrophic low Arctic ponds. Our study confirms that the consequences of higher DOC can reduce light availability for primary production and favor microbial activity in the water column, with cascading effects to the higher trophic levels.
Supplementary Materials
Download Zip (49.7 KB)Acknowledgments
We thank Tim Howe and Norma Haubenstock at the Alaska Stable Isotope Facility for their assistance with stable isotope analyses; Nils Willumsen for chemical analyses and assistance in the laboratory; as well as Nina Reuss, Jacob Yde, and Jasmine Saros for constructive comments on an earlier version of the manuscript.
Additional information
Funding
References
- AMAP. 2011. Snow, water, ice and permafrost in the Arctic (SWIPA): Climate change and the cryosphere. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP).
- Anderson, N. J., and C. A. Stedmon. 2007. The effect of evapoconcentration on dissolved organic carbon concentration and quality in lakes of SW Greenland. Freshwater Biology 52:280–89. doi:https://doi.org/10.1111/j.1365-2427.2006.01688.x.
- Ask, J., J. Karlsson, L. Persson, and P. Ask. 2009a. Terrestrial organic matter and light penetration: Effects on bacterial and primary production in lakes. Limnology and Oceanography 54:2034–40. doi:https://doi.org/10.4319/lo.2009.54.6.2034.
- Ask, J., J. Karlsson, L. Persson, P. Ask, and M. Kansson. 2009b. Whole-lake estimates of carbon flux through algae and bacteria in benthic and pelagic habitats of clear-water lakes. Ecology 90:1923–32. doi:https://doi.org/10.1890/07-1855.1.
- Berg, M.B.. 1995. Larval food and feeding behaviour. In The Chironomidaeed. P. D. Armitage, P. S. Cranston, and L. C. V. Pinder, 136–168. Dordrecht: Springer.
- Berggren, M., H. Laudon, M. Haei, L. Ström, and M. Jansson. 2010. Efficient aquatic bacterial metabolism of dissolved low-molecular-weight compounds from terrestrial sources. ISME Journal 4:408–16. doi:https://doi.org/10.1038/ismej.2009.120.
- Berggren, M., S. E. Ziegler, N. F. St-Gelais, B. Beisner, and P. A. del Giorgio. 2014. Contrasting patterns of allochthony among three major groups of crustacean zooplankton in boreal and temperate lakes. Ecology 97(5): 1–16.
- Biasi, C., O. Rusalimova, and H. Meyer. 2005. Temperature-dependent shift from labile to recalcitrant carbon sources of arctic heterotrophs. Rapid Communication in Mass Spectrometry 19:1401–8. doi:https://doi.org/10.1002/rcm.1911.
- Burpee, B., J. E. Saros, R. M. Northington, and K. S. Simon. 2016. Microbial nutrient limitation in Arctic lakes in a permafrost landscape of southwest Greenland. Biogeosciences 13:365–74. doi:https://doi.org/10.5194/bg-13-365-2016.
- Cazzanelli, M., L. Försstöm, A. Muchelsen, and K. Christoffersen. 2012. Benthic production is the key to Daphnia middendorffiana survival in a high arctic pond. Freshwater Biology 57:541–51. doi:https://doi.org/10.1111/j.1365-2427.2011.02722.x.
- Christoffersen, K. 2001. Predation on Daphnia pulex by Lepidurus arcticus. Hydrobiologia 442:223–29. doi:https://doi.org/10.1023/A:1017584928657.
- Christoffersen, K., C. Bernard, and J. Ekebom. 1996. A comparison of the ability of different heterotrophic nanoflagellates to incorporate dissolved organic macromolecules. Archives Hydrobiology Special Issues Advances in Limnology 48:73–84.
- Christoffersen, K., and J. M. González. 2003. An approach to measure ciliate grazing on living heterotrophic nanoflagellates. Hydrobiologia 491:159–66. doi:https://doi.org/10.1023/A:1024459605469.
- Cole, J. J., and C. T. Solomon. 2012. Terrestrial support of zebra mussels and Hudson River food web: A multi-isotope Bayesian analysis. Limnology and Oceanography 55 (6):1802–15. doi:https://doi.org/10.4319/lo.2012.57.6.1802.
- Cory, R. M., C. P. Ward, B. C. Crump, and G. W. Kling. 2014. Sunlight controls water column processing of carbon in arctic fresh waters. Science 345 (6199):925–28. doi:https://doi.org/10.1126/science.1253119.
- Culler, L. E., M. P. Ayres, and R. A. Virginia. 2015. In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proceedings of the Royal Society B: Biological Sciences 282. 20151549. doi:https://doi.org/10.1098/rspb.2015.1549.
- Demott, W. R. 1990. Daphnia, ceriodaphnia, diaphanosoma, chydorus. NATO Advances Science Institutes Series 20:569–94.
- Forsström, L., M. Rautio, M. Cusson, S. Sorvari, R. L. Albert, M. Kumagai, and A. Korhola. 2015. Dissolved organic matter concentration, optical parameters and attenuation of solar radiation in high-latitude lakes across three vegetation zones. Ecoscience 22:17–31. doi:https://doi.org/10.1080/11956860.2015.1047137.
- Forsström, L., T. Roiha, and M. Rautio. 2013. Responses of microbial food web to increased allochthonous DOM in an oligotrophic subarctic lake. Aquatic Microbology Ecology 68:171–84. doi:https://doi.org/10.3354/ame01614.
- Grey, J., and R. I. Jones. 2001. Seasonal changes in the importance of the source of organic matter to the diet of zooplankton in Loch Ness, as indicated by stable isotope analysis. Limnology and Oceanography 46:505–13. doi:https://doi.org/10.4319/lo.2001.46.3.0505.
- Grosbois, G., P. A. del Giorgio, and M. Rautio. 2017. Zooplankton allochthony is spatially heterogeneous in a small boreal lake. Freshwater Biology 62(3):474–90. doi:https://doi.org/10.1111/fwb.12879.
- Guillemette, F., S. L. Mccallister, and P. A. del Giorgio. 2013. Differentiating the degradation dynamics of algal and terrestrial carbon within complex natural dissolved organic carbon in temperate lakes. Journal of Geophysical Research Biogeosciences 118:963–73. doi:https://doi.org/10.1002/jgrg.20077.
- Jansson, M., L. Persson, A. M. Roos, R. I. Jones, and L. J. Tranvik. 2007. Terrestrial carbon and intraspecific size-variation shape lake ecosystems. Trends in Ecology and Evolution 22 (6):316–22. doi:https://doi.org/10.1016/j.tree.2007.02.015.
- Jassby, A. D., and T. Platt. 1976. Mathematical formulation of the relationship photosynthesis and light for phytoplankton. Limnology and Oceanography 21:540–47. doi:https://doi.org/10.4319/lo.1976.21.4.0540.
- Johansson, E., S. Berglund, T. Lindborg, J. Petrone, D. Van As, L. G. Gustafsson, J. O. Näslund, and H. Laudon. 2015. Hydrological and meteorological investigations in a periglacial lake catchment near Kangerlussuaq, west Greenland: Presentation of a new multi-parameter data set. Earth System Science Data 7 (1):93–108. doi:https://doi.org/10.5194/essd-7-93-2015.
- Karlsson, J., P. Byström, J. Ask, P. Ask, L. Person, and M. Janson. 2009. Light limitation of nutrient-poor lake ecosystems. Nature 460:506–9. doi:https://doi.org/10.1038/nature08179.
- Knap, A., A. Michael, A. Close, H. Ducklow, and A. Dickson. 1994. Protocols for the Joint Global Ocean Flux Study (JGOFS) core measurments. Paris: UNESCO Intergovernmental Oceanographic Commission.
- Mariash, H. L., S. P. Devlin, L. Forsström, R. I. Jones, and M. Rautio. 2014. Benthic mats offer a potential subsidy to pelagic consumers in tundra pond food webs. Limnology and Oceanography 59:733–44. doi:https://doi.org/10.4319/lo.2014.59.3.0733.
- Middelburg, J. J. 2014. Stable isotopes dissect aquatic food webs from the top to the bottom. Biogeosciences 11:2357–71. doi:https://doi.org/10.5194/bg-11-2357-2014.
- O’Brien, D. M., and M. J. Wooller. 2007. Tracking human travel using stable oxygen and hydrogen isotope analyses of hair and urine. Rapid Communications in Mass Spectrometry 21:2422–30. doi:https://doi.org/10.1002/rcm.3108.
- Pace, M. L., S. R. Carpenter, J. J. Cole, J. J. Coloso, J. F. Kitchell, J. R. Hodgson, J. J. Middelburg, N. D. Preston, C. T. Solomon, and B. C. Weidel. 2007. Does terrestrial organic carbon subsidize the planktonic food web in a clear-water lake? Limnology and Oceanography 52 (5):2177–89. doi:https://doi.org/10.4319/lo.2007.52.5.2177.
- Pace, M. L., J. J. Cole, S. R. Carpenter, J. F. Kitchell, J. R. Hodgson, M. C. Van de Bogert, D. L. Bade, E. S. Kritzberg, and D. Bastviken. 2004. Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature 427:240–43. doi:https://doi.org/10.1038/nature02227.
- Parnell, A., and A. L. Jackson. 2013. siar: Stable isotope analysis in R. Vienna, Austria. http://cran.r-project.org/package=siar
- Platt, T., C. Gallegos, and W. Harrison. 1980. Photoinkibition of photosynthesis in natural assemblages of marine phytoplankton. Journal of Marine Research 38:687–701.
- R Core Team. 2014. R: A language and environment for statistical computing. Version 2.1.4. www.R-project.org.
- Rae, R., and W. F. Vincent. 1998. Phytoplankton production in subarctic lake and river exosystems: Development of a photosysnthesis-temperature-irradiance model. Journal of Plankton Research 20:1293–312. doi:https://doi.org/10.1093/plankt/20.7.1293.
- Rantala, M. V., L. Nevalainen, M. Rautio, A. Galkin, and T. P. Luoto. 2016. Sources and controls of organic carbon in lakes across the subarctic treeline. Biogeochemistry 129:235–53. doi:https://doi.org/10.1007/s10533-016-0229-1.
- Rautio, M., F. Dufresne, I. Laurion, S. Bonilla, W. F. Vincent, and K. S. Christoffersen. 2011b. Shallow freshwater ecosystems of the circumpolar Arctic. Ecoscience 18:204–22. doi:https://doi.org/10.2980/18-3-3463.
- Rautio, M., H. L. Mariash, and L. Forsström. 2011a. Seasonal shifts between autochthonous and allochthonous carbon contrubutions to zooplankton diets in a subarctic lake. Limnology and Oceanography 56 (4):1513–24. doi:https://doi.org/10.4319/lo.2011.56.4.1513.
- Rautio, M., and W. Vincent. 2007. Isotopic analysis of the sources of organic carbon for zooplankton in shallow subarctic and arctic waters. Ecography 30:77–87. doi:https://doi.org/10.1111/eco.2007.30.issue-1.
- Rautio, M., and W. F. Vincent. 2006. Benthic and pelagic food resources for zooplankton in shallow high-latitude lakes and ponds. Freshwater Biology 51:1038–52. doi:https://doi.org/10.1111/fwb.2006.51.issue-6.
- Reuss, N. S., L. Hamerlik, G. Velle, A. Michelsen, O. Pedersen, and K. P. Brodersen. 2013. Stable isotopes reveal that chironomids occypy several trophic levels within West Greenland lakes: Implication for food web studies. Limnology and Oceanography 58 (3):1023–34. doi:https://doi.org/10.4319/lo.2013.58.3.1023.
- Riis, T., K. S. Christoffersen, and A. Baattrup-Pedersen. 2015. Mosses in high-Arctic lakes: In situ measurements of annual primary production and decomposition. Polar Biology 39:543–52. doi:https://doi.org/10.1007/s00300-015-1806-9.
- Roiha, T., I. Laurion, and M. Rautio. 2015. Carbon dynamics in highly heterotrophic subarctic thaw ponds. Biogeosciences 12:7223–37. doi:https://doi.org/10.5194/bg-12-7223-2015.
- Roiha, T., S. Peura, M. Cusson, and M. Rautio. 2016. Allochthonous carbon is a major contributor to bacterial growth and community composition in subarctic freshwaters. Scientific Reports 6:34456. doi:https://doi.org/10.1038/srep34456.
- Roiha, T., M. Tiirola, M. Cazzanelli, and M. Rautio. 2012. Carbon quantity defines productivity while its quality defines community composition of bacterioplankton in subarctic ponds. Aquatic Sciences 74:513–25. doi:https://doi.org/10.1007/s00027-011-0244-1.
- Saros, J. E., C. L. Osburn, R. M. Northington, S. D. Birkel, J. D. Auger, C. A. Stedmon, and N. J. Anderson. 2015. Recent decrease in DOC concentration in Arctic lakes of southwest Greenland. Geophysical Research Letters 42:1–7.doi:https://doi.org/10.1002/2015GL065075
- Schindler, D. E., W. P. J. Curtis, B. R. Parker, and M. P. Stainton. 1996. Consequences of climate warming and lake acidification for UV-B penetration in North American boreal lakes. Nature 379:705–7. doi:https://doi.org/10.1038/379705a0.
- Schindler, D. W. 2009. Lakes as sentials and integrators for the effects of climate change on watersheds, airsheds, and landscapes. Limnology and Oceanography 56 (6):2349–2358. doi:https://doi.org/10.4319/lo.2009.54.6_part_2.2349.
- Solomon, C. T., S. R. Carpenter, M. K. Clayton, J. J. Cole, J. J. Coloso, M. L. Pace, J. M. Vader Zaden, and B. C. Weidel. 2011. Terrestial, benthic, and pelagic resource use in lakes: Results form a three-isotope Bayesian mixing model. Ecology 92 (5):1115–25. doi:https://doi.org/10.1890/10-1185.1.
- Solomon, C. T., S. E. Jones, B. C. Weidel, I. Buffam, M. L. Fork, J. Karlsson, S. Larsen, J. T. Lennon, J. S. Read, S. Sadro, et al. 2015. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges. Ecosystems 18:376–89. doi:https://doi.org/10.1007/s10021-015-9848-y.
- Vadeboncoeur, Y., J. Kalff, K. Christoffersen, and E. Jeppesen. 2006. Substratum as a driver of variation in periphyton chlorophyll and productivity in lakes. Journal of North American Benthological Society 25:379–92. doi:https://doi.org/10.1899/0887-3593(2006)25[379:SAADOV]2.0.CO;2.
- Vadeboncoeur, Y., G. Peterson, J. M. Vander Zanden, and J. Kalff. 2008. Benthic algal production across lake size gradients: Interaction among morphometry, nutrients, and light. Ecology 89:2542–52. doi:https://doi.org/10.1890/07-1058.1.
- Vincent, W. F., and J. Laybourn-Parry. 2008. Polar lakes and rivers. Oxford: Oxford University Press.
- Vonk, J. E., S. E. Tank, W. B. Bowden, I. Laurion, W. F. Vincent, P. Alekseychik, M. Amyot, M. E. Billet, J. Carnario, R. M. Cory, et al. 2015. Reviews and syntheses: Effects of permafrost thaw on arctic aquatic ecosystems. Biogeosciences 12:10719–815. doi:https://doi.org/10.5194/bgd-12-10719-2015.
- Wassenaar, L. I., and K. A. Hobson. 2003. Comparative equilibration and online technique for determination of non- exchangeable hydrogen of keratins for use in animal migration studies. Isotopes in Environmental and Health Studies 39:211–17. doi:https://doi.org/10.1080/1025601031000096781.
- Weishaar, J. L., G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fuji, and K. Mopper. 2003. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environmental Science and Technology 37:4702–8. doi:https://doi.org/10.1021/es030360x.
- Wickham, H. 2009. ggplot2: Elegant graphics for data anaylsis. New York: Springer.
- Wilkinson, G. M., S. R. Carpenter, and J. J. Cole. 2013. Terrestrial support of pelagic consumers: Patterns and variability revealed by a multilake study. Freshwater Biology 58:2037–49. doi:https://doi.org/10.1111/fwb.12189.
- Williamson, C. E., E. P. Overholt, R. M. Pilla, T. H. Leach, J. A. Brentrup, L. B. Knoll, E. M. Metter, and R. E. Moeller. 2015. Ecological consequences of long-term browning in lakes. Scientific Reports 5:18666. doi:https://doi.org/10.1038/srep18666.
- Yang, C., G. Wilkinson, J. Cole, S. A. Macko, and M. L. Pace. 2014. Assigning hydrogen, carbon, and nitrogen isotope values for phytoplankton and terrestrial detritus in aquatic food web studies. Inland Waters 4:233–42. doi:https://doi.org/10.5268/IW.