ABSTRACT
Rapid climate change in the Arctic may increase sexual reproduction in plants because of changes in both abiotic factors, such as temperature, and biotic factors, such as pollination. Pollination may currently limit plant reproduction in the Arctic, where cold temperatures hinder pollinator activity. To understand how warming may affect pollination and plant reproduction, we studied three plant species in western Greenland. Two species were hermaphroditic and insect-pollinated (Vaccinium uliginosum and Chamerion latifolium), and one was dioecious and insect- and wind-pollinated (Salix glauca). We measured how pollinator visitation and plant reproduction varied across three temperature zones. We also conducted pollinator exclusion and pollen supplementation experiments to measure pollinator dependence and pollen limitation. Proportion of fruit set in Vaccinium and Salix was pollen limited in every temperature zone, and Vaccinium and Chamerion depended on pollinator-mediated outcrossing for maximum reproductive success. Furthermore, higher pollinator visitation to Vaccinium in the warmer temperature zones mirrored lower pollen limitation and higher fruit set, suggesting that temperature zone indirectly influenced reproduction via changes in pollination. Taken together, our results demonstrate that both abiotic factors and pollination are important in limiting reproduction in the Arctic and that plant–pollinator interactions can mediate the response of plant reproduction to warming.
Introduction
Climate change is expected to cause an increase in the growth and reproduction of many plant species in the Arctic (Arft et al. Citation1999; Chapin and Shaver Citation1996; Wookey et al. Citation1993). Given the rapid rate of warming in the Arctic, some of these changes are already occurring (Forbes, Fauria, and Zetterberg Citation2010; Sturm, Racine, and Tape Citation2001), and a general “greening” of the Arctic is predicted to continue (Normand et al. Citation2013; Pearson et al. Citation2013; but see Phoenix and Bjerke Citation2016). Much of the knowledge of the mechanisms underlying the response of vegetation to warming comes from small-scale experiments that manipulate local temperatures (Dormann and Woodin Citation2002; Elmendorf et al. Citation2012). While providing valuable information on the effects of warming across the Arctic, these small-scale experiments are limited in understanding how climate change may affect interactions between plants and mobile organisms (Jones, Bay, and Nordenhäll Citation1997; Richardson, Hartley, and Press Citation2000), such as herbivores and pollinators, which are also responding to climate change and may influence the response of plants to warming. For example, plants may be limited not only by cold temperatures but also by a lack of pollinators, and warming temperatures may increase plant reproduction via increasing pollinator visitation. Studying the combined effects of abiotic and biotic factors is important because they may have nonadditive and potentially unexpected effects on plant reproduction (Angert, LaDeau, and Ostfeld Citation2013).
In the Arctic, plants face short growing seasons with low air and soil temperatures that can limit plant growth and reproduction (Billings Citation1987). Demands on plant maintenance and growth often come at the expense of sexual reproduction (Stenström and Jónsdóttir Citation1997; Wookey et al. Citation1995). For example, temperatures near species-specific thermal limits impede flower formation, the development of pollen and ovules, fertilization, and seed maturation (Bykova et al. Citation2012). In addition to abiotic factors, pollinators also have the potential to limit sexual reproduction of flowering plants in the Arctic. Globally, 88 percent of flowering species require or benefit from pollinators for fruit and seed production, although the proportion of animal-pollinated species decreases with latitude (Ollerton, Winfree, and Tarrant Citation2011), and pollen limitation is a widespread phenomenon (Ashman et al. Citation2004). Autogamous pollination, wind pollination, and self-fertilization may buffer low pollinator visitation in the Arctic and elsewhere, as predicted by the reproductive assurance hypothesis (Carlson, Gisler, and Kelso Citation2008; Haag and Ebert Citation2004; Kalisz, Vogler, and Hanley Citation2004; Lloyd Citation1980; Regal Citation1982; Wada Citation1999). However, pollen limitation in the Arctic has been demonstrated in some cases (Fulkerson, Whittall, and Carlson Citation2012; Philipp et al. Citation1996; Stenström and Molau Citation1992).
Because pollinators may limit plant reproduction in the Arctic, they also have the potential to mediate how plant species respond to rapid warming in this region. There are several non-mutually exclusive pathways through which changes in pollination services could occur. Pollinators may directly respond to climate change through changes in population size and foraging activity (Hegland et al. Citation2009). For example, increases in summer temperatures may increase pollinator visitation rates, which are particularly low in the Arctic partly because of cold temperatures that produce unfavorable flight conditions (Fulkerson, Whittall, and Carlson Citation2012; McCall and Primack Citation1992; Totland Citation1994). Warming may also indirectly affect pollination services via altering plant attractive traits, such as flower size and nectar volume, and flowering phenology (Høye et al. Citation2013; Scaven and Rafferty Citation2013). Moreover, warming is expected to alter plant community composition and cover (Post and Pedersen Citation2008; Walker et al. Citation2006), which can influence pollinator visitation to any one species. For example, an increase in flowering plant cover may attract more pollinators to an area (Ghazoul Citation2006) or further increase competition for pollinators (Mitchell et al. Citation2009), which is already typical of Arctic ecosystems (Hocking Citation1968). Combined, these effects of climate change may influence the number and composition of pollinators visiting flowers, and may ultimately affect plant reproduction, either positively or negatively.
In this study, we took advantage of a natural temperature gradient in western Greenland, adjacent to the Greenland Ice Sheet. To gain an overall understanding of how warming during the growing season may affect pollination and plant reproduction in the Arctic, we studied three plant species that varied in their flowering phenology (two early season, one late season), morphology (two shrubs, one herbaceous), and breeding and pollination systems. We predicted that the warmer end of the temperature gradient would be associated with higher pollinator visitation and fruit and seed set. We also predicted that plants would be less pollen limited for reproduction at the warm end of the gradient, assuming that the plants were not resource limited. Alternatively, if the abiotic stress of cold temperatures is more important than pollinator visitation in limiting plant reproduction, then we predicted that pollen limitation would be lowest at the cold end of the gradient. Understanding how plant reproduction in the Arctic will respond to climate change is important because the distribution and abundance of species are closely tied to their reproductive success (Bykova et al. Citation2012).
Methods
Study system and focal species
We conducted this study between the margin of the Greenland Ice Sheet and the fjord Kangerlussuaq in western Greenland. The study area is classified as low-shrub tundra that is dominated by Betula nana (Betulaceae), Salix glauca (Salicaceae), and graminoids (Walker et al. 2005). The mean annual temperature is −5.7°C, the mean growing season temperature is 9.2°C, and the average annual precipitation is 140 mm (NOAA Citation2015). The dominant floral visitors in the area include flies (Dipterans), especially from the families Muscidae and Syrphidae (Urbanowicz et al. Citation2017). There is a well-established natural temperature gradient running from the ice sheet to the fjord (Bradley-Cook et al. Citation2011); based on two weather stations with data from 2011 to 2014 (Geological Survey of Denmark and Greenland Citation2014; Wunderground Citation2015) and nine temperature loggers with data from 2015 and 2016 (Urbanowicz, unpublished data), areas within 3 km of the ice sheet are on average approximately 3ºC cooler than areas 40 km away near the fjord during the growing season. This temperature gradient is in line with the predicted 3ºC increase in temperature in western Greenland during the next seventy years (Aðalgeirsdóttir Citation2008), making the temperature gradient ecologically relevant. Our study area was deglaciated from approximately 7.3 to 6.8 thousand years before present (Levy et al. Citation2012), and an area of primary succession from the retreat of the ice sheet after the Little Ice Age was not included in our study area. All of the data for this study were collected during the 2014 growing season.
We studied three pan-Arctic plant species: Vaccinium uliginosum (Ericaceae, bog blueberry; hereafter Vaccinium), which flowers in June, is a weakly protandrous, insect-pollinated shrub (Jacquemart and Thompson Citation1996). In other locations, this Vaccinium species is self-compatible but with reduced fruit set after self-pollination compared to outcrossed hand-pollination (Bingham and Orthner Citation1998; Jacquemart and Thompson Citation1996). Salix glauca (Salicaceae, grayleaf willow; hereafter Salix), which also flowers in June, is a dioecious shrub that is wind- and insect-pollinated (eFloras Citation2008). Chamerion latifolium (Onagraceae, dwarf fireweed; hereafter Chamerion), which flowers in July and August, is a protandrous, insect-pollinated herb (Swales Citation1979). Kevan (Citation1972) found that C. latifolium is not dependent on pollinators for maximum seed set in northern Canada.
Study sites
We partitioned the study area into three temperature zones based on distance to the ice sheet: cold (adjacent to the ice sheet, centered at 67.14°, −50.14°), intermediate (halfway between the ice sheet and the fjord, centered at 67.08°, −50.37°), and warm (approximately 40 km away from the cold zone and adjacent to the fjord, centered at 67.02°, −50.68°). For Vaccinium and Salix, we selected three 20 m × 20 m sites per temperature zone that were in close proximity to randomly generated coordinates. All sites within a temperature zone were at least 500 m from each other. Because Vaccinium and Salix co-occur, we could use the same sites for these two species. For Chamerion, we identified multiple populations in each zone and then randomly selected a subset of these populations. We selected three Chamerion sites in the intermediate and warm zones and five sites in the cold zone. We used five sites in the cold zone because we were concerned that some cold sites would be washed away because of their proximity to the ice-fed headwater. These sites remained intact, so we present data from all five cold sites. We recognize that we used only one climatic gradient in this study. However, small-scale variation in the abiotic environment and spatial separation of our study sites helped reduce pseudo-replication (Jacquemart and Thompson Citation1996).
Environmental variables
Surrounding total vegetation and conspecific cover may alter the abiotic environment and compete for or attract pollinators (Laverty Citation1992). Conspecific cover can also influence reproduction through altering mate availability (Delmas et al. Citation2016). We established two transects across the length of each site and recorded the vegetation in a 0.5 m × 0.5 m quadrat placed every 2 m along the transects. For each site, we calculated the mean percent vegetation cover and mean percent cover of conspecific plants. Given that it is difficult to determine the number of individuals in low-lying continuous shrub cover, we use cover rather than density. We also measured soil volumetric water content in the active layer to a depth of approximately 6 cm, using a Theta Probe ML2x and a HH2 moisture meter (Delta T Devices, Cambridge, UK). Five uniformly spaced measurements were taken per site approximately one week after the flowering period in each site, and mean soil moisture was calculated. We used separate ANOVAs to test for differences in these environmental variables among temperature zones.
Pollinator visitation
During peak flowering, we observed insects visiting the flowers of the focal plant species within fifteen randomly placed 0.25 m × 0.25 m quadrats. We identified floral visitors to the lowest taxonomic resolution possible on the wing, including Muscoidea (the superfamily that includes Anthomyiidae and Muscidae), Syrphidae, and Culicidae. For Vaccinium and Chamerion, we only recorded visitors that contacted the sexual parts of flowers, suggesting they were likely pollinators. For Salix, we only observed insects visiting female catkins and counted the number of visits to catkins rather than individual flowers. We observed each quadrat for four minutes, for a total of one hour of observation per site visit. Vaccinium sites were observed once, Salix sites two times, and Chamerion sites four times, resulting in a total of seventy-one hours of pollinator observations. During each site visit, we measured wind speed 1 m off the ground, using a digital anemometer (Kestrel 1000, Nielsen-Kellerman, Boothwyn, PA, USA). We conducted observations only on days when the average wind speed was below 4 m/s and the sky was less than 50 percent cloudy. For each site, we calculated the mean number of visitors per flower (or Salix catkin) per hour and the mean visit duration. For each plant species, we used generalized linear mixed models (GLMMs) to test for differences in the pollinator visitation rate among temperature zones. Site was included as a random effect, and we used a negative binomial error distribution. We used linear mixed models to test for differences in mean visit duration among temperature zones. We log(x + 1) transformed mean visit duration to meet assumptions of normality and used site as a random effect. We tested the significance of temperature zone using likelihood ratio tests (Dobson and Barnett Citation2008).
To supplement the Vaccinium and Chamerion pollinator observations, we used stigma pollen receipt as a proxy for pollinator visitation and pollination (Engel and Irwin Citation2003). Stigma pollen receipt integrates across the number of visits to a plant and the efficiency of pollinators at depositing pollen on stigmas. At the tail end of the flowering period at each site, we collected forty randomly selected Vaccinium stigmas, two each from twenty plants, and twenty randomly selected Chamerion stigmas, one from each of twenty plants. We stained the stigmas with basic fuchsin dye (Kearns and Inouye Citation1993) and counted the number of conspecific pollen tetrads (in the case of Vaccinium) and pollen grains (in the case of Chamerion) on each stigma under a compound microscope. Because of logistical constraints, we did not measure pollen receipt on Salix. For each site, we calculated the mean pollen receipt per stigma. For each plant species, we used separate generalized linear mixed models to test for differences in pollen receipt among temperature zones. We used plant nested within site as a random effect for the Vaccinium model and site as a random effect for the Chamerion model. We used a negative binomial error distribution, which is appropriate for count data (O’Hara and Kotze 2010).
Dependence on pollinators, pollen limitation, and plant reproduction
Dependence on pollinators
We tested whether Vaccinium and Chamerion, which are capable of autonomous self-pollination in other systems (Jacquemart and Thompson Citation1996; Kevan Citation1972), depended on pollinators for maximum fruit and seed set. We bagged buds just before they opened with fine mesh fabric to exclude pollinators (self-pollination treatment). We compared the self-pollination treatment to flowers that were hand pollinated with outcrossed pollen and then bagged to control for the effect of the bag, akin to the methods in Windus and Snow (Citation1993). For each outcrossed-pollinated Vaccinium flower, we collected three flowers from shrubs at least 5 m away and pinched the top of their corollas to produce a vibration that released the pollen onto the focal stigmas. For Chamerion, we collected anthers from ten flowers at least 5 m away, placed these anthers into a microcentrifuge tube, and vibrated the bottom of the tube with an electric toothbrush to loosen the pollen, which was held together with viscin threads, from the anthers. We then applied the pollen to the focal stigmas with a camel-hair paintbrush until pollen was visible on the stigma. We collected more pollen as needed to treat all the hand-pollinated flowers. The self-pollination treatment was applied to fifteen haphazardly selected branches with from two to twenty flowers of spatially distinct Vaccinium shrubs and to fifteen inflorescences with from two to ten flowers of separate Chamerion stems. The outcrossed pollination treatment was applied to five haphazardly selected branches of Vaccinium shrubs and five Chamerion stems. We used fewer branches in the outcrossed pollination treatment because of a lack of pollen donors at some sites.
Pollen limitation
We tested the degree of pollen limitation of plant reproduction by comparing open-pollinated flowers to supplemental hand-pollinated flowers. For Vaccinium and Salix sites, we haphazardly selected fifteen shrubs of each species and assigned separate branches on each shrub to either the open-pollination or supplemental-pollination treatments. The Vaccinium branches had between two and twenty flowers, and the Salix branches between two and five female catkins. For Chamerion, we haphazardly assigned fifteen stems to each treatment, and all flowers that had receptive stigmas during site visits were treated. We supplied pollen to Vaccinium and Chamerion as described previously (see “Methods: Dependence on Pollinators”). For each hand-pollinated Salix catkin, we collected three male dehiscing catkins from shrubs at least 5 m away and directly brushed the receptive female catkins with the male catkins, following the methods in Fox (Citation1992). We visited each site at least twice and hand pollinated all open flowers during each site visit. Open-pollinated flowers were handled but not hand pollinated, and both open-pollination and supplemental-pollination treatments were open to insect visitors.
Plant reproduction
We collected all fruits when they were mature and counted the number of developed fruits and flowers that did not develop into fruits. For Salix, each capsule was considered a fruit, and the number of developed capsules per catkin was recorded. We counted the number of seeds within each fruit under a dissecting microscope. We visually classified seeds as developed seeds, aborted seeds, or aborted ovules. For each Salix catkin, we counted the number of seeds in three haphazardly selected capsules, following the methods in Fox (Citation1992). Our final measures of plant reproduction were proportion fruit set (number of developed fruits/total flowers in treatment), seed set per fruit (number of developed seeds per fruit), and proportion seed set (number of developed seeds/total ovules). Seed set per fruit and proportion seed set provide an assessment of the ability of the plant to utilize the pollen deposited on stigmas, and seed set per fruit provides information on reproductive output (Waser and Price Citation1989).
Statistical analyses of plant reproduction
For both the pollinator dependence and pollen limitation experiments, we used GLMMs to analyze the response of reproductive success to pollination treatment and temperature zones (glmmADMB package; Fournier et al. Citation2012; Millar Citation2011). We used AICc values to compare candidate models and selected the best-fitting model, favoring the simpler model if delta AIC was less than two (AICtab function in bblme package; Bolker Citation2016; Burnham and Anderson Citation2002). The candidate models included a full model with pollination treatment, temperature zone, and their interaction; a model with both main effects; models with only one main effect; and a null model with no main effects. In all models, site was included as a random effect. In the models for Vaccinium and Salix pollen limitation, plant identity was nested within site to account for pairing the supplemental-pollination and open-pollination treatments on each shrub. To model proportion fruit set or proportion seed set, we used a binomial error distribution. If the models were over-dispersed, we included an observation-level random effect (Harrison 2014). We tested the significance of the fixed effects in the best-fitting models using likelihood ratio tests (Dobson and Barnett Citation2008). When temperature zone was significant, differences among zones were tested with post hoc Tukey pairwise comparisons. We verified that including environmental variables (percent vegetation cover, percent conspecific cover, and soil moisture) in the best-fitting models did not qualitatively alter our conclusions by changing the magnitude or direction of coefficients (following Theobald, Gabrielyan, and HilleRisLambers Citation2016). For Chamerion models, we did not include soil moisture in this step because the significant variation in soil moisture among temperature zones (see “Results”) would introduce multicollinearity in the models.
To test the direct effect of temperature zone on reproduction, in the absence of changes in pollination, we analyzed the response of pollen-supplemented branches to temperature zone. We used a GLMM to relate each measure of reproductive success to temperature zone. We included site as a random effect and, for seed set and proportion seed set, plant identity as a random effect.
Results
Environmental variables
For all three focal species, percent vegetation cover and percent conspecific cover did not vary among temperature zones. For Vaccinium and Salix, soil moisture also did not vary among temperature zones. However, soil moisture in Chamerion sites varied significantly among temperature zones (F2,8 = 6.04, p = 0.025), with the warm zone having a lower mean soil moisture (2.6% ± 1.0 SE) than the cold zone (8.3% ± 1.1, Tukey’s contrast, p = 0.036). Soil moisture in the intermediate zone (3.4 ± .3) was not significantly different from the cold or warm zones (Tukey’s contrasts, p = 0.92 and p = 0.068, respectively).
Pollinator visitation
During seventy-one hours of pollinator observations, we observed a total of 189 insects visiting the flowers of our three focal species. The dominant visitors to Vaccinium and Chamerion were syrphid flies (39% and 58% of all visits, respectively), and the dominant visitors to Salix were muscoid flies (33%).
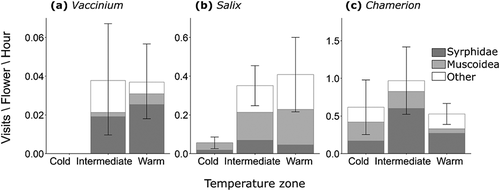
Pollinator visitation rate to Vaccinium varied significantly among temperature zones (χ22 = 9.52, p = 0.009), with no pollinators observed visiting Vaccinium in the cold zone and an average of 0.04 ± 0.02 SE visits per flower per hour in the intermediate and warm zones (). We found no difference in mean visit duration to Vaccinium among temperature zones (χ22 = 0.46, p = 0.50). Flower visitation rates to Chamerion and Salix were an order of magnitude higher than to Vaccinium, and there was no significant difference in pollinator visitation rate or mean visit duration among temperature zones in these two species (p > 0.05 in all cases; , ).
Figure 1. Pollinator visitation rate in three temperature zones for (A) Vaccinium uliginosum, (B) Salix gluaca, and (C) Chamerion latifolium. The shades within the bars represent different groups of visiting insects: Syrphidae (dark gray), Muscoidea (families Anthomyiidae and Muscidae; light gray), and other insects (white). The height of the bars represents mean visitation rate ± 1 SE across all insects. Note the different scales on the y axes
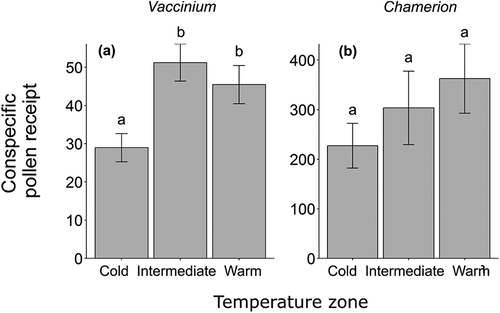
Total conspecific stigma pollen receipt significantly varied between temperature zones for Vaccinium (χ22 = 11.18, p = 0.0037; ). Stigmas in the intermediate and warm zones had nearly 2 times and 1.5 times more conspecific pollen than those in the cold zone (Tukey’s contrasts, p < 0.0001 and p = 0.0019, respectively), but the warm zone did not differ significantly from the intermediate zone (Tukey’s contrast, p = 0.64). In Chamerion, total conspecific pollen receipt did not vary with temperature zone (χ22 = 3.64, p = 0.16; ).
Figure 2. Conspecific stigma pollen receipt in three temperature zones for (A) Vaccinium uliginsoum pollen tetrads and (B) Chamerion latifolium pollen grains. Bars represent mean values ± 1 SE. Different letters indicate significant differences among treatments according to Tukey’s test at the 0.05 significance level. Note the different scales on the y axes
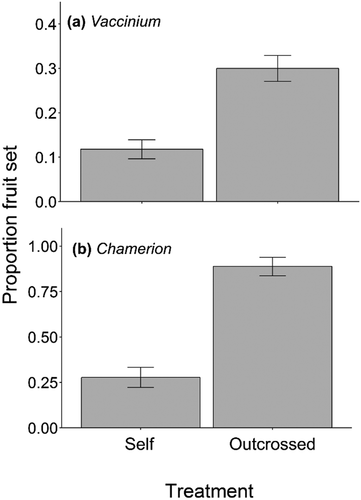
Dependence on pollinators
The best-fitting model for proportion fruit set in Vaccinium and Chamerion included outcrossed pollination treatment but not temperature zone. Proportion fruit set in both species was from two to three times greater in the outcrossed pollination treatment than in flowers that were bagged to test for autonomous self-pollination (Vaccinium: χ21 = 15.2, p < 0.001; Chamerion: χ21 = 49.6, p < 0.001; ). There was no difference in Vaccinium seed set per fruit or proportion seed set between the two pollination treatments or temperature zones (null models were best-fitting models). For seed set per fruit and proportion seed set in Chamerion, the full models were the best-fitting models. In both models, there was a significant interaction between pollination treatment and temperature zone, suggesting that the degree of pollinator dependence varied by temperature zone (seed set per fruit: χ22 = 7.14, p = 0.028; proportion seed set: χ22 = 11.01, p = 0.0041; Figure A1, Supplementary Materials). In the intermediate and cold zones, but not the warm zone, seed set per fruit and proportion seed set were more than three times greater in the outcrossed pollination treatment than in the self-pollination treatment. Taken together, these results suggest that Vaccinium and Chamerion depended on pollinator-mediated pollination for maximum reproductive success.
Pollen limitation and plant reproduction
In Vaccinium, the effect of pollen supplementation on proportion fruit set was dependent on temperature zone (pollination treatment × temperature zone interaction χ22 = 6.3, p = 0.043; ). While proportion fruit set was pollen limited in all temperature zones, proportion fruit set in the cold zone was 1.3 times more pollen limited than in the intermediate zone and 1.5 times more pollen limited than in the warm zone. Neither pollination treatment nor temperature zone explained variation in Vaccinium seed set per fruit or proportion seed set (null models were best-fitting models; Figure A2 in the Supplementary Materials (available online) and ). There was no effect of temperature zone on fruit set, seed set per fruit, or proportion seed set in pollen-supplemented branches, indicating no direct effect of temperature zone on reproductive success (p > 0.29 in all cases). These results suggest that Vaccinium fruit set was pollen limited, the degree of pollen limitation depended on temperature zone, and there was no direct effect of temperature zone on Vaccinium reproductive success.
Figure 4. Plant reproduction, measured as proportion fruit set and proportion seed set for (A, B) Vaccinium uliginosum, (C.D) Salix glauca, and (E, F) Chamerion latifolium. Plant reproduction was measured across three temperature zones and two experimental treatments: open pollinated (white bars) and hand-pollinated with supplemental pollen (gray bars). Bars represent mean ± 1 SE. Note the different scales on the y axes
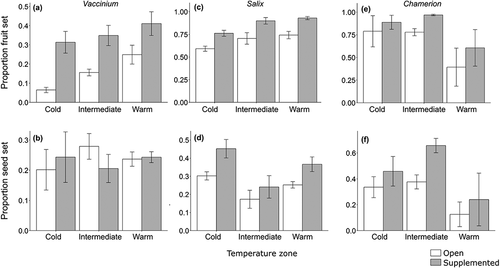
In Salix, proportion fruit set varied significantly with pollen-supplementation treatment (χ21 = 80.5, p < 0.001) and temperature zone (χ22 = 7.70, p = 0.021; ), but their interaction was not in the best-fitting model. Proportion fruit set was 1.3 times greater in pollen-supplemented branches than in open-pollinated branches. Additionally, proportion fruit set was greater in the intermediate and warm zones than in the cold zone (Tukey’s contrasts, p = 0.024 and 0.004, respectively). Salix seed set per fruit responded to the interaction between treatment and temperature zone (χ2 = 6.04, p = 0.048; Figure A2 in the Supplementary Materials). Seed set per fruit was pollen limited in the cold and warm zones but not in the intermediate zone, and there was no significant difference in pollen limitation between the cold and warm zones (Tukey’s contrast, p > 0.05). Proportion seed set varied significantly with both pollen-supplementation treatment (χ21 = 66.4, p < 0.001) and temperature zone (χ22 = 9.20, p = 0.010; ), but their interaction was not in the best-fitting model. Proportion seed set was on average 1.4 times greater in pollen-supplemented flowers than in open-pollinated flowers, and 2.4 times greater in the cold zone than in the intermediate zone (Tukey’s contrast, p = 0.001).
Proportion fruit set in pollen-supplemented Salix branches varied significantly by temperature zone, (χ22 = 12.63, p = 0.0018), indicating a direct effect of temperature zone, with the cold zone having a 15 percent lower fruit set than the intermediate zone (Tukey’s contrast, p = 0.0039) and an 18 percent lower fruit set than the warm zone (Tukey’s contrast, p < 0.001). Similarly, pollen-supplemented seed set per fruit and proportion seed set varied significantly by temperature zone (χ22 = 6.56, p = 0.038, χ22 = 7.67, p = 0.022, respectively), with the intermediate zone but not the warm zone having higher seed set per fruit and proportion seed set than the cold zone (Tukey’s contrasts, p = 0.010 and p = 0.003, respectively). In summary, all three measures of Salix reproductive success were pollen limited, the degree of pollen limitation of seed set per fruit varied by temperature zone, and there was a direct effect of temperature zone on all three measures of reproductive success.
In Chamerion, proportion fruit set was 1.3 times higher in pollen-supplemented than open-pollinated plants (χ21 = 27.41, p < 0.001; ). Temperature zone was not in the best-fitting model for proportion fruit set. Both Chamerion seed set per fruit (Figure A2 in the Supplementary Materials) and proportion seed set () responded to the interaction between treatment and temperature zone (seed set per fruit: χ22 = 9.56, p = 0.008; proportion seed set: χ22 = 14.6, p < 0.001) and were only pollen limited in the intermediate zone. Proportion fruit set in pollen-supplemented plants varied significantly by temperature zone (χ22 = 8.74, p = 0.013), with the warm zone having 30 percent lower fruit set than the cold and intermediate zones (Tukey’s contrasts, p = 0.02, p = 0.02, respectively), indicating a direct negative effect of temperature zone. There was no direct effect of temperature zone on pollen-supplemented seed set per fruit (χ22 = 4.00, p = 0.14) or proportion seed set (χ22 = 3.99, p = 0.14). In summary, all three measures of Chamerion reproductive success were only pollen limited in the intermediate zone, and there was a direct effect of temperature zone on proportion fruit set.
For all species, inclusion of vegetation cover, conspecific cover, and soil moisture produced worse-fitting models in all cases.
Discussion
By studying plant–pollinator interactions and plant reproduction across three temperature zones, our study revealed how warming during the growing season in the Arctic may alter plant reproduction. Pollinator visitation to Vaccinium was higher in the warm and intermediate zones than in the cold zone. This difference in pollinator visitation was mirrored by lower pollen limitation and higher Vaccinium fruit set in the warmer temperature zones, suggesting an indirect effect of temperature zone on reproduction via altering pollination. We also found evidence of a direct positive effect of temperature zone on proportion fruit set in Salix and a negative effect of temperature zone in Chamerion, with the warm zone having the lowest fruit set. Despite species-specific responses, our results indicate that warming in the Arctic has the potential to alter plant reproduction directly and indirectly, through changes in pollination services.
Our results agree with previous findings that temperature is associated with a direct positive effect on reproduction in the Arctic for some species (Arft et al. Citation1999; Hedhly, Hormaza, and Herrero Citation2009). In particular, Salix and other erect shrubs generally show a positive response to experimental warming in terms of growth and reproduction (Dormann and Woodin Citation2002; Klady, Henry, and Lemay Citation2011; Post and Pedersen Citation2008). It is therefore not surprising that we found evidence of a direct effect of temperature zone on Salix, with the pollen-supplemented treatment (thus controlling for pollen supply) showing higher proportion fruit set in the warmer zone. However, in Vaccinium, the reproductive success of pollen-supplemented branches did not vary among temperature zones, suggesting that there was no direct effect of temperature zone on this species. This result is in line with previous studies that have shown Vaccinium uliginosum to be insensitive to experimental summer warming in terms of growth and reproduction (Anadon-Rosell et al. Citation2017; Natali, Schuur, and Rubin Citation2012; Wahren, Walker, and Bret-Harte Citation2005). There was also evidence that temperature zone directly affected Chamerion reproduction, with the warm zone having the lowest fruit set in pollen-supplemented plants. It is possible that any benefit of the warm zone came at a cost of low soil moisture, as the well-drained mineral soil found in Chamerion sites was more than three times drier in the warm zone than in the cold zone. There was no significant variation in soil moisture across temperature zones for Vaccinium or Salix, which are found on soils with higher organic matter content (Bradley-Cook and Virginia Citation2016) and, because they flower early in the season, may not be prone to evaporative loss (Rowntree Citation1997).
We found that proportion fruit set in Vaccinium and Salix was pollen limited in every temperature zone. Moreover, we found that Chamerion and Vaccinium (the two hermaphroditic species) depended on pollinator-mediated outcrossing for maximum reproductive success. Because flowers could autonomously self-pollinate, excluding pollinators did not prevent fruit production but did reduce proportion fruit set threefold compared to the outcrossing treatment. Thus, there is limited ability for autonomous selfing to buffer low pollinator visitation rates in these species (Hargreaves, Weiner, and Eckert Citation2015). Our findings suggest that pollination has the potential to be a limiting factor in the Arctic and could be an important factor in explaining the observed spatial variation in shrub expansion across the Arctic (Myers-Smith et al. Citation2011).
Our finding of concurrent changes in pollinator visitation and pollen limitation in Vaccinium suggests that pollinators may mediate how plant reproduction responds to warming in the Arctic. Not only was Vaccinium proportion fruit set pollen limited in every temperature zone, but it was also less pollen limited in the warm zone than the cold zone. Lower pollen limitation in the warm zone was associated with higher pollinator visitation, as evidenced by both pollinator observations and stigma pollen receipt. Establishing a link between pollinator visitation and pollen limitation is important because changes in pollen limitation can also be caused by variation in resource availability (HilleRisLambers et al. Citation2013; Totland Citation2001). It is important to note that given that we only observed pollinators visiting Vaccinium for one hour at each site, more observations are necessary to confirm that pollinator visitation is indeed higher in the warm zone and that this difference is the result of temperature and not random chance. The mechanisms underlying the apparent higher pollinator visitation in the warmer temperature zones remain unclear. Pollinator activity or abundance may have directly benefitted from warmer average temperatures (Hegland et al. Citation2009), or the plant may have been more attractive to pollinators in the warmer temperature zones. It is also possible that co-occurring species, such as Salix glauca, influenced pollinator abundance by competing for or attracting pollinators (Ghazoul Citation2006).
There were no clear associations between pollinator visitation and pollen limitation of Salix and Chamerion fruit and seed set or Vaccinium seed set. Differences between species are likely the result of differences in pollination modes and resource limitation. Salix is both wind- and insect-(eFloras Citation2008), so it is possible that any patterns of pollen limitation because of pollinator visitation were obscured by wind pollination. Moreover, pollen limitation of Salix proportion fruit set in all three temperature zones may have been because of female-biased sex ratios (Myers-Smith and Hik Citation2012; Urbanowicz, unpublished data) and long distances to male plants (Myers-Smith and Hik Citation2012), which can lower pollen receipt for both wind- and insect-mediated pollination (Davis et al. Citation2004; de Jong, Batenburg, and Klinkhamer Citation2005). Salix seed set per fruit and proportion seed set were not pollen limited in the cold and warm zones, where reproduction was also the highest. Chamerion reproduction was only pollen limited in the intermediate zone, where reproduction was also the highest, potentially because of adequate soil moisture and a release from cold temperatures. Finally, while Vaccinium fruit set was pollen limited, seed set per fruit and proportion seed set were not pollen limited. Supplemental pollen treatments often lead to increased fruit set but not seed set (Burd Citation1994). The low proportion seed set in Vaccinium across all temperature zones may be because of bet hedging, in which a plant produces many ovules to take advantage of stochastic increases in pollinator visitation, especially in highly variable environments such as the Arctic (Ashman et al. Citation2004; Burd et al. Citation2009).
There are several caveats to the interpretation of these results. First, we applied our pollen supplementation treatment to Vaccinium and Salix branches rather than entire plants, which may have led to resource reallocation and inflated pollen limitation estimates (Zimmerman and Pyke Citation1988). However, in several species of Salix, there is evidence that catkins on the same plant are unaffected by each other’s pollen receipt or seed set (Fox Citation1992; Totland and Sottocornola Citation2001). Second, because plants may reallocate resources over time, it would be useful to evaluate reproduction throughout several seasons (Ashman et al. Citation2004). Third, there are confounding variables that may have changed along the temperature gradient, such as water and nutrient availability (Bonito et al. Citation2003) and windspeed. Fourth, while there was a clear difference in temperature between the ice sheet and the fjord, it is unknown if temperatures changed linearly with distance from the ice sheet. Future studies could gain additional ecological insight by studying replicated temperature gradients.
Fruit and seed production are necessary for dispersal and recruitment, maintenance of genetic diversity, and in situ adaptation (Bykova et al. Citation2012; Hargreaves and Eckert Citation2014). Yet, sexual reproduction is often overlooked in the literature on plant responses to climate change, particularly in the Arctic (but see Klady, Henry, and Lemay Citation2011; Natali, Schuur, and Rubin Citation2012). Our study highlights the need to consider both abiotic and biotic factors when investigating the response of plant reproduction to climate change in the Arctic.
Supplementary Materials
Download Zip (115.2 KB)Acknowledgments
We thank Rebecca Novello and Emily Snowden for field assistance and Zachary Joseph and Nekesa Masibo for lab assistance. We also thank members of the Irwin Lab for comments on the manuscript. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF.
Supplemental data
Supplemental data for this article can be accessed publisher’s website.
Additional information
Funding
References
- Aðalgeirsdóttir, G. 2008. Danish Climate Centre Report 08-06. Copenhagen: Danish Meteorological Institute.
- Anadon-Rosell, A., J. M. Ninot, S. Palacio, O. Grau, S. Nogués, E. Navarro, M. C. Sancho, and E. Carrillo. 2017. Four years of experimental warming do not modify the interaction between subalpine shrub species. Oecologia 183:1167–81. doi:https://doi.org/10.1007/s00442-017-3830-7.
- Angert, A. L., S. L. LaDeau, and R. S. Ostfeld. 2013. Climate change and species interactions: Ways forward. Annals of the New York Academy of Sciences 1297:1–7. doi:https://doi.org/10.1111/nyas.12286.
- Arft, A. M., M. D. Walker, J. Gurevitch, J. M. Alatalo, M. S. Bret-Harte, M. Dale, M. Diemer, F. Gugerli, G. H. R. Henry, M. H. Jones, et al. 1999. Responses of tundra plants to experimental warming: Meta-analysis of the international tundra experiment. Ecological Monographs 69:491–511. doi:https://doi.org/10.1890/0012-9615(1999)069[0491:ROTPTE]2.0.CO;2.
- Ashman, T.-L., T. M. Knight, J. A. Steets, P. Amarasekare, M. Burd, D. R. Campbell, M. R. Dudash, M. O. Johnston, S. J. Mazer, R. J. Mitchell, et al. 2004. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 85:2408–21. doi:https://doi.org/10.1890/03-8024.
- Billings, W. D. 1987. Constraints to plant growth, reproduction, and establishment in Arctic environments. Arctic and Alpine Research 19:357–65. doi:https://doi.org/10.2307/1551400.
- Bingham, R. A., and A. R. Orthner. 1998. Efficient pollination of alpine plants. Nature 391:238–39. doi:https://doi.org/10.1038/34564.
- Bolker, B. 2016. Package “bbmle”: Tools for General Maximum Likelihood Estimation. Available: http://CRAN.R-project.org/package=bbmle.
- Bonito, G. M., D. C. Coleman, B. L. Haines, and M. L. Cabrera. 2003. Can nitrogen budgets explain differences in soil nitrogen mineralization rates of forest stands along an elevation gradient? Forest Ecology and Management 176:563–74. doi:https://doi.org/10.1016/S0378-1127(02)00234-7.
- Bradley-Cook, J. I., A. Burzynski, C. R. Hammond, and R. A. Virginia. 2011. Land cover heterogeneity and soil respiration in a west Greenland tundra landscape. AGU Fall Meeting Abstracts 41. Accessed November 28, 2016. http://adsabs.harvard.edu/abs/2011AGUFM.C41B0406B.
- Bradley-Cook, J. I., and R. A. Virginia. 2016. Soil carbon storage, respiration potential, and organic matter quality across an age and climate gradient in southwestern Greenland. Polar Biology 39:1283–95. doi:https://doi.org/10.1007/s00300-015-1853-2.
- Burd, M. 1994. Bateman’s principle and plant reproduction: The role of pollen limitation in fruit and seed set. Botanical Review 60:83–139. doi:https://doi.org/10.1007/BF02856594.
- Burd, M., T.-L. Ashman, D. R. Campbell, M. R. Dudash, M. O. Johnston, T. M. Knight, S. J. Mazer, R. J. Mitchell, J. A. Steets, and J. C. Vamosi. 2009. Ovule number per flower in a world of unpredictable pollination. American Journal of Botany 96:1159–67. doi:https://doi.org/10.3732/ajb.0800183.
- Burnham, K., and D. Anderson. 2002. Model selection and multimodel inference: A practical information-theoretical approach. New York, NY: Springer.
- Bykova, O., I. Chuine, X. Morin, and S. I. Higgins. 2012. Temperature dependence of the reproduction niche and its relevance for plant species distributions. Journal of Biogeography 39:2191–200. doi:https://doi.org/10.1111/j.1365-2699.2012.02764.x.
- Carlson, M. L., S. D. Gisler, and S. Kelso. 2008. The role of reproductive assurance in the Arctic: A comparative study of a homostylous and distylous species pair. Arctic, Antarctic, and Alpine Research 40:39–47. doi:https://doi.org/10.1657/1523-0430(06-080)[CARLSON]2.0.CO;2.
- Chapin, F. S., and G. R. Shaver. 1996. Physiological and growth responses of arctic plants to a field experiment simulating climatic change. Ecology 77:822–40. doi:https://doi.org/10.2307/2265504.
- Davis, H. G., C. M. Taylor, J. G. Lambrinos, and D. R. Strong. 2004. Pollen limitation causes an Allee effect in a wind-pollinated invasive grass (Spartina alterniflora). Proceedings of the National Academy of Sciences of the United States of America 101:13804–7. doi:https://doi.org/10.1073/pnas.0405230101.
- de Jong, T. J., J. C. Batenburg, and P. G. L. Klinkhamer. 2005. Distance-dependent pollen limitation of seed set in some insect-pollinated dioecious plants. Acta Oecologica 28:331–35. doi:https://doi.org/10.1016/j.actao.2005.07.001.
- Delmas, C. E. L., T. L. C. Fort, N. Escaravage, and A. Pornon. 2016. Pollen transfer in fragmented plant populations: Insight from the pollen loads of pollinators and stigmas in a mass‐flowering species. Ecology and Evolution 6:5663–73. doi:https://doi.org/10.1002/ece3.2280.
- Dobson, A. J., and A. Barnett. 2008. An introduction to generalized linear models. Boca Raton, FL: CRC Press.
- Dormann, C. F., and S. J. Woodin. 2002. Climate change in the Arctic: Using plant functional types in a meta-analysis of field experiments. Functional Ecology 16:4–17. doi:https://doi.org/10.1046/j.0269-8463.2001.00596.x.
- eFloras. 2008. eFloras. Accessed January 7, 2015. http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=200005841.
- Elmendorf, S. C., G. H. R. Henry, R. D. Hollister, R. G. Björk, A. D. Bjorkman, T. V. Callaghan, L. S. Collier, E. J. Cooper, J. H. C. Cornelissen, T. A. Day, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecology Letters 15:164–75. doi:https://doi.org/10.1111/j.1461-0248.2011.01716.x.
- Engel, E. C., and R. E. Irwin. 2003. Linking pollinator visitation rate and pollen receipt. American Journal of Botany 90(11):1612–18.
- Forbes, B. C., M. M. Fauria, and P. Zetterberg. 2010. Russian Arctic warming and “greening” are closely tracked by tundra shrub willows. Global Change Biology 16:1542–54. doi:https://doi.org/10.1111/j.1365-2486.2009.02047.x.
- Fournier, D. A., H. J. Skaug, J. Ancheta, J. Ianelli, A. Magnusson, M. N. Maunder, A. Nielsen, and J. Sibert. 2012. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods and Software 27:233–49. doi:https://doi.org/10.1080/10556788.2011.597854.
- Fox, J. F. 1992. Pollen limitation of reproductive effort in willows. Oecologia 90:283–87. doi:https://doi.org/10.1007/BF00317187.
- Fulkerson, J. R., J. B. Whittall, and M. L. Carlson. 2012. Reproductive ecology and severe pollen limitation in the polychromic tundra plant, Parrya nudicaulis (Brassicaceae). PLoS ONE 7:e32790. doi:https://doi.org/10.1371/journal.pone.0032790.
- Geological Survey of Denmark and Greenland. 2014. PROMICE: Programme for Monitoring of the Greenland Ice Sheet. Accessed November 21, 2014. http://promice.org/DataDownload.html.
- Ghazoul, J. 2006. Floral diversity and the facilitation of pollination. Journal of Ecology 94:295–304. doi:https://doi.org/10.1111/j.1365-2745.2006.01098.x.
- Haag, C. R., and D. Ebert. 2004. A new hypothesis to explain geographic parthenogenesis. Annales Zoologici Fennici 41:539–44.
- Hargreaves, A. L., and C. G. Eckert. 2014. Evolution of dispersal and mating systems along geographic gradients: Implications for shifting ranges. Functional Ecology 28:5–21. doi:https://doi.org/10.1111/1365-2435.12170.
- Hargreaves, A. L., J. L. Weiner, and C. G. Eckert. 2015. High-elevation range limit of an annual herb is neither caused nor reinforced by declining pollinator service. Journal of Ecology 103:572–84. doi:https://doi.org/10.1111/1365-2745.12377.
- Hedhly, A., J. I. Hormaza, and M. Herrero. 2009. Global warming and sexual plant reproduction. Trends in Plant Science 14:30–36. doi:https://doi.org/10.1016/j.tplants.2008.11.001.
- Hegland, S. J., A. Nielsen, A. Lázaro, A.-L. Bjerknes, and Ø. Totland. 2009. How does climate warming affect plant-pollinator interactions? Ecology Letters 12:184–95. doi:https://doi.org/10.1111/j.1461-0248.2008.01269.x.
- HilleRisLambers, J., M. A. Harsch, A. K. Ettinger, K. R. Ford, and E. J. Theobald. 2013. How will biotic interactions influence climate change–induced range shifts? Annals of the New York Academy of Sciences 1297:112–25. doi:https://doi.org/10.1111/nyas.12182.
- Hocking, B. 1968. Insect-flower associations in the high arctic with special reference to nectar. Oikos 19:359–87. doi:https://doi.org/10.2307/3565022.
- Høye, T. T., E. Post, N. M. Schmidt, K. Trøjelsgaard, and M. C. Forchhammer. 2013. Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nature Climate Change 3:759–63. doi:https://doi.org/10.1038/nclimate1909.
- Jacquemart, A.-L., and J. D. Thompson. 1996. Floral and pollination biology of three sympatric Vaccinium (Ericaceae) species in the Upper Ardennes, Belgium. Canadian Journal of Botany 74:210–21. doi:https://doi.org/10.1139/b96-025.
- Jones, M. H., C. Bay, and U. Nordenhäll. 1997. Effects of experimental warming on arctic willows (Salix spp.): A comparison of responses from the Canadian High Arctic, Alaskan Arctic, and Swedish Subarctic. Global Change Biology 3:55–60. doi:https://doi.org/10.1111/j.1365-2486.1997.gcb135.x.
- Kalisz, S., D. W. Vogler, and K. M. Hanley. 2004. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature 430:884–87. doi:https://doi.org/10.1038/nature02776.
- Kearns, C. A., and D. W., Inouye. 1993. Techniques for pollination biologists. Niwot, CO: University Press of Colorado.
- Kevan, P. G. 1972. Insect pollination of high arctic flowers. Journal of Ecology 60:831–47. doi:https://doi.org/10.2307/2258569.
- Klady, R. A., G. H. R. Henry, and V. Lemay. 2011. Changes in high arctic tundra plant reproduction in response to long-term experimental warming. Global Change Biology 17:1611–24. doi:https://doi.org/10.1111/j.1365-2486.2010.02319.x.
- Laverty, T. M. 1992. Plant interactions for pollinator visits: A test of the magnet species effect. Oecologia 89:502–8. doi:https://doi.org/10.1007/BF00317156.
- Levy, L. B., M. A. Kelly, J. A. Howley, and R. A. Virginia. 2012. Age of the Ørkendalen moraines, Kangerlussuaq, Greenland: Constraints on the extent of the southwestern margin of the Greenland Ice Sheet during the Holocene. Quaternary Science Reviews 52:1–5. doi:https://doi.org/10.1016/j.quascirev.2012.07.021.
- Lloyd, D. 1980. Demographic factors and mating patterns in angiosperms. In Demography and Evolution in Plant Populations, O. T. Solbrig (ed.). Berkeley, CA: University of California Press.
- McCall, C., and R. B. Primack. 1992. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. American Journal of Botany 79:434–42. doi:https://doi.org/10.2307/2445156.
- Millar, R. B. 2011. Maximum likelihood estimation and inference: With examples in R, SAS and ADMB. Hoboken, NJ: John Wiley & Sons.
- Mitchell, R. J., R. J. Flanagan, B. J. Brown, N. M. Waser, and J. D. Karron. 2009. New frontiers in competition for pollination. Annals of Botany 103:1403–13. doi:https://doi.org/10.1093/aob/mcp062.
- Myers-Smith, I. H., B. C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K. D. Tape, M. Macias-Fauria, U. Sass-Klaassen, E. Lévesque, et al. 2011. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters 6:45509. doi:https://doi.org/10.1088/1748-9326/6/4/045509.
- Myers-Smith, I. H., and D. S. Hik. 2012. Uniform female-biased sex ratios in alpine willows. American Journal of Botany 99:1243–48. doi:https://doi.org/10.3732/ajb.1200107.
- Natali, S. M., E. A. G. Schuur, and R. L. Rubin. 2012. Increased plant productivity in Alaskan tundra as a result of experimental warming of soil and permafrost. Journal of Ecology 100:488–98. doi:https://doi.org/10.1111/j.1365-2745.2011.01925.x.
- NOAA. 2015. The Global Climate Observing System (GCOS). National Oceanic and Atmospheric Administration. Available: https://www.ncdc.noaa.gov/gosic/global-climate-observing-system-gcos.
- Normand, S., C. Randin, R. Ohlemüller, C. Bay, T. T. Høye, E. D. Kjær, C. Körner, H. Lischke, L. Maiorano, J. Paulsen, et al. 2013. A greener Greenland? Climatic potential and long-term constraints on future expansions of trees and shrubs. Phiosophical Transactions of the Royal Society B: Biological Sciences 368:20120479. doi:https://doi.org/10.1098/rstb.2012.0479.
- Ollerton, J., R. Winfree, and S. Tarrant. 2011. How many flowering plants are pollinated by animals? Oikos 120:321–26. doi:https://doi.org/10.1111/j.1600-0706.2010.18644.x.
- Pearson, R. G., S. J. Phillips, M. M. Loranty, P. S. A. Beck, T. Damoulas, S. J. Knight, and S. J. Goetz. 2013. Shifts in Arctic vegetation and associated feedbacks under climate change. Nature Climate Change 3:673–77. doi:https://doi.org/10.1038/nclimate1858.
- Philipp, M., S. R. J. Woodell, J. Böcher, and O. Mattsson. 1996. Reproductive biology of four species of Pedicularis (Scrophulariaceae) in West Greenland. Arctic and Alpine Research 28:403–13. doi:https://doi.org/10.2307/1551851.
- Phoenix, G. K., and J. W. Bjerke. 2016. Arctic browning: Extreme events and trends reversing arctic greening. Global Change Biology 22:2960–62. doi:https://doi.org/10.1111/gcb.13261.
- Post, E., and C. Pedersen. 2008. Opposing plant community responses to warming with and without herbivores. Proceedings of the National Academy of Sciences 105:12353–58. doi:https://doi.org/10.1073/pnas.0802421105.
- Regal, P. J. 1982. Pollination by wind and animals: Ecology of geographic patterns. Annual Review of Ecology and Systematics 13:497–524. doi:https://doi.org/10.1146/annurev.es.13.110182.002433.
- Richardson, S. J., S. E. Hartley, and M. C. Press. 2000. Climate warming experiments: Are tents a potential barrier to interpretation? Ecological Entomology 25:367–70. doi:https://doi.org/10.1046/j.1365-2311.2000.00263.x.
- Rowntree, P. R. 1997. Global and regional patterns of climate change: Recent predictions for the Arctic. In Global change and Arctic terrestrial ecosystems: Ecological studies, edited by W. C. Oechel, T. V. Callaghan, T. G. Gilmanov, J. I. Holten, B. Maxwell, U. Molau, and B. Sveinbjörnsson. 82–109. New York: Springer. doi:https://doi.org/10.1007/978-1-4612-2240-8_5.
- Scaven, V. L., and N. E. Rafferty. 2013. Physiological effects of climate warming on flowering plants and insect pollinators and potential consequences for their interactions. Current Zoology 59:418–26. doi:https://doi.org/10.1093/czoolo/59.3.418.
- Stenström, A., and I. S. Jónsdóttir. 1997. Responses of the clonal sedge, Carex bigelowii, to two seasons of simulated climate change. Global Change Biology 3:89–96. doi:https://doi.org/10.1111/j.1365-2486.1997.gcb134.x.
- Stenström, M., and U. Molau. 1992. Reproductive ecology of saxifraga oppositifolia: Phenology, mating system, and reproductive success. Arctic and Alpine Research 24:337–43. doi:https://doi.org/10.2307/1551289.
- Sturm, M., C. Racine, and K. Tape. 2001. Climate change: Increasing shrub abundance in the Arctic. Nature 411:546–47. doi:https://doi.org/10.1038/35079180.
- Swales, D. E. 1979. Nectaries of certain arctic and sub-arctic plants with notes on pollination. Rhodora 81:363–407.
- Theobald, E. J., H. Gabrielyan, and J. HilleRisLambers. 2016. Lilies at the limit: Variation in plant-pollinator interactions across an elevational range. American Journal of Botany 103:189–97. doi:https://doi.org/10.3732/ajb.1500416.
- Totland, Ø. 1994. Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arctic and Alpine Research 26:66–71. doi:https://doi.org/10.2307/1551879.
- Totland, Ø. 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–44. doi:https://doi.org/10.2307/2680228.
- Totland, Ø., and M. Sottocornola. 2001. Pollen limitation of reproductive success in two sympatric alpine willows (Salicaceae) with contrasting pollination strategies. American Journal of Botany 88:1011–15. doi:https://doi.org/10.2307/2657082.
- Urbanowicz, C., R. A. Virginia, and R. E. Irwin. 2017. The response of pollen-transport networks to landscape-scale climate variation. Polar Biology 1–11. doi:https://doi.org/10.1007/s00300-017-2138-8.
- Wada, N. 1999. Factors affecting the seed-setting success of Dryas octopetala in front of Brøggerbreen (Brøgger Glacier) in the high Arctic, Ny-Ålesund, Svalbard. Polar Research 18:261–68. doi:https://doi.org/10.1111/j.1751-8369.1999.tb00302.x.
- Wagenius, S., and S. P. Lyon. 2010. Reproduction of Echinacea angustifolia in fragmented prairie is pollen limited but not pollinator-limited. Ecology 91:733–42. doi:https://doi.org/10.1890/08-1375.1.
- Wahren, C.-H. A., M. D. Walker, and M. S. Bret-Harte. 2005. Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Global Change Biology 11:537–52. doi:https://doi.org/10.1111/j.1365-2486.2005.00927.x.
- Walker, D. A., M. K. Raynolds, F. J. Daniëls, E. Einarsson, A. Elvebakk, W. A. Gould, et al. 2005. The circumpolar Arctic vegetation map. Journal of Vegetation Science 16(3):267–82.
- Walker, M. D., C. H. Wahren, R. D. Hollister, G. H. R. Henry, L. E. Ahlquist, J. M. Alatalo, M. S. Bret-Harte, M. P. Calef, T. V. Callaghan, A. B. Carroll, et al. 2006. Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences 103:1342–46. doi:https://doi.org/10.1073/pnas.0503198103.
- Waser, N. M., and M. V. Price. 1989. Optimal outcrossing in Ipomopsis aggregata: Seed set and offspring fitness. Evolution 43:1097–109. doi:https://doi.org/10.2307/2409589.
- Wunderground. 2015. Weather History for Kangerlussuaq, Greenland. Weather Underground. Available: https://www.wunderground.com/history/airport/BGSF/.
- Windus, J. L., and A. A. Snow. 1993. Fruit set and seed predation in an Ohio population of Gentiana saponaria. The American Midland Naturalist 129:346–51. doi:https://doi.org/10.2307/2426515.
- Wookey, P. A., A. N. Parsons, J. M. Welker, J. A. Potter, T. V. Callaghan, J. A. Lee, and M. C. Press. 1993. Comparative responses of phenology and reproductive development to simulated environmental change in Sub-Arctic and High Arctic plants. Oikos 67:490–502. doi:https://doi.org/10.2307/3545361.
- Wookey, P. A., C. H. Robinson, A. N. Parsons, J. M. Welker, M. C. Press, T. V. Callaghan, and J. A. Lee. 1995. Environmental constraints on the growth, photosynthesis and reproductive development of Dryas octopetala at a high Arctic polar semi-desert, Svalbard. Oecologia 102:478–89. doi:https://doi.org/10.1007/BF00341360.
- Zimmerman, M., and G. H. Pyke. 1988. Reproduction in Polemonium: Assessing the factors limiting seed set. The American Naturalist 131:723–38. doi:https://doi.org/10.1086/284815.