ABSTRACT
Stable isotope compositions of organic carbon (δ13Corg) and nitrogen (δ15N) in macrophytes and sediments are useful in assessing sources of lake productivity and diagenesis of organic matter from formation through sedimentation to decomposition. Despite the increasing importance of high-latitude landscapes to carbon cycling under amplified and accelerating warming in the Arctic, the high density of small closed-basin lakes in this landscape, and the utility of stable isotopes in the study of carbon dynamics, limited data are available on within-lake spatial variability of δ13Corg and δ15N in these systems. The goal of this study was to investigate the spatial variability in stable isotopic composition of three dominant macrophyte species (Hippuris vulgaris, Eriophorum angustifolium, Warnstorfia exannulata) and sediments from littoral and profundal areas of a single closed-basin system among the common small Arctic lakes that populate the ice-free margin of Greenland. The range in δ13Corg of macrophytes (−33.9‰ to −27.1‰) was within the typical range of plants utilizing the C3 pathway for carbon fixation. No notable differences were observed in δ13Corg between segments of the individual macrophytes (emergent, submergent, and root tissues), indicating that the isotopic fractionation of carbon was similar throughout the plant. Between-species variations in δ13Corg were small, but significant (p < 0.01), with the moss most depleted in 13C. The range of δ15N in littoral and profundal sediments (−0.52‰ to 1.33‰) was small, with littoral surface sediments 1‰ less enriched in 15N than surface sediments in the profundal zone. The C/N ratios of macrophytes (mean ± SD: 27.0 ± 12.6), littoral sediments (mean ± SD: 11.0 ± 1.0), and profundal sediments (mean ± SD: 9.1 ± 0.9) point to diagenetic alteration. Combined isotopic and elemental (C/N) compositions of littoral and profundal sediments suggest that organic matter accumulating in the study lake originate primarily from in-lake primary production of macrophytes. Terrestrial sources are likely minor because of the hydrologically closed basin and limited aeolian inputs, suggesting that the majority of organic matter produced by the dominant littoral macrophyte community was decomposed between production and sediment deposition.
Introduction
Lakes and associated wetlands are known to play an important role in the global carbon cycle through greenhouse gas (GHG) production and carbon sequestration (Battin et al. Citation2009; Sobek et al. Citation2014). Small lakes (< 10 km2) represent about half of the total area of surface water in Arctic environments (Abnizova et al. Citation2012) and emit more greenhouse gases (CO2 and CH4) per unit area than larger lakes (Bastviken et al. Citation2004; Cole et al. Citation2007; Downing Citation2010; Juutinen et al. Citation2009). Northern latitudes are experiencing amplified temperature increases (Cohen et al. Citation2014), with this trend predicted to continue throughout the next century (Meehl et al. Citation2007). Recent analyses of methane emissions based on stable isotopes further constrain fossil fuel contributions to greenhouse gases, lending further urgency to research on the accelerated warming of northern landscapes and enhanced biogenic emissions (Schwietzke et al. Citation2016). As part of the effort to understand positive feedbacks in the Arctic, it is crucial to gain a better understanding of the carbon dynamics in the extensive lake and wetland systems of this region (Wik et al. Citation2016).
Primary producers and heterotrophic microbes contribute to the carbon budget of lakes through photosynthesis and decomposition, respectively (Boschker et al. Citation1995; Keeley and Sandquist Citation1992; Meyers Citation1994). These processes also have a direct effect on the composition of the sedimentary organic matter (OM) throughout the system (Aichner, Herzschuh, and Wilkes Citation2010; Boschker et al. Citation1995). Stable carbon isotope (δ13Corg) values of lake OM are frequently used as proxies to trace biologic sources, lake productivity, and carbon burial (Brenner et al. Citation2006; Liu et al. Citation2013; Meyers and Ishiwatari Citation1993; Osmond Citation1981; Sobek et al. Citation2014). Although the input of the δ13Corg of roots and falling litter has been shown to directly influence the δ13Corg of soil OM (Ehleringer, Buchmann, and Flanagan Citation2000), the relationship between the δ13Corg of lake sediments and freshwater macrophytes is confounded by variation in relative inputs of terrestrial and aquatic organic carbon (Aichner, Herzschuh, and Wilkes Citation2010; Boschker et al. Citation1995; Keeley and Sandquist Citation1992; LaZerte and Szalados Citation1982; Osmond Citation1981). This relationship is more complex among macrophytes versus terrestrial plants because of the variability of the δ13Corg signatures of the source carbon between environments, the form of the inorganic carbon assimilated, and the differences in diffusional resistances related to the degree of submergence (Raven Citation1970; Keeley and Sandquist Citation1992; Leng et al. Citation2006). In closed-basin lakes where terrestrial organic carbon inputs are highly constrained, the δ13Corg may be directly controlled by isotopic variation in macrophytes and other aquatic plants within the system (Leng et al. Citation2006; Leng and Marshall Citation2004).
Two principal plant groups contribute to OM in lacustrine systems: (1) nonvascular plants lacking woody and cellulosic tissues, and (2) vascular plants, which include these tissues (Meyers and Ishiwatari Citation1993). Both groups are capable of utilizing the C3, C4, and CAM photosynthetic pathways for carbon fixation, with contributions to the system influenced by lake morphology, watershed topography, and relative abundances of lake and watershed plants (Meyers and Ishiwatari Citation1993). Inorganic carbon sources used by macrophytes during photosynthesis include carbon dioxide (CO2) and/or bicarbonate (HCO3−; Allen and Spence Citation1981; Aichner, Herzschuh, and Wilkes Citation2010; Keeley and Sandquist Citation1992; Meyers Citation1994; Osmond Citation1981). These sources are distinguished by unique isotopic signatures (CO2~ −7‰ vs. HCO3−~ +1‰), which affect the δ13Corg value of the macrophyte and allow for the primary inorganic carbon source to be deduced (Osmond Citation1981; Keeley and Sandquist Citation1992; Meyers Citation1994). Nitrogen isotope composition (δ15N) can also be useful in determining sources of OM (Brenner et al. Citation2006). Organic matter typically retains a δ15N signature similar to the source nitrogen (Meyers and Ishiwatari Citation1993). Therefore, OM from terrestrial C3 plants utilizing atmospheric N2 will have a δ15N value of approximately 1‰, while autochthonous sources of OM, which utilize dissolved nitrate as the nitrogen source, will have a δ15N value of approximately 8‰ (Meyers and Ishiwatari Citation1993).
Sedimentary OM consists of a mixture, which can include OM from primary producers within the lake (i.e., macrophytes, algae) and from the surrounding watershed (i.e., terrestrial vegetation and soils in catchment) (Boschker et al. Citation1995; Brenner et al. Citation2006; Kuzyakov and Domanski Citation2000; Liu et al. Citation2013; Meyers and Ishiwatari Citation1993). In the time between origin and sediment burial, OM can be altered by selective degradation, making the amount incorporated into subsurface sediments only a fraction of the original OM introduced to the surface waters (Meyers and Ishiwatari Citation1993). This results in sedimentary OM that is markedly different from the OM produced by the biota in and around the lake (Eadie et al. Citation1984; Meyers Citation1994; Meyers and Ishiwatari Citation1993). Stable carbon isotopes of OM are largely unaffected by diagenesis, making them a useful resource in determining the source carbon of the system (Boschker et al. Citation1995; Meyers and Ishiwatari Citation1993). However, inputs from multiple carbon sources with varying isotopic signatures can make it difficult to constrain the primary source of OM to sediments. It is therefore useful to include multiple parameters, such as nitrogen isotopes (δ15N) and C/N ratios, to increase confidence in analysis (Bernasconi, Barbieri, and Simona Citation1997; Meyers and Lallier-Vergès Citation1999; Boschker et al. Citation1995; Sampei and Matsumoto Citation2001).
West Greenland contains hundreds of thousands of Holocene lakes underlain by continuous permafrost and isolated from regional water flow (Anderson et al. Citation2001; Leng and Anderson Citation2003; Anderson and Stedmon Citation2007; Jørgensen and Andreasen Citation2007). Paleolimnological studies of lake sediments have been particularly important to the current understanding of regional environmental variability in Greenland (Anderson and Leng Citation2004; Anderson et al. Citation2008; Olsen, Anderson, and Leng Citation2013; Olsen et al. Citation2012). These studies have surveyed multiple lakes in this region, with sampling at the maximum water depth. In particular, Olsen, Anderson, and Leng (Citation2013) demonstrated that stable isotope composition of lake sediments can only inform past environmental change when informed by the context of lake-specific limnological processes. Yet little is known about the spatial variability in biogeochemistry within these systems and how this might influence sediment composition. In this study we focus on spatial variability in a single small Arctic lake that is part of a population common to the ice-free margin of Greenland. We investigate the spatial variation in the δ13Corg and δ15N of OM, including δ13Corg from macrophytes and associated littoral and profundal sediments. We hypothesized that variation would be low, with the majority of OM in the system originating from autochthonous littoral sources, because of the limited terrestrial vegetation in the surrounding landscape, a lack of allochthonous carbon transport to the lake because of closed-basin hydrology, the absence of drainage inputs, and the presence of continuous permafrost.
Methods
Site description
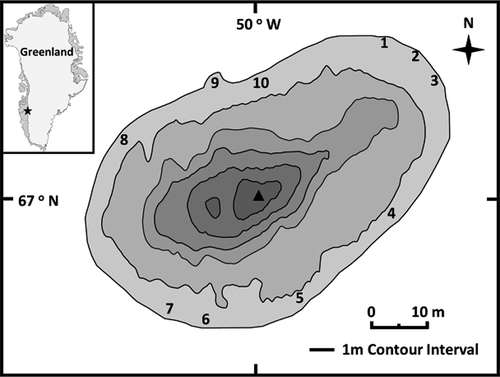
This study focused on a small lacustrine system (informally referred to as EVV Upper lake) with a fringing wetland located near Kangerlussuaq, Greenland (67°05ʹ16ʺ N, 50°17ʹ14ʺ W) between the Søndre Strømfjord and Russell Glacier (). This lake is part of a larger study of seven small lakes in the area that investigated seasonal and spatial variations in biogeochemical dynamics within these lakes during winter and summer and the effects of these dynamics on methane cycling (see Cadieux Citation2015; Cadieux et al. Citation2016). Morphometric characteristics were presented in detail elsewhere (Colcord et al. Citation2015; Goldman et al. Citation2015; Cadieux et al. Citation2016; ), while limnological parameters were recorded every 1 m up the water column at maximum depth (5.5 m; ). Lake hydrology is dominated by melting snowpack with no through-drainage and limited groundwater seepage because of negative regional precipitation-evaporation balance and the presence of continuous permafrost (Willemse et al. Citation2004). The littoral zone of the system is abundant in macrophytic vegetation that is spatially limited to the shallow margin of the lake. The littoral community is dominated by a forb Hippuris vulgaris (common mare’s tail), a graminoid Eriophorum angustifolium (tall cottongrass), and a moss tentatively identified as Warnstorfia exannulata (warnstorfia moss; Guo et al. Citation2013).
Table 1. Morphometric characteristics of EVV Upper lake (from Cadieux et al. Citation2016)
Table 2. Limnological properties for EVV Upper lake, including temperature, pH, dissolved oxygen (DO), dissolved organic carbon (DOC), and conductivity during summer 2014
Figure 1. Bathymetric map of EVV Upper lake (modified from Cadieux et al. Citation2016) indicating sampling sites for macrophytes and littoral sediments and profundal sediment core (triangle). Inset of Greenland marking Kangerlussuaq
The continuous permafrost has a seasonably active layer extending to less than 50 cm below the surface (Jørgensen and Andreasen Citation2007) and the area has a Low Arctic continental climate with low annual precipitation (<150 mm yr−1; Anderson et al. Citation2001). Local flora is described as an Arctic Mountain Tundra community (UNEP/GRID-Arendal Citation2006) and includes mixed dwarf shrubs (Salix glauca, Vaccinium uliginosum), mixed graminoid (Carex concolor, Calamagrostis lapponica), vibrant graminoid (Calamagrostis lapponica), and aquatic forb (H. vulgaris). Mean annual air temperature in the area is −6°C (Anderson et al. Citation2001), with maximum temperatures of 20°C from June to early August when mean temperatures are higher than 8°C. Complexly deformed Archean felsic gneisses interlayered with garnetiferous mafic gneiss, pegmatite lenses, and rare pyroxenites are characteristic of the bedrock surrounding the study lake.
Field sampling
The three vegetative species that dominate the littoral community in EVV Upper lake and associated surface sediments were collected on July 17, 2014, at ten sampling sites along the shallow edge of the littoral zone (). Sampling locations were chosen based on eleven 0.5 × 0.4 m plots denoted by high-density polyethylene (HDPE) frames being utilized during a recent methane flux chamber study. Two individual samples of each plant species were collected from within each plot. Vascular species Eriophorum and Hippuris were separated by emergent, submergent, and root segments for discrete analyses. However, because of the small size of the nonvascular moss Warnstorfia, the biomass of leaf/stem collections was pooled for tissue analyses. Littoral surface sediments associated with macrophyte sampling sites were sampled from under a layer of water and bryophytic mat ranging in depth from 10 cm to 30 cm. Surface sediment grab samples were collected from each plot by cutting through the peat mat until the sediment surface was exposed and filling a presterilized 50 mL centrifuge tube with the sample. A 50 cm deep sediment core was taken at maximum water depth (Zmax) with a WaterMark® Universal Corer Sediment Sampler, capped, and transported to the lab for processing.
Following same-day return to the Kangerlussuaq International Science Support (KISS) lab, littoral sediment samples were immediately frozen for preservation. Plant samples were rinsed with deionized water to remove sediment and debris, dried at 35°C for 24 hrs, and stored in Ziploc® bags for transport to Indiana University for analysis. The profundal sediment core was sectioned into 5 cm-depth discs, stripped of dissolved CH4 in the pore water using the methods described in Cadieux et al. (Citation2016), and transferred to 50 mL centrifuge tubes. Samples were frozen and transported to Indiana University for analysis.
Laboratory analysis
Dried plant samples were flash frozen in liquid nitrogen and ground to a fine powder to ensure homogeneity. Sediment samples were dried, ground, and sieved to remove rock and plant particulate matter. Three sediment samples were randomly chosen to test for carbonates using 10 percent HCl. The absence of bubbling indicated that no carbonates were present, and it was determined that no acid treatment was necessary for the remaining samples. Plant and sediment samples were analyzed for percent organic carbon (%OC), percent nitrogen (%N), nitrogen isotope ratio (δ15N), and organic carbon isotope ratio (δ13Corg) in the Stable Isotope Research Facility (SIRF) lab at Indiana University on a Costech ECS4010 Elemental Analyzer (EA-IRMS). Instrumental precision, using acetanilide, EDTA, and cornstarch standards, was determined to be ±0.2‰, with reproducibility of δ13Corg and δ15N within ± 0.6‰ and ± 0.4‰, respectively. Isotope ratios are reported in conventional delta notation (δ) with units as per mil (‰) relative to the Vienna Pee Dee Belemnite (VPDB) standard for carbon and atmospheric N2 (AIR) for nitrogen.
Quantitative analysis
The C/N mass ratios were calculated for macrophyte segments and sediment samples. Concentrations of dissolved carbonate species were estimated using a chemical equilibrium model (MINEQL+ v. 4.6). Field measurements of water temperature, partial pressure of CO2, pH, and alkalinity were used as inputs in MINEQL+ to determine equilibrium concentrations of carbonate species and to calculate the degree of saturation with respect to atmospheric partial pressure. Correlation analyses were conducted using linear regression and between-species variation was determined using one-way ANOVA analysis. Statistical analysis was performed using Microsoft Excel for Mac 2011 (Microsoft Corp., Excel for Mac OS X. Version 14.4.6; www.microsoft.com/mac) and StatPlus (AnalystSoft Inc., StatPlus:mac-statistical analysis program for Mac OS, Version v5; www.analysoft.com/en/).
Results and discussion
δ13Corg, C/N, and %OC of macrophyte tissues
The three vegetative species that dominate the littoral community in EVV Upper lake, H. vulgaris, E. angustifolium, and W. exannulata, were subsampled and analyzed for δ13Corg and C/N (). Pooled tissues of each species exhibited δ13Corg values that ranged between −33.9‰ and −27.1‰, with median values for H. vulgaris, E. angustifolium, and W. exannulata of −27.7‰, −27.9‰, and −31.7‰, respectively (; Table S1). The range in values for δ13Corg are similar to data reported for macrophytes in previous studies (Syväranta, Hämäläinen, and Jones Citation2006). They are similar to δ13Corg of OM produced from fixation of atmospheric CO2 by terrestrial plants utilizing the C3 photosynthetic pathway (Bender Citation1971; Brenner et al. Citation2006; Meyers Citation1994; Meyers and Ishiwatari Citation1993; O’Leary Citation1988; Osmond Citation1981), including Arctic terrestrial C3 plants (Körner, Farquhar, and Wong Citation1991). Although each of these species utilizes the C3 pathway, some macrophytes are known to be capable of HCO3− uptake (Allen and Spence Citation1981; Keeley and Sandquist Citation1992; Madsen and Maberly Citation2003), which can lead to enriched δ13Corg values similar to C4 plants. The more depleted δ13Corg values of EVV Upper lake macrophytes indicate that HCO3− is not being utilized as a primary inorganic carbon source, even though HCO3− is a dominant form of dissolved inorganic carbon (DIC) between pH 6 and 8 (Wetzel Citation2001).
Figure 2. Composition of pooled tissue samples from dominant macrophyte species: (A) organic carbon isotope value (δ13Corg) and (B) carbon-to-nitrogen ratio (C/N). Box plots include mean (small square inside box), median (horizontal line inside box), interquartile range (25th and 75th percentiles as box ends), minimum and maximum (vertical lines), and range from 1st to 99th percentile (X)
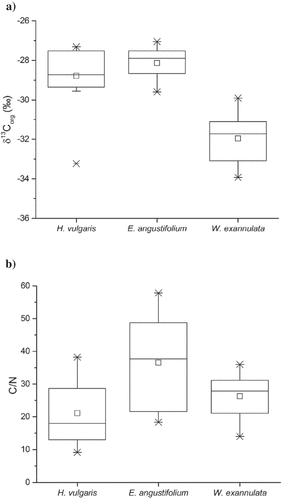
The C/N ratios of pooled tissues in macrophyte samples ranged from 9 to 58, with median values for H. vulgaris, E. angustifolium, and W. exannulata of 18, 38, and 28, respectively (). Bryophytes typically contain degradation/decomposition-resistant tissues, which would lead to higher C/N ratios relative to forbs and graminoids (Hájek et al. Citation2011; Kroken, Graham, and Cook Citation1996). Several studies report average C/N ratios between 4 and 10 (Meyers Citation1994; Meyers and Ishiwatari Citation1993; Meyers and Teranes Citation2002). The C/N ratios for the nonvascular bryophyte in EVV Upper lake (W. exannulata) ranged from 14 to 36. Variability in C/N in this study was high for all three plant species, with relative standard deviations for H. vulgaris, E. angustifolium, and W. exannulata of 49, 41, and 27 percent, respectively. The high variability in C/N likely resulted from the use of EA-IRMS to simultaneously determine elemental C and N concentrations and stable isotopic composition, which requires relatively small sample mass.
The carbon isotopic composition of the individual segments of vascular macrophytes, H. vulgaris and E. angustifolium, varied only by about 2‰ (δ13Corg of emergent, submergent, and root tissues; and Table S1).
Figure 3. δ13Corg versus %OC for macrophyte tissues and surface sediments collected from littoral sampling sites. Macrophyte tissues for Eriophorum and Hippuris are presented separately for emergent, submergent, and root portions of each plant collected; for Warnstorfia the leaf/stem tissues were pooled for each sample
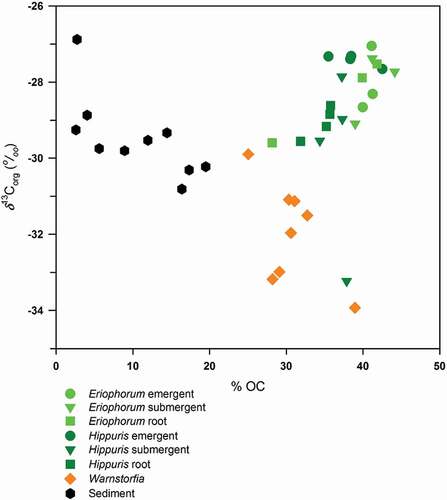
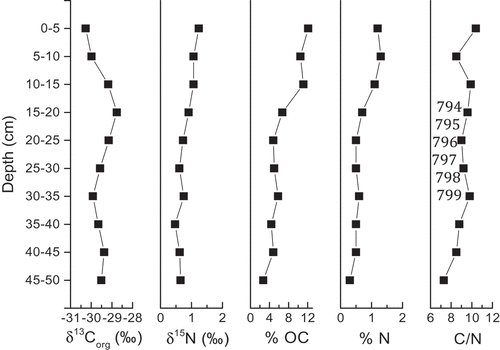
Figure 4. Sediment depth profile of δ13Corg, δ15N, %OC, %N, and C/N ratios for the 50 cm profundal sediment core
This range is similar to Ehleringer, Buchmann, and Flanagan (Citation2000), who reported differences between foliage, wood, fruit, and roots of terrestrial plants of 2–3‰. Previous studies have reported variation of carbon isotopic signatures of whole macrophytes related to water depth (Farquhar, Ehleringer, and Hubick Citation1989; Liu et al. Citation2013; O’Leary Citation1988). The depth range of the littoral zone of EVV Upper lake is narrow, and macrophyte densities are sparse in deeper areas. Consequently, these areas were not sampled. Rapid light extinction (vertical light attenuation coefficient, kd = 2.3 m−1; Cadieux Citation2015) associated with high water-column DOC (median = 48 mg L−1; ) likely is the cause for the limited distribution of the littoral community to shallow water-column depths. Thus, depth of submergence could not be evaluated as a factor affecting carbon isotopic discrimination in individual plant segments. We do note that the emergent tissues of H. vulgaris were about 1‰ more enriched in 13C than the submergent and root tissues, while no effect of submergence was observed for E. angustifolium ().
While within-species variation of δ13Corg values was limited, significant differences were observed between the vascular macrophytes and the nonvascular moss (p < 0.01). Vascular macrophyte species displayed mean δ13Corg values of −28.8 ± 1.6‰ (H. vulgaris) and −28.1 ± 0.8‰ (E. angustifolium), while the nonvascular moss species (W. exannulata) was greater than 2‰ less enriched in 13C; mean δ13Corg = −33.0 ± 1.3‰ (). Greater carbon isotopic discriminiation in W. exannulata could be attributed to several factors. Microbial symbiosis between Sphagnum sp. and methanotrophs has been shown to allow the moss to utilize methane as a carbon source, leading to greater depletion of 13C in the fixed carbon of moss plant tissues (Kip et al. Citation2010; Raghoebarsing et al. Citation2005). Spatial variation in methane isotopic composition and its overall contribution to fixed carbon within the moss mats could also account for the relatively large variation in δ13Corg we observed for W. exannulata (). However, W. exannulata in EVV Upper lake exhibited considerably greater depletion in 13C of plant tissue than was reported for Sphagnum sp. in the studies cited earlier. An alternative explanation for the more negative δ13Corg values in moss tissues is that these plants have unlimited access to dissolved CO2 in the surface water of the littoral zone they inhabit. Many aquatic bryophytes will fix only dissolved CO2 (Bain and Proctor Citation1980; Turetsky Citation2003). Isotopic fractionation in macrophytes has been shown to vary with CO2 availability (Kerby and Raven Citation1985; O’Leary Citation1988), with greater fractionation observed when dissolved CO2 is unlimited. The concentration of dissolved CO2 in the surface waters of EVV Upper lake was calculated to be more than four times the concentrations in equilibrium with the partial pressure of atmospheric CO2, making the system supersaturated. Supersaturation is likely caused by the production of carbon dioxide from decomposition at a rate that exceeds rates of degassing from the water column. EVV Upper lake has a small surface area (0.2 ha; ) and limited wind mixing, particularly during midsummer. Elevated rates of CO2 production from decomposition and kinetically constrained evasion rates likely lead to supersaturated concentrations of CO2 during midsummer. More negative δ13Corg values would be expected under supersaturated CO2 conditions because of an increase in 12C availability (Kelley Citation1987; Kling et al. Citation1991).
Several noteworthy distinctions were observed in the relationship between the δ13Corg and %OC of the different littoral plant tissues in EVV Upper lake (). Generally, in macrophyte tissues from EVV Upper lake, the δ13Corg increased with increasing organic carbon content, likely because of the preferential fixation of 12C over 13C during photosynthesis (Farquhar, Ehleringer, and Hubick Citation1989). Note that the distinctly lighter carbon isotopic compositions of the forb (Hippuris) and graminoid (Eriophorum) tissues versus moss (Warnstorfia) tissues were also associated with higher organic carbon content ().
δ13Corg, δ15N, C/N, and %OC of littoral sediments
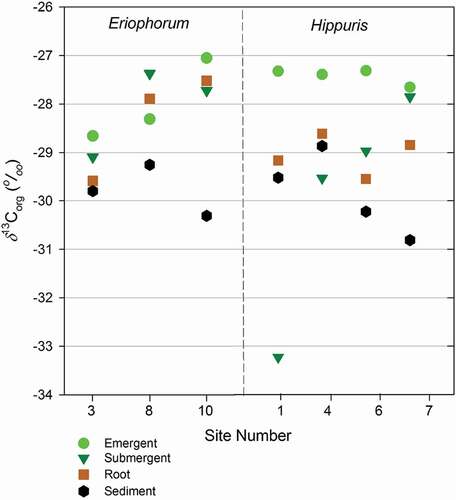
Stable carbon isotope values of littoral surface sediments associated with macrophyte sampling sites ranged from −30.8‰ to −26.9‰ (mean ± SD: −29.6 ± 0.97), and are similar to values reported in previous lake sediment studies (Ho and Meyers Citation1994; Meyers and Ishiwatari Citation1993; Qiu et al. Citation1993). We note that the δ13Corg values of littoral sediments were bracketed by the range of the dominant plants in the littoral community, particularly by the emergent species, H. vulgaris and E. angustifolium (). The dominant moss species, W. exannulata, exhibited δ13Corg values consistently more depleted in 13C than the sediments beneath. For both the Eriophorum-dominated sites (nos. 3, 8, 10) and Hippuris-dominated sites (nos. 1, 4, 6, 7), plant tissues were consistently isotopically enriched in 13C relative to the sediment directly below (). We did not find systematic variation in δ13Corg from emergent to submergent to root for Eriophorum, although emergent tissues of Hippuris were consistently more enriched in 13C relative to its submergent and root tissues. Note that submergent tissue for Site 1 may be an outlier. Syväranta, Hämäläinen, and Jones (Citation2006) also measured variations in the δ13Corg of macrophytes, comparing emergent, floating, and submerged species in a lake in central Finland. They found large spatial variability, with submerged species approximately 3‰ more isotopically enriched than emergent and floating species. As with other studies on the isotopic composition of aquatic plants, Syväranta, Hämäläinen, and Jones (Citation2006) attribute differences in δ13Corg to differences in the inorganic carbon pools being accessed (aqueous DIC versus atmospheric CO2). Although there may be seasonal variation in the DIC pool in the littoral zone that affects the δ13Corg of macrophyte biomass, the isotopic signal that is transferred to sediment pools is likely to be an integration of the isotopic composition of carbon fixed during the course of the growing season. The pooled result is the biomass that senesces and is added to the sediment pool in the fall. Although we are not sampling during fall senescence, the time period of our sampling corresponds with the near maximum in macrophyte growth for the season. Thus, we believe our plant samples reasonably approximate the integrated seasonal value of δ13Corg of macrophytes that is ultimately transferred to littoral sediments.
Figure 5. δ13Corg versus site number for Eriophorum- and Hippuris-dominated sites. Data are presented separately for emergent, submergent, and root portions of each plant and for surface sediments collected beneath
The isotopic composition of OM in littoral surface sediments is likely to reflect differences in plant tissue inputs and in diagenetic alternation of OM (Gälman, Rydberg, and Bigler Citation2009). Comparing the δ13Corg with %OC for littoral sites, we found that carbon isotopic composition of surficial sediment OM became heavier with decreasing %OC (). Preferential decomposition of isotopically lighter carbon substrates may be driving these changes even in shallow sediments of the littoral zone analogous to diagenetic alternation described for longer sediment cores representing longer time frames (Gälman, Rydberg, and Bigler Citation2009; Sobek et al. Citation2014). The carbon isotopic composition of littoral sediments was also similar to nearby surface soils (Colcord, Pearson, and Brassell Citation2017); however, inferring the source of sedimentary OM using δ13Corg exclusively can be misleading (Aichner, Herzschuh, and Wilkes Citation2010; Lehmann et al. Citation2002).
Surface sediment δ15N values ranged from −0.52‰ to 1.33‰, with samples becoming more enriched in 15N on the western side of the lake (Table S1). These values are consistent with results from other lacustrine systems. For example, Jones et al. (Citation2004), in a survey of thirty lakes from upland United Kingdom, reported a range in the δ15N of surface sediments from −0.2‰ to 6.1‰; values were very similar to the δ15N of macrophytes from these sites. Variations in nitrogen isotope ratios in lake nitrogen pools often are attributed to differences in the isotopic composition of inputs and from biologically mediated fractionations within the system (Jones et al. Citation2004). For example, depleted signatures of δ15N have been associated with preferential diagenesis (Meyers and Ishiwatari Citation1993) and selective discrimination during nitrate assimilation (Meyers and Lallier-Vergès Citation1999; Cloern, Canuel, and Harris Citation2002). Without comprehensive spatial analysis of δ15N values for plant tissues, we are unable to relate spatial patterns in sediment δ15N to littoral plants.
The C/N ratios in surface sediments of the littoral zone ranged from 8.5 to 12.4 (mean ± SD: 11.0 ± 1.0; Table S2). The C/N ratio in plant biomass ranges from higher values in terrestrial plants (C/N of 20–80) to lower values in aquatic algae (C/N of 4–10; Meyers and Ishiwatari Citation1993). Our observations fall in the typical range of autochthonous sources in freshwater lakes, including littoral macrophytes (Brenner et al. Citation2006) and periphytic algae from oligotrophic systems (Fink, Peters, and Von Elert Citation2006) and below terrestrial plants. Similar to previous observations of lakes in the surrounding area, the δ13Corg values and C/N ratios of EVV Upper lake surface sediments indicate that OM most likely originated from in-lake primary production (Sobek et al. Citation2014). Although observed values of these parameters could also indicate an input of OM from terrestrial C3 plants, this is unlikely because of the absence of drainage inputs associated with the closed-basin hydrology of EVV Upper lake. Aeolian inputs may also be important to the biogeochemistry of certain lakes in the region (Olsen, Anderson, and Leng Citation2013; Rydberg et al. Citation2016); however, we suggest that these sources are likely limited because of the very small surface area of the lake (A = 0.2 ha; ) and protection from wind by topographic relief on three sides.
δ13Corg, δ15N, and C/N of profundal sediments
The profundal sediment core collected from EVV Upper lake measured 50 cm in depth and was characterized by a gradual change from darker layers in shallow intervals to lighter layers in deeper intervals. However, distinct laminations were not visually evident. Although this specific core was not dated, sediment dating of EVV Upper lake and an immediately adjacent lake (EVV Lower lake) was completed on cores collected in 2012 (Colcord et al. Citation2015). The sediment accumulation rate for EVV Upper lake was approximately 0.015 cm yr−1. Anderson et al. (Citation2008) report sedimentation rates for other small lakes in the region of about 0.02 cm yr−1 for the upper 50 cm, representing approximately 2,500 yr. The δ13Corg in sediments ranged from −30.3‰ to −29.2‰ (mean ± SD: −29.5 ± 0.5) with the most 13C depleted isotopic values measured in the shallowest intervals (). The values and the depth-dependent trend are very similar to those reported from the core collected at EVV Upper lake in 2012 by another laboratory (Colcord, Pearson, and Brassell Citation2017). Collected concurrently, surface soils (upper 10 cm) surrounding EVV Upper lake had an average δ13Corg of −28.9‰ (Colcord, Pearson, and Brassell Citation2017). The results from nearby EVV Lower lake, only 60 m to the west of EVV Upper lake, were notably different, where δ13Corg in sediments ranged from −22.8‰ at the surface to −25.6‰ down core, with the most 13C depleted isotopic values occurring at depth; the mean δ13Corg in soils nearby was −23.7‰. Colcord, Pearson, and Brassell (Citation2017) suggest that the δ13Corg of surface sediments was influenced by inputs from local soils. Surface sediments from larger Two Boat Lake, located 5 km northeast of EVV Upper lake, also contained similar δ13Corg values (Rydberg et al. Citation2016). Sobek et al. (Citation2014), in a study of similar lakes in the surrounding area, found more enrichment in δ13Corg values below 5–10 cm depth, while Funder (Citation1978) reported no trends in δ13Corg with depth in east Greenland lake-sediment cores. As these studies show, there is considerable variation in patterns of δ13Corg with sediment depth in this region. However, a negative offset in sediment δ13Corg values relative to OM inputs is likely because of discrimination against 13C during microbial decomposition in sediments (Gälman, Rydberg, and Bigler Citation2009; Lehmann et al. Citation2002).
Combining δ15N measurements with sediment C/N and δ13Corg data can enhance our understanding of watershed and in-lake processes contributing to OM deposition and preservation in sediments (Gälman, Rydberg, and Bigler Citation2009; Olsen, Anderson, and Leng Citation2013). Selective degradation of OM through microbial modification of specific components during early diagenesis can alter C/N ratios and the C and N isotopic composition of the residual OM (Bernasconi, Barbieri, and Simona Citation1997; Lehmann et al. Citation2002; Meyers Citation1994; Meyers and Ishiwatari Citation1993). The median δ15N of littoral surface sediments in EVV Upper lake was 0.1‰ (Table S1), while δ15N in the profundal sediment core decreased with core depth from 1.2‰ to 0.7‰ (). The littoral surface sediments were about 1‰ less enriched in 15N than surface sediments in the profundal zone. Although the difference is small, it suggests some diagenetic alternation of OM between deposition in littoral sediments and initial burial in profundal areas. This is supported by the C/N ratio in the upper 5 cm of profundal sediments (10.4; ) and the mean value for littoral surface sediments (11.4; Table S2). Similarity in δ13Corg and C/N ratios of littoral surface sediments and surface intervals of the profundal sediments also supports the idea that OM sourcing to the profundal sediments is primarily from the littoral zone. This is consistent with the organic matter cycling of closed-basin lakes, where the primary input of OM to profundal sediments may be autochthonous supply from littoral areas (Leng and Marshall Citation2004; Olsen, Anderson, and Leng Citation2013).
Conclusions
This study focuses on a single small closed-basin lake near Kangerlussuaq, southwestern Greenland. We demonstrate low spatial variability in elemental and isotopic composition of littoral plants and associated sedimentary OM within three dominant macrophyte communities that comprise the littoral areas. Further, we show that the δ13Corg of plant tissues from different segments of the dominant macrophytes (emergent, submergent, root) differed by less than 6‰, indicating that isotopic fractionation associated with carbon fixation is similar for each macrophyte species and consistent throughout the community. Further, plant tissue δ13Corg matches values for C3 plants utilizing atmospheric CO2 for carbon fixation.
The δ13Corg and δ15N of OM from surficial sediments in the littoral zone exhibited low spatial variability throughout the system, differing by less than 4‰ and less than 2‰, respectively. The isotopic composition and C/N ratios of littoral plants and associated sediments and profundal sediments suggest that the inputs of OM to profundal sediments are dominated by sourcing from littoral macrophytes. The influence of terrestrial sources of OM are likely limited because of the hydrologically closed basin and small surface area of the lake. While small, hydrologically isolated lakes currently dominate west Greenland, Arctic lakes with through-drainage are likely to have a complex variety of OM sources that contribute to higher spatial variability of isotopes of OM. More work is required to understand how representative this site is of closed-basin systems in the Arctic and how small closed-basin lakes compare with open-basin lakes throughout the Arctic.
Supplementary Materials
Download Zip (15 KB)Acknowledgments
Special thanks to Ruth Droppo, Polar Field Services, Inc., and Kangerlussuaq International Science Support (KISS) for logistical support while in Greenland, and Dr. Peter E. Sauer of the Stable Isotope Research Facility at IU for assistance with isotopic analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abnizova, A., J. Siemens, M. Langer, and J. Boike. 2012. Small ponds with major impact: The relevance of ponds and lakes in permafrost landscapes to carbon dioxide emissions. Global Biogeochemical Cycles 26 (2):1. doi:https://doi.org/10.1029/2011GB004237.
- Aichner, B., U. Herzschuh, and H. Wilkes. 2010. Influence of aquatic macrophytes on the stable carbon isotopic signatures of sedimentary organic matter in lakes on the Tibetan Plateau. Organic Geochemistry 41 (7):706–12. doi:https://doi.org/10.1016/j.orggeochem.2010.02.002.
- Allen, E. D., and D. H. N. Spence. 1981. The differential ability of aquatic plants to utilize the inorganic carbon supply in fresh water. New Phytolology 87 (2):269–83. doi:https://doi.org/10.1111/j.1469-8137.1981.tb03198.x.
- Anderson, N. J., and C. A. Stedmon. 2007. The effect of evapoconcentration on dissolved organic carbon concentration and quality in lakes of SW Greenland. Freshwater Biology 52 (2):280–89. doi:https://doi.org/10.1111/j.1365-2427.2006.01688.x.
- Anderson, N. J., K. P. Brodersen, D. B. Ryves, S. McGowan, L. S. Johansson, E. Jeppesen, and M. J. Leng. 2008. Climate versus in-lake processes as controls on the development of community structure in a low-arctic lake (South-West Greenland). Ecosystems (New York, N.Y.) 11 (2):307–24. doi:https://doi.org/10.1007/s10021-007-9123-y.
- Anderson, N. J., R. Harriman, D. B. Ryves, and S. T. Patrick. 2001. Dominant factors controlling variability in the ionic composition of West Greenland lakes. Arctic, Antarctic, and Alpine Research 33 (4):418–25. doi:https://doi.org/10.2307/1552551.
- Anderson, N. J., and M. J. Leng. 2004. Increased aridity during the early Holocene in West Greenland inferred from stable isotopes in laminated-lake sediments. Quaternary Science Reviews 23 (7–8):841–49.
- Bain, J. T., and M. C. F. Proctor. 1980. The requirement of aquatic bryophytes for free CO2 as an inorganic carbon source: Some experimental evidence. New Phytology 86:393–400. doi:https://doi.org/10.1111/j.1469-8137.1980.tb01680.x.
- Bastviken, D., J. Cole, M. Pace, and L. Tranvik. 2004. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochemical Cycles 18:GB4009. doi:https://doi.org/10.1029/2004GB002238.
- Battin, T. J., S. Luyssaert, L. A. Kaplan, A. K. Aufdenkampe, A. Richter, and L. J. Tranvik. 2009. The boundless carbon cycle. Nature Geoscience 2 (9):598–600. doi:https://doi.org/10.1038/ngeo618.
- Bender, M. 1971. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 10 (6):1239–44. doi:https://doi.org/10.1016/S0031-9422(00)84324-1.
- Bernasconi, S. M., A. Barbieri, and M. Simona. 1997. Carbon and nitrogen isotope variations in sedimenting organic matter in Lake Lugano. Limnology and Oceanography 42 (8):1755–65. doi:https://doi.org/10.4319/lo.1997.42.8.1755.
- Boschker, H. T. S., E. M. J. Dekkers, R. Pel, and T. E. Cappenberg. 1995. Sources of organic carbon in the littoral of Lake Gooimeer as indicated by stable carbon isotope and carbohydrate compositions. Biogeochemistry 29 (1):89–105. doi:https://doi.org/10.1007/BF00002596.
- Brenner, M., D. A. Hodell, B. W. Leyden, J. H. Curtis, W. F. Kenney, B. Gu, and J. M. Newman. 2006. Mechanisms for organic matter and phosphorus burial in sediments of a shallow, subtropical, macrophyte-dominated lake. Journal of Paleolimnology 35 (1):129–48. doi:https://doi.org/10.1007/s10933-005-7881-0.
- Cadieux, S. B. 2015. Biogeochemical cycling of methane within adjacent closed-basin lakes on the margin of the Greenland Ice Sheet. Doctoral thesis, Indiana University, Bloomington, IN.
- Cadieux, S. B., J. R. White, P. E. Sauer, Y. Peng, A. E. Goldman, and L. M. Pratt. 2016. Large fractionations of C and H isotopes related to methane oxidation in Arctic lakes. Geochimica Et Cosmochimica Acta 187:141–55. doi:https://doi.org/10.1016/j.gca.2016.05.004.
- Cloern, J. E., E. A. Canuel, and D. Harris. 2002. Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnology and Oceanography 47 (3):713–29. doi:https://doi.org/10.4319/lo.2002.47.3.0713.
- Cohen, J., J. A. Screen, J. C. Furtado, M. Barlow, D. Whittleston, D. Coumou, J. Francis, K. Dethloff, D. Entekhabi, J. Overland, et al. 2014. Recent Arctic amplification and extreme mid-latitude weather. Nature Geoscience 7 (9):627–37. doi:https://doi.org/10.1038/ngeo2234.
- Colcord, D. E., S. B. Cadieux, S. C. Brassell, I. S. Castañeda, L. M. Pratt, and J. R. White. 2015. Assessment of branched GDGTs as temperature proxies in sedimentary records from several small lakes in southwestern Greenland. Organic Geochemistry 82:33–41. doi:https://doi.org/10.1016/j.orggeochem.2015.02.005.
- Colcord, D. E., A. Pearson, and S. C. Brassell. 2017. Carbon isotopic composition of intact branched GDGT core lipids in Greenland lake sediments and soils. Organic Geochemistry 110:25–31. doi:https://doi.org/10.1016/j.orggeochem.2017.04.008.
- Cole, J., Y. T. Prairie, N. F. Caraco, W. H. McDowell, L. Tranvik, R. G. Striegl, C. M. Duarte, P. Kortelainen, J. A. Downing, J. J. Middleburg, et al. 2007. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems (New York, N.Y.) 10:171–84. doi:https://doi.org/10.1007/s10021-006-9013-8.
- Downing, J. A. 2010. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 29 (1):9–24.
- Eadie, B. J., R. L. Chambers, W. S. Gardner, and G. L. Bell. 1984. Sediment trap studies in Lake Michigan: Resuspension and chemical fluxes in the southern basin. Journal of Great Lakes Research 10 (3):307–21. doi:https://doi.org/10.1016/S0380-1330(84)71844-2.
- Ehleringer, J. R., N. Buchmann, and L. B. Flanagan. 2000. Carbon isotope ratios in belowground carbon cycle processes. Ecological Applications 10 (2):412–22. doi:https://doi.org/10.1890/1051-0761(2000)010[0412:CIRIBC]2.0.CO;2.
- Farquhar, G. D., J. R. Ehleringer, and K. T. Hubick. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Biology 40 (1):503–37. doi:https://doi.org/10.1146/annurev.pp.40.060189.002443.
- Fink, P., L. Peters, and E. Von Elert. 2006. Stoichiometric mismatch between littoral invertebrates and their periphyton food. Archiv Für Hydrobiologie 165 (2):145–65. doi:https://doi.org/10.1127/0003-9136/2006/0165-0145.
- Funder, S. 1978. Holocene stratigraphy and vegetation history in the Scoresby Sund area, East Greenland. Grønlands Geologiske Undersøgelse Bulletin 129:1–65.
- Gälman, V., J. Rydberg, and C. Bigler. 2009. Decadal diagenetic effects on δ13C and δ15N studied in varved lake sediment. Limnology and Oceanography 54 (3):917–24. doi:https://doi.org/10.4319/lo.2009.54.3.0917.
- Goldman, A. E., S. B. Cadieux, J. R. White, and L. M. Pratt. 2015. Passive sampling method for high-resolution concentration and isotopic composition of dissolved methane in Arctic lakes. Limnology and Oceanography, Methods 14 (2):69–78. doi:https://doi.org/10.1002/lom3.10070.
- Guo, C., R. Ochyra, P. Wu, R. D. Seppelt, Y. Yao, L. Bian, S. Li, and C. Li. 2013. Warnstorfia exannulata, an aquatic moss in the Arctic: Seasonal growth responses. Climatic Change 119:407–19. doi:https://doi.org/10.1007/s10584-013-0724-5.
- Hájek, T., S. Ballance, J. Limpens, M. Zijlstra, and J. T. Verhoeven. 2011. Cell-wall polysaccharides play an important role in decay resistance of Sphagnum and actively depressed decomposition in vitro. Biogeochemistry 103 (1–3):45–57. doi:https://doi.org/10.1007/s10533-010-9444-3.
- Ho, E. S., and P. A. Meyers. 1994. Variability of early diagenesis in lake sediments: Evidence from the sedimentary geolipid record in an isolated tarn. Chemical Geology 112 (3):309–24. doi:https://doi.org/10.1016/0009-2541(94)90031-0.
- Jones, R. I., L. King, M. M. Dent, S. C. Maberly, and C. E. Gibson. 2004. Nitrogen stable isotope ratios in surface sediments, epilithon and macrophytes from upland lakes with differing nutrient status. Freshwater Biology 49:382–91. doi:https://doi.org/10.1111/fwb.2004.49.issue-4.
- Jørgensen, A. S., and F. Andreasen. 2007. Mapping of permafrost surface using ground-penetrating radar at Kangerlussuaq Airport, western Greenland. Cold Regions Science and Technology 48 (1):64–72. doi:https://doi.org/10.1016/j.coldregions.2006.10.007.
- Juutinen, S., M. Rantakari, P. Kortelainen, J. T. Huttunen, T. Larmola, J. Alm, J. Silvola, and P. J. Martikainen. 2009. Methane dynamics in different boreal lake types. Biogeosciences (Online) 6 (2):209–23. doi:https://doi.org/10.5194/bg-6-209-2009.
- Keeley, J. E., and D. R. Sandquist. 1992. Carbon: Freshwater plants. Plant, Cell, and Environment 15 (9):1021–35. doi:https://doi.org/10.1111/pce.1992.15.issue-9.
- Kelley, J. J. 1987. Carbon dioxide in the Arctic environment. Journal of Earth Sciences 35 (2):341–54.
- Kerby, N. W., and J. A. Raven. 1985. Transport and fixation of inorganic carbon by marine algae. Advances in Botanical Research 11:71–123.
- Kling, G. W., G. W. Kipphut, and M. C. Miller. 1991. Arctic lakes and streams as gas conduits to the atmosphere: implications for tundra carbon budgets. Science 251 (4991):298–301.
- Kip, N., J. F. van Winden, Y. Pan, L. Bodrossy, G. J. Reichart, A. J. Smolders, M. S. Jetten, J. S. Damsté, and H. J. Op den Camp. 2010. Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nature Geoscience 3 (9):617–21. doi:https://doi.org/10.1038/ngeo939.
- Körner, C., G. D. Farquhar, and S. C. Wong. 1991. Carbon isotope discrimination by plants following latitudinal and altitudinal trends. Oecologia 88 (1):30–40. doi:https://doi.org/10.1007/BF00328400.
- Kroken, S. B., L. E. Graham, and M. E. Cook. 1996. Occurrence and evolutionary significance of resistant cell walls in charophytes and bryophytes. American Journal of Botany 83:1241–54. doi:https://doi.org/10.2307/2446108.
- Kuzyakov, Y., and G. Domanski. 2000. Carbon input by plants into the soil: Review. Journal of Plant Nutrition and Soil Science 163 (4):421–31. doi:https://doi.org/10.1002/(ISSN)1522-2624.
- LaZerte, B. D., and J. E. Szalados. 1982. Stable carbon isotope ratio of submerged freshwater macrophytes. Limnology and Oceanography 27 (3):413–18. doi:https://doi.org/10.4319/lo.1982.27.3.0413.
- Lehmann, M. F., S. M. Bernasconi, A. Barbieri, and J. A. McKenzie. 2002. Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis. Geochimica Et Cosmochimica Acta 66 (20):3573–84. doi:https://doi.org/10.1016/S0016-7037(02)00968-7.
- Leng, M. J., and N. J. Anderson. 2003. Isotopic variation in modern lake waters from western Greenland. The Holocene 13 (4):605–11. doi:https://doi.org/10.1191/0959683603hl620rr.
- Leng, M. J., A. L. Lamb, T. H. Heaton, J. D. Marshall, B. B. Wolfe, M. D. Jones, J. A. Holmes, and C. Arrowsmith. 2006. Isotopes in lake sediments. In Isotopes in palaeoenvironmental research, ed. M. J. Leng, 147–84. Netherlands: Springer.
- Leng, M. J., and J. D. Marshall. 2004. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quaternary Science Reviews 23:811–31. doi:https://doi.org/10.1016/j.quascirev.2003.06.012.
- Liu, W., X. Li, Z. An, L. Xu, and Q. Zhang. 2013. Total organic carbon isotopes: A novel proxy of lake level from Lake Qinghai in the Qinghai-Tibet Plateau, China. Chemical Geology 347:153–60. doi:https://doi.org/10.1016/j.chemgeo.2013.04.009.
- Madsen, T. V., and S. C. Maberly. 2003. High internal resistance to CO2 uptake by submerged macrophytes that use HCO3−: Measurements in air, nitrogen and helium. Photosynthesis Research 77 (2):183–90. doi:https://doi.org/10.1023/A:1025813515956.
- Meehl, G. A., T. F. Stocker, W. D. Collins, P. Friedlingstein, A. T. Gaye, J. M. Gregory, A. Kitoh, R. Knutti, J. M. Murphy, A. Noda, et al. 2007. Global climate projections. In Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, ed. S. Solomon, D. Quin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, and H. L. Miller. Cambridge, UK: Cambridge University Press.
- Meyers, P. A. 1994. Preservation of the elemental and isotopic source identification of sedimentary organic matter. Chemical Geology 114:289–302. doi:https://doi.org/10.1016/0009-2541(94)90059-0.
- Meyers, P. A., and R. Ishiwatari. 1993. Lacustrine organic geochemistry: An overview of indicators of organic matter sources and diagenesis in lake sediments. Organic Geochemistry 20 (7):167–900. doi:https://doi.org/10.1016/0146-6380(93)90100-P.
- Meyers, P.A., and E. Lallier-Vergès. 1999. Lacustrine sedimentary organic matter records of late Quaternary paleoclimates. Journal of Paleolimnology 21 (3):345–72.
- Meyers, P. A., and J. L. Teranes. 2002. Sediment organic matter. In Tracking environmental changes using lake sediments, volume 2: Physical and geochemical methods, ed. W. M. Last and J. P. Smol, 239–69. Dordrecht: Springer.
- O’Leary, M. H. 1988. Carbon isotopes in photosynthesis. Bioscience 38 (5):328–36. doi:https://doi.org/10.2307/1310735.
- Olsen, J., N. J. Anderson, and M. J. Leng. 2013. Limnological controls on stable isotope records of late-Holocene palaeoenvironment change in SW Greenland: A paired lake study. Quaternary Science Reviews 66:85–95. doi:https://doi.org/10.1016/j.quascirev.2012.10.043.
- Olsen, J., K. H. Kjær, S. Funder, N. K. Larsen, and A. Ludikova. 2012. High-Arctic climate conditions for the last 7000 years inferred from multi-proxy analysis of the Bliss Lake record, North Greenland. Journal of Quaternary Science 27 (3):318–27. doi:https://doi.org/10.1002/jqs.1548.
- Osmond, C. B. 1981. Photorespiration and photoinhibition: Some implications for the energetics of photosynthesis. Biochimica Et Biophysica Acta (BBA)-Reviews on Bioenergetics 639 (2):77–98. doi:https://doi.org/10.1016/0304-4173(81)90006-9.
- Qiu, L., D. F. Williams, A. Gvorzdov, E. Karabanov, and M. Shimaraeva. 1993. Biogenic silica accumulation and paleoproductivity in the northern basin of Lake Baikal during the Holocene. Geology 21:25–28. doi:https://doi.org/10.1130/0091-7613(1993)021<0025:BSAAPI>2.3.CO;2.
- Raghoebarsing, A. A., A. J. Smolders, M. C. Schmid, W. I. C. Rijpstra, M. Wolters-Arts, J. Derksen, M. S. Jetten, S. Schouten, J. S. Sinninghe Damsté, L. P. Lamers, et al. 2005. Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 436 (7054):1153–56. doi:https://doi.org/10.1038/nature03802.
- Raven, J. A. 1970. Exogenous inorganic carbon sources in plant photosynthesis. Biological Reviews 45:167–221. doi:https://doi.org/10.1111/j.1469-185X.1970.tb01629.x.
- Rydberg, J., T. Lindborg, G. Sohlenius, N. Reuss, J. Olsen, and H. Laudon. 2016. The importance of eolian input on lake-sediment geochemical composition in the dry proglacial landscape of western Greenland. Arctic, Antarctic, and Alpine Research 48 (1):93–109. doi:https://doi.org/10.1657/AAAR0015-009.
- Sampei, Y., and E. Matsumoto. 2001. C/N ratios in a sediment core from Nakaumi Lagoon, southwest Japan: Usefulness as an organic source indicator. Geochemical Journal 35:189–205. doi:https://doi.org/10.2343/geochemj.35.189.
- Schwietzke, S., O. A. Sherwood, L. M. Bruhwiler, J. B. Miller, G. Etiope, E. J. Dlugokencky, S. E. Michel, V. A. Arling, B. H. Vaughn, J. W. White, et al. 2016. Upward revision of global fossil fuel methane emissions based on isotope database. Nature 538 (7623):88–91. doi:https://doi.org/10.1038/nature19797.
- Sobek, S., N. J. Anderson, S. M. Bernasconi, and T. Del Sontro. 2014. Low organic carbon burial efficiency in the arctic lake sediments. Journal of Geophysical Research-Biogeochemistry 119 (6):1231–43. doi:https://doi.org/10.1002/2014JG002612.
- Syväranta, J., H. Hämäläinen, and R. I. Jones. 2006. Within-lake variability in carbon and nitrogen stable isotope signatures. Freshwater Biology 51 (6):1090–102. doi:https://doi.org/10.1111/j.1365-2427.2006.01557.x.
- Turetsky, M. R. 2003. The role of bryophytes in carbon and nitrogen cycling. The Bryologist 106 (3):395–409. doi:https://doi.org/10.1639/05.
- UNEP/GRID-Arendal. 2006. Arctic vegetation zones Arendal, Norway Accessed July 10, 2015. http://www.grida.no/graphicslib/detail/arctic-vegetation-zones_b1d3#.
- Wetzel, R. G. 2001. Limnology, Lake and River Ecosystems. Academic Press. USA.
- Wik, M., R. K. Varner, K. W. Anthony, S. MacIntyre, and D. Bastviken. 2016. Climate-sensitive northern lakes and ponds are critical components of methane release. Nature Geoscience 9:99–105. doi:https://doi.org/10.1038/ngeo2578.
- Willemse, N. W., O. van Dam, P. van Helvoort, R. Dankers, M. Brommer, J. Schokker, T. E. Valstar, and H. de Wolf. 2004. Physical and chemical limnology of a subsaline athalassic lake in West Greenland. Hydrobiologia 524:167–92. doi:https://doi.org/10.1023/B:HYDR.0000036132.96154.01.
