ABSTRACT
In west Greenland, an approximate chronosequence of landscape evolution and weathering exists between the coast, which has been ice free for long periods, and more recently deglaciated areas along the present day ice margin. Traditional geochemical and isotopic analyses (δ18O, δ2H, 3H, δ34S/δ18O (SO4), and 87Sr/86Sr) along with novel isotopic tools, such as δ37Cl and δ81Br, were used to provide new insights into lake geochemical processes along a transect of lakes from the coast to the ice margin in the Kangerlussuaq region. Evaporation was found to be a key process impacting lake chemistry and isotopic signatures in the ice marginal area, with decreasing importance toward the coast. Evaporative processes were apparent in the δ37Cl and δ81Br isotopic signatures of lake-water chemistry. Consistent with previous work elsewhere (e.g., Blum and Erel, 1995) on increased biotite weathering in glaciated environments, 87Sr/86Sr isotopic ratios were found to be more radiogenic (>0.73) in lakes found in more recently glaciated terrain. Sulfide oxidation was the main source of sulfur (as sulfate) in lakes in the ice marginal area, while the influence of marine aerosols and bacterial sulfate reduction increased further away from the ice sheet around the fjord Kangerlussuaq. Groundwater discharge significant enough to impact lake chemistry was not observed in any of the lakes studied, suggesting that little groundwater–surface water interaction occurs in the study area or that recharge conditions are present in the majority of the lakes studied.
KEYWORDS:
Introduction
The geochemical composition of lake water reflects catchment geology, regional climate, vegetation, and soil development and, importantly, varies on a range of spatial and temporal scales (Kling et al. Citation2000). The interaction and dominance of these factors will vary over time as climate and vegetation successional states change. Landscape change over time, that is, ontogeny (Fritz and Anderson Citation2013; Leng et al. Citation2012), exerts strong controls on biogeochemical processing (Engstrom et al. Citation2000), but so does position in the landscape, which influences hydrological connectivity, both surface and groundwater (Johansson et al. Citation2015; Webster et al. Citation2000). At lower latitudes landscape perspectives of lake biogeochemistry have tended to focus on nutrients, in part because of catchment cultural disturbance (Kendall Citation1998; Wagner et al. Citation2011), and DOC, because of the dominance of lakes in boreal landscapes and the key role terrestrially derived dissolved organic carbon (DOC) plays in aquatic ecosystem functioning (Jansson et al. Citation2007). In many of these studies, the use of multiple isotopic tracers has proven invaluable in understanding landscape evolution, particularly in terms of chemical interactions.
Landscape position has been shown to strongly influence groundwater effect on lakewater chemistry, even in areas of low relief (Webster et al. Citation1996, Citation2000). In contrast to temperate and boreal zones, Arctic lakes have reduced terrestrial vegetation biomass, limited cultural impacts, and, importantly, permafrost strongly reduces groundwater hydrological connectivity. In areas of continuous permafrost, taliks provide one of the few pathways for groundwater–surface water interaction. Taliks are created in permafrost environments by lakes that do not freeze to the bottom during the winter, resulting in the warming of the ground around the lake bottom to temperatures above 0°C. Taliks have been documented beneath lakes in permafrost environments in both the Arctic (Burn Citation2002; Kokelj, Zijdlik, and Thompson Citation2009) and Antarctic (Cartwright and Harris Citation1981; Matsubaya et al. Citation1979). Subpermafrost waters may only receive recharge from lakes with taliks that fully penetrate the permafrost (through taliks) and from subglacial meltwaters where permafrost is absent. Subpermafrost groundwater discharge will occur in lakes with through taliks, or as discharge into the ocean (e.g., DeFoor et al. Citation2011).
As well as the overarching effects of low mean annual air temperature, climate can impact Arctic lakes directly through processes such as precipitation and evaporation, as well as indirectly through catchment inputs associated with chemical and physical weathering and impacts on biota (Anderson et al. Citation2001; Birks, Jones, and Rose Citation2004). Landscape relief also influences local air temperature and evaporation rates, which can strongly affect vegetation development within a catchment (Anderson Citation2014). The interplay between vegetation and local climate can influence hydrological retention time and hydrochemistry (Turner et al. Citation2014). In west Greenland these processes result in immediately adjacent lakes having contrasting major ion chemistry: evaporatively concentrated oligosaline lakes are interspersed among freshwater ones (Anderson et al. Citation2001; Johnson et al. Citation2000; Shand et al. Citation2007).
Isotopic tracers such as δ18O and δ2H, as well as 87Sr/86Sr and δ34S-δ18O (SO4), can reveal whether groundwater discharge is providing a significant component of the water balance within a lake catchment (Gibson, Birks, and Yi Citation2016; Gibson and Edwards Citation2002; Gibson et al. Citation2005; Hagerty and Webb Citation2008). Additional insight into lake evolution can be gained by using novel isotopic tools, such as δ37Cl and δ81Br (Eggenkamp Citation2014), in combination with more traditional geochemical and isotopic analyses (δ18O, δ2H, 3H, δ34S/δ18O (SO4), and 87Sr/86Sr). Use of these isotopic parameters has been applied elsewhere to provide increased understanding of evaporation (Gibson, Birks, and Yi Citation2016; Leng and Anderson Citation2003), glaciation/deglaciation (Blum and Erel Citation1997), weathering (Anderson, Drever, and Humphrey Citation1997; Blum and Erel Citation1997; Sharp, Creaser, and Skidmore Citation2002), aeolian transport (Hanlon Citation2015; Hanlon et al. Citation2017), marine inputs (McArthur et al. Citation1989; Negrel and Roy Citation1998; Wood and Sanford Citation1995), groundwater–surface water interaction (Bohlke and Denver Citation1995; Bullen and Kendall Citation1998; Hagerty and Webb Citation2008; Kendall and McDonnell Citation1998; Lyons et al. Citation1995; Negrel et al. Citation2003), and microbial processes (van Everdingen and Krouse Citation1985) in glaciated areas.
The Kangerlussuaq area of west Greenland is an excellent natural experimental area in which to address some of these controls on lake geochemistry at the landscape scale using stable isotopes. There are strong gradients in climate (both precipitation and temperature), which are reflected in both soil and vegetation differences as well as limnology, especially conductivity and DOC (Whiteford et al. Citation2016), with a number of closed-basin oligosaline lakes close to the head of the fjord (). Regional geology is relatively homogenous, but there is a gradient in lake age from the coast (formed >10,000 yr BP) to those adjacent to the present ice margin, which are approximately 4,000 years old, and very young lakes formed recently at the edge of the outlet glaciers. A final regionally variable process influencing geochemistry is dust deflation from the outwash plains and its deposition on lakes (Anderson et al. Citation2017). Here we use novel stable isotope analysis of samples from lakes covering the environmental gradients in the Kangerlussuaq area to provide insight into how lake chemistry evolves at 100–1,000-year timescales following deglaciation. Additionally, two boreholes drilled as part of the Greenland Analogue Project (GAP) provide information on the geochemical and isotopic composition of groundwaters in the study area (Claesson Liljedahl et al. Citation2016; Harper et al. Citation2016), allowing the extent to which groundwater–surface water interaction affects lake chemistry in the Kangerlussuaq region to be examined.
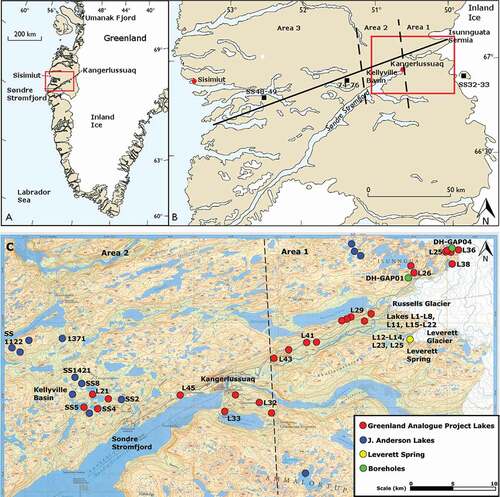
Figure 1. (A) Location of study area in the south west of Greenland. (B) Transect of studied lakes extending to coast. (C) Map detailing most heavily sampled area and indicating which lakes were sampled for the Greenland Analogue Project and which were sampled by Dr. John Anderson. The study area is divided into three regions: ice marginal (area 1), upper fjord (area 2), and coastal (area 3). (A) and (B) are adapted from Anderson and Brodersen (Citation2001)
Study area
The study area centers on the town of Kangerlussuaq (), located about 125 km inland from the west coast and 25 km west of the Greenland Ice Sheet and encompassing both a low-Arctic maritime climatic regime in the coastal region and a low-Arctic continental inland regime (Anderson et al. Citation2001). Inland, continuous permafrost may be greater than 300 m thick near the ice sheet (Harper et al. Citation2016; Kern Hansen Citation1990), while closer to the head of the fjord it is reported to be approximately 100–150 m thick (van Tatenhove and Olesen Citation1994). Toward the coast, permafrost becomes discontinuous around 50 km west of Kangerlussuaq (Weidick Citation1975). Mean annual temperature (measured 1977–2011) at Kangerlussuaq is −5.1°C and varies from −40°C in winter to 18–20°C in summer, with an annual precipitation of 173 mm (Cappelen Citation2012). In coastal areas, summer temperatures are cooler and mean annual precipitation is higher: 383 mm per year on the coast at Sisimiut (; long-term normal 1961–1990; Cappelen Citation2012).
The geology of the study area consists of Archean gneisses reworked in the Paleoproterozoic with an east-notheast structural trend. The area is structurally complex, having gone through several episodes of deformation (Engström and Klint Citation2014), with lakes often occurring along structural lineaments and within closed basins. The dominant rock type is quartzo-feldspathic gneiss that is quartz rich and commonly biotite bearing.
Previous studies have examined lake chemistry, paleolimnological history, and ecology in the Kangerlussuaq region (Aebly and Fritz Citation2009; Anderson et al. Citation2008, Citation2001; Anderson and Leng Citation2004; Bocher Citation1949; Eisner et al. Citation1995; Fredskild Citation1977; Fritz and Anderson Citation2013; Jensen Citation1889; Leng and Anderson Citation2003; Leng et al. Citation2012; McGowan, Ryves, and Anderson Citation2003; Olsen, Anderson, and Leng Citation2013; Williams Citation1991). In the present study, a series of lakes, from the coast to the ice front in the Kangerlussuaq region of southwest Greenland, were sampled ().
Lakes in this region are fresh (<1,000 mg/L) to brackish (1,000–10,000 mg/L) based on the classification of S.N. Davis (Krieger Citation1964). Most lakes have an electrical conductivity (EC) of less than 800 μS/cm. However, previous limnological studies in the region used a classification of less than 800 μS/cm (approximately 500 mg/L TDS) as dilute and more than 800 μS/cm as “oligosaline”; thus, saline is used in a relative sense when comparing the more brackish lakes to the dilute lakes (Anderson et al. Citation2001; Leng and Anderson Citation2003). The convention of referring to lakes with EC greater than 800 μS/cm as “oligosaline” will be used in this article (hereafter, saline). In general, the Kangerlussuaq region of Greenland lacks the very high TDS lakes (TDS > 10,000 mg/L) that may be found in other polar environments, such as the Canadian Arctic (Ouellet, Dickman, and Page Citation1989) and Antarctica (e.g., Lyons et al. Citation2002; Matsubaya et al. Citation1979), which were formed as the result of isostatic uplift and trapping of saltwater. In contrast, saline lakes in the Kangerlussuaq region are primarily closed-basin lakes where evaporation has concentrated salts in the lake water throughout the past 6,000–11,000 years since deglaciation (Anderson and Leng Citation2004).
Methods
Originally, lake samples were taken for the Greenland Analogue Project (GAP; see Claesson Liljedahl et al. Citation2016; Harper et al. Citation2016) in the area from the head of the fjord Kangerlussuaq (Søndre Strømfjord) and extending to the ice sheet (). Samples for the GAP were acquired between 2008 and 2013. The aim of the GAP was to study the impact of a modern ice sheet on the groundwater system. During the initial phase of the study, the lake sampling program was designed to examine the potential for groundwater discharge to, or recharge from, lakes in the periglacial area. Data collected as part of this study and the GAP include surface-water samples, subpermafrost groundwater samples, spring water samples, and samples of glacial meltwater taken from sub- and supraglacial flow at Isunnguata Sermia, Russell Glacier, and Leverett Glacier ().
Water samples from lakes collected for the GAP were generally taken from the shoreline, using nalgene bottles. Care was taken to sample in deeper locations to avoid poorly mixed shallow waters. In some instances, samples were taken at various depths, either from a boat or from holes drilled through ice (depending on the time of year). Sample depths are indicated in the sample name for samples not acquired from the surface. Samples taken at depth were acquired using a kemerer or van Dorn sampler. pH, conductivity, and lake temperature were measured in the field using a portable Oakton pH/conductivity meter. Cation samples were filtered (0.45 µm) and acidified in the field, while anion and isotope samples were untreated. Samples were refrigerated at approximately 4°C, except for brief periods during shipping.
Additional archived water samples were used to extend the study area and complete a transect of lakes from the ice sheet to the coast (). These samples consisted of historical samples (2001–2012) from previous studies in the region (see Anderson and Stedman Citation2007). Archived samples were from tightly sealed bottles, and the possibility of sample deterioration in these older samples was considered unlikely to be an issue, particularly for the isotopic analyses used in this study.
Two research boreholes installed for the GAP provided subpermafrost and talik groundwater samples with which to compare the surface waters (Harper et al. Citation2016). DH-GAP01 was drilled to intersect a talik beneath lake L26 () and is a total of 221.6 m long and 191 m deep (vertical depth). DH-GAP04 was drilled at the edge of the ice margin at the glacier Isunnguata Sermia. DH-GAP04 is 687 m long and has a vertical depth of 645 m. DH-GAP04 has three sampling sections between the base of the permafrost, at 400 m borehole length, and the bottom of the borehole. The lowest section (604.5–687 m borehole length) of the DH-GAP04 borehole is free of drilling water contamination, while the upper and middle sections still contained significant drilling water contamination (33 and 29 percent, respectively) at the time of sampling in 2014. Both δ18O and δ2H results presented here for the upper and middle sections of DH-GAP04 are calculated using a linear mixing model and drilling-fluid tracer concentrations (Claesson Liljedahl et al. Citation2016). The drill-fluid tracer allows the proportion of the sample that is drilling fluid, which has a known composition, to be determined and allows the unknown proportion and composition of the groundwater to be calculated (see Harper et al. Citation2016 for details). Other isotopic results for these sections (δ34S-δ18O of SO4, δ37Cl, δ81Br, and 87Sr/86Sr) can be considered representative of groundwater isotopic values because of much higher concentrations of these ions in the groundwaters compared to the very dilute solute composition of the drilling fluids.
The highly soluble mineral gypsum (CaSO4 • 2H2O) was found as an abundant fracture infilling mineral in the DH-GAP04 core, predominantly below 300 m of borehole length (Harper et al. Citation2016; Henkemans Citation2016; Pere Citation2014). Samples of fracture gypsum were obtained using dental tools under a binocular microscope and were analyzed for δ34S and δ18O of SO4 as well as 87Sr/86Sr. Sulfide minerals, pyrite (FeS2), and chalcopyrite ([CuFe]S2), from gneissic bedrock from the DH-GAP01 borehole were extracted and analyzed for δ34S.
Samples of salt crusts that had formed on soils near lake L21 (Hunde Sø; SS3 in the Anderson et al. Citation2001 numbering system) and lake L32 (Store Saltsø; SS17) were preserved in sample bags and analyzed by X-ray diffraction (XRD). XRD was used to determine what salts formed these crusts.
Isotopic and geochemical analyses
Isotope analyses were performed at the University of Waterloo Environmental Isotope Laboratory. Tritium preanalysis was determined using the electrolytic enrichment method of Taylor (Citation1977). After enrichment, samples were counted in a LKB Wallac 1220 Quantalus liquid scintillation counter with a detection limit of 0.8 ± 0.8 TU. Deuterium determinations were made following the Cr reduction method outlined by Morrison et al. (Citation2001) and were analyzed on an Isoprime IRMS coupled to a Eurolectron elemental analyzer. Oxygen isotope analysis was performed on an IsoPrime continuous flow isotope ratio mass spectrometer (CF-IRMS), using the preparation procedures of Epstein and Mayeda (Citation1953) with Moser’s modification (Moser Citation1977). Results for δ18O and δ2H are reported based on standard corrections to VSMOW (Vienna Standard Mean Ocean Water) and VSLAP (Vienna Standard Light Antarctic Precipitation) from the International Atomic Energy Agency (IAEA). Analytical reproducibility of δ18O and δ2H are ±0.2‰ and ±0.8‰, respectively.
Sulphate is extracted using BaCl2, converted to SO2 by combustion with Nb2O5, and analyzed on a Micromass IsoChrom-IRMS. For analysis of 18O in SO42-, the BaSO4 is combusted to produce CO2 and is analyzed in a GVI IsoPrime-IRMS (±0.5‰; Morrison Citation1997). Both 18O and 34S are corrected to BaSO4 IAEA-SO5, IAEA-SO-6, and NBS-127.
Chlorine and bromine stable isotope determinations are performed by first precipitating Cl− or Br− as AgCl or AgBr using AgNO3. Analyses for 37Cl and δ81Br are carried out on CH3Cl and CH3Br, respectively, after reacting the silver chloride/bromide with methyl iodide. Both 37Cl and δ81Br were analyzed using continuous flow technology on a Micromass IsoPrime IRMS (±0.2‰), using the methods outlined in Eggenkamp (Citation1994), Kaufmann et al. (1984), and Shouakar-Stash et al. (2005a, 2005b). The reference material used for δ37Cl analysis is Standard Mean Ocean Chloride (SMOC), and that used for δ81Br analysis is Standard Mean Ocean Bromide (SMOB).
Analysis of 87Sr/86Sr isotopic ratios was performed using thermal ionization mass spectrometry (TIMS). Samples are passed through a strontium-specific resin. The isolated strontium is loaded onto a double rhenium filament and analyzed using a Thermo Finnegan Scientific TRITON TIMS and calibrated against the NBS international standard material NIST SRM 987 (Dicken Citation2000).
Geochemistry samples were analyzed at Labtium Oy (via Geological Survey of Finland) in 2008 and 2010–2013 and at the TVO (Teollisuuden Voima Oy) laboratory in Finland in 2009. Geochemical analyses were performed using the following methods at Labtium Oy: alkalinity was measured using a titrimetric determination, anions were measured by Ion Chromatography (IC), and cation multielement determination was performed by a combination of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES). At TVO, multielement analyses were performed using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) and ICP-MS analysis. Results with charge balances exceeding 10 percent were discarded, with the exception of the sample from lake L20 (01/07/2008), which had a charge balance error of −12.5 percent. Both Cl− and SO42- concentrations from the 2008 L20 sample (L20-1) are used in order to include L20 δ34S and δ18O (SO4) isotopic values in and . Sampling and analytical methods for historical samples are documented in Anderson et al. (Citation2001).
Results
Geochemical
The dilute lakes were generally of Ca or Mg -HCO3 or of Ca or Mg-HCO3-SO4 type, while the more saline lakes tended to be of Na or Mg-HCO3, Cl-type waters. A small subset of lakes contained a high percentage of anions as sulfate: lake L20 from the GAP and lakes SS70–76 in Anderson et al. (Citation2001). Lake L20 was highly acidic, with a pH of 3.5, whereas the majority of lakes had a pH greater than 7.
Lakes were divided into four categories based on size, salinity, and water source. (1) Ponds are lakes that are approximately 2 m deep or less, and may evaporate completely in the summer or freeze to the bottom in the winter. Ponds may show seasonal salinity variations because of evaporation. For example, lake L20 had an electrical conductivity of 354 µS/cm when sampled in early June 2008, and 1,750 µS/cm when sampled in early September 2010. Lake L20 had visibly shrunk by a significant amount when sampled in 2010. (2) Dilute lakes are those that were unlikely to freeze to the bottom in winter (>2 m deep) and had conductivities of less than 800 µS/cm, following the classification of Anderson et al. 2001. (3) Saline lakes were also of sufficient size to not freeze to the bottom in winter and had conductivities of more than 800 µS/cm. In general, saline lakes are located in closed basinal settings with no visible outflow. (4) Meltwater/thaw lakes include glacial meltwater-fed lakes (e.g., lake L6) and thaw lakes that derive water from the melting of frozen till and precipitation. Thaw lakes include lakes L12–14 and L23–24, all of which are located on the till plain in front of Leverett Glacier (). While the thaw lakes are likely to be more geochemically evolved than the meltwater-fed lakes, they are similar in terms of low conductivity and depleted δ18O/δ2H isotopic signatures () and access to relatively fresh glacial sediment. Meltwater and thaw lakes tend to plot together in many of the figures.
Table 1. Summary of major geochemical and isotopic results. Alkalinity is expressed as mmol/L as CaCO3. Meltwater lakes, lakes that receive meltwater directly, and the thaw lakes on the Leverett Glacier till plain are separated here to illustrate the similarity between these two groups
A summary of average, minimum, and maximum values for concentrations of dissolved species, EC, pH, alkalinity, as well as a subset of isotopic results are presented by group (ponds, dilute lakes, saline lakes, and meltwater/thaw lakes) in . presents results for a representative subset of lakes with chemistry typical of each of the areas described in (ice marginal, upper fjord, and coastal).
Table 2. Summary of representative chemistry and isotopic results. P = pond, DL = dilute lake, SL = saline lake, and MW = meltwater lake. *Indicates that the results for dissolved chemical species are average results throughout multiple years as measured by John Anderson (ex., Anderson et al. Citation2001). Alkalinity is expressed as mg/L as CaCO3. NM = not measured
Isotopic results
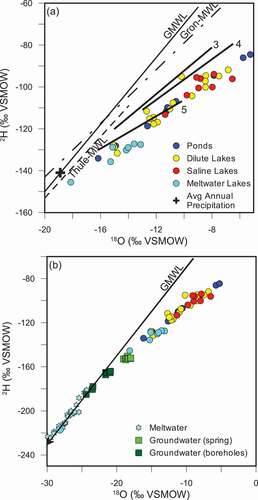
When considering the isotopes of water, lakes fall along local evaporation lines (LELs) similar to those described by Leng and Anderson (Citation2003) for lakes close to the ice (zone 5) and lakes around the upper fjord (zone 4; . Meltwater lakes tend to show greater isotopic depletion and fall along an evaporation line with lower deuterium-excess (D-excess) relative to the LELs described by Leng and Anderson (Citation2003; . The precipitation average of δ18O was estimated to be −19‰ by Leng and Anderson (Citation2003), based on the intercepts of the LELs with the GMWL and Kangerlussuaq’s position between the IAEA-monitoring stations at Grønnedal and Thule. A snowfall sample taken July 1, 2009, near the ice sheet had a δ18O of −20.16 ‰ and a tritium concentration of 13.8 TU. Lakes had a range of tritium concentrations from 6.6 to 13.2 TU (mean of 9.6 TU for all lakes). Average, minimum, and maximum tritium concentrations are broken down for each lake grouping in .
Figure 2. (A) Isotopic composition of lakes. Local evaporation lines are adapted from Leng and Anderson (Citation2003) for zones 3 (mid fjord), 4 (upper fjord), and 5 (close to ice sheet). The zones from Leng and Anderson (Citation2003) correspond to area 3 (zone 3), area 2 (zone 4), and area 1 (zone 5). Average annual precipitation estimated from intercept of LEL with GMWL. Local meteoric water lines for Grönnedal and Thule were generated using isotopic monitoring data from IAEA stations. (B) Isotopic composition of lakes compared to groundwaters and meltwaters from Kangerlussuaq region
The 87Sr/86Sr isotopic ratios in lakes have a large range of values from 0.7154 to 0.7580 (subset of results in ). Both δ37Cl and δ81Br are measured against standard mean oceanic chloride (SMOC) and bromide (SMOB), respectively, and thus, marine chlorine and bromine have isotopic signatures of 0‰. Fifteen lakes were analyzed for δ37Cl, which ranged from −0.41‰ to 0.04‰ (). Six lakes were analyzed for δ81Br, which ranged from −0.06‰ to 1.76‰ ().
Table 3. Results of δ37Cl and δ81Br analyses on lakes and borehole groundwaters. Analytical uncertainty on δ37Cl and δ81Br is ±0.2‰. Locations with * next to the name indicate average lake chemistries. NM = not measured
Groundwaters tend to be of Ca-Na-SO4 type and are depleted in δ18O and δ2H relative to lake waters. DH-GAP01 intersects the talik beneath lake L26. Pressure measurements indicating a downward gradient in hydraulic head suggest recharge conditions in the talik (Johansson et al. Citation2015). Both δ18O and δ2H isotopic values that are more enriched than the DH-GAP04 groundwaters and plot below the GMWL further support the recharge and mixing of lake water into the talik (). Groundwater from DH-GAP04 indicates that subpermafrost groundwater at the ice margin is brackish to a depth of 450 m and contains concentrations of sulfate up to 1,900 mg/L and Cl concentrations of 176 mg/L. The 87Sr/86Sr ratios of groundwater (0.7033–0.7075) reflect that of fracture minerals, such as gypsum (0.7023–0.7080), and are less radiogenic than surface waters (0.7154–0.7580; Henkemans Citation2016).
Lake sulfate values have a much larger range of δ34S and δ18O (SO4) values than groundwaters or sulfide and sulfate minerals. Full results for δ34S and δ18O (SO4) are given in .
Table 4. Isotopic composition and concentration of sulfate in surface waters and groundwaters. Sulfate minerals (predominantly gypsum) from fracture infillings and sulfide minerals from the rock matrix are included from the DH-GAP01 and DH-GAP04 cores. Gypsum was found abundantly as a fracture filling below a depth of 300 m in the DH-GAP04 borehole, while only one occurrence of gypsum was noted in the DH-GAP01 borehole. NM is not measured because of insufficient sample material. NA = not applicable
Salt crusts collected from the vicinity of L21 (Hunde Sø) and L32 (Store Saltsø) were analyzed by X-ray diffraction. Salts around lake L21 included antarcticite (CaCl2 • 6H2O), calcite (CaCO3), and hydrohalite (NaCl • 2H2O), while around L32 (Store Saltsø) the salt crust was composed primarily of gypsum (CaSO4 • 2H2O).
Discussion
The lakes sampled represent a transect () from the coast north of the fjord Kangerlussuaq to the ice sheet east of Kangerlussuaq. The transect represents both a climatic gradient from maritime to continental interior (Anderson et al. Citation2012; Leng et al. Citation2012) as well as a chronosequence of lake and lake catchment development since glacial retreat (Fritz and Anderson Citation2013). The lakes along this transect can be roughly divided into the three areas shown on . The first (area 1) is from the ice margin to the head of the fjord (ice marginal). The second (area 2) is the area around the head of the fjord that Leng and Anderson (Citation2003) termed “upper fjord,” and the third (area 3) is coastal and covers from the head of the fjord to the west coast ().
From the coastal to ice marginal areas, the processes that affect the chemical evolution of lakes vary in importance. Evaporation decreases toward the coast as humidity increases (Anderson et al. Citation2012; Leng and Anderson Citation2003). Weathering rates are assumed to decrease in the less vegetated, cooler, and dryer areas close to the ice sheet (Anderson and Brodersen Citation2001; Anderson, Drever, and Humphrey Citation1997). Microbial activity, such as that of sulfate-reducing bacteria, increases in the warmer temperatures around the upper fjord. Toward the coast there is increased influence of marine aerosols (Anderson et al. Citation2001). These processes and others are discussed in detail further on.
Evaporation and salts
The influence of distance from the coast, which controls marine inputs and the relative influence of evaporation versus precipitation, is apparent in several isotopic systems related to lake-water composition (Leng and Anderson Citation2003). Anderson et al. (Citation2001) indicate that the change to negative effective precipitation occurs approximately halfway between the coast and head of the fjord (). Together with the balance between evaporation and precipitation, the presence of a lake outlet appears to be the main control on lake salinity. Lakes can be isolated during periods of low precipitation as lake levels fall and eventually become cut off from drainage routes, forming closed basin lakes where salinity increases over time because of evaporation (Aebly and Fritz Citation2009; Anderson et al. Citation2001; Leng and Anderson Citation2003; Williams Citation1991). Evaporation in closed-basin lakes is an important source of salinity elsewhere in Greenland (Williams Citation1991) and in the McMurdo Dry Valleys, Antarctica (Gooseff et al. Citation2006; Matsubaya et al. Citation1979).
Evaporation is a major control of the isotopic signature (δ18O/δ2H) of lakes in the region. Leng and Anderson (Citation2003) analyzed δ18O and δ2H in lakes from the coast to the ice sheet and found that lakes fall along LELs, which vary in slope by region (). Coastal lakes have an LEL with a slope (s = 5.4–5.5) closest to the slope of the GMWL (s = 8) because of higher humidity (Gibson, Birks, and Yi Citation2016), while in the dry, low precipitation regions close to the ice sheet, the slope was much lower (s = 3.9). Continental climates at high latitudes can produce highly evaportive signatures because of pronounced evaporation under arid conditions during the seasonal thaw period (Gibson, Birks, and Yi Citation2016). Depending on the volume of precipitation, surface area, and lake depth, the δ18O isotopic signature can vary annually by more than 2‰ (Leng and Anderson Citation2003). The lakes sampled for the GAP show similar trends to those sampled by Anderson et al. (Citation2001; . Evaporative effects are greatest in the ice-marginal lakes (area 1) and decrease toward the coast (area 3). Lakes for the GAP were sampled in the ice-marginal (area 1) and upper fjord (area 2) regions, which coincide with areas 4 and 5 in Leng and Anderson (Citation2003; .
The majority of lakes whose main water source is glacial meltwater have a lower D-excess and plot below the LEL for the ice-marginal lakes (area 1; shown as line 5 in , because the initial source of water for the meltwater lakes would have the more depleted isotopic signature observed for meltwaters (Figure 2B; Gibson, Birks, and Yi Citation2016).
Evaporation in shallow areas around lakes or from soils can lead to salt-crust formation. An XRD analysis of salt crusts found around lakes L21 and L32 showed the presence of gypsum as well as antarcticite and hydrohalite: chloride salts that occur in cold-climate conditions. Salt crusts may be redissolved during precipitation events or snowmelt, contributing to lake salinity. Salts may also be removed by wind and transported out of the lake catchment basin.
The stable isotopic ratio of chlorine (chloride), 37/35Cl, can be used to identify sources of chloride salts and processes. Chlorine-37 is preferentially incorporated into the solid phase during halite (or other chloride salt) precipitation, causing the residual solution to become progressively lighter as halite precipitates (Magenheim et al. Citation1995; Wood, Sanford, and Frape Citation2005). In a closed system, where halite is precipitated and then redissolved, the δ37Cl signature should not change significantly from the initial value. In the case of the lakes in the Kangerlussuaq region, Cl− is likely to be of marine origin from sea-salt aerosols and have a value of approximately 0‰ (Eggenkamp Citation1994; Eggenkamp, Kreulen, and Koster van Groos Citation1995; Zhang et al. Citation2007). In an open system, for example, if halite is removed by aeolian activity, δ37Cl should be depleted relative to the marine value, yielding negative isotopic signatures. Alternatively, lakes with positive δ37Cl values may be receiving δ37Cl-enriched chloride, deflated from halite precipitated from waters whose initial chloride input was marine.
δ81Br follows the opposite trend to δ37Cl, becoming more enriched in the residual solution (Hanlon Citation2015; Hanlon et al. Citation2017). In the Sand Hills of Nebraska, δ81Br was found to be more enriched than δ37Cl in the waters, while δ37Cl in salt crusts was found to be more enriched than δ81Br. δ37Cl in salts crusts was slightly enriched relative to δ37Cl in waters, while δ81Br in salt crusts was depleted relative to the waters (Frape, Stotler, and Harvey Citation2013; Hanlon Citation2015; Hanlon et al. Citation2017).
Lakes analyzed for δ37Cl and δ81Br are within areas 1 and 2, because the dilute coastal lakes (area 3) had insufficient concentrations of halides to perform the analyses. Both the ice-marginal and upper fjord areas were predicted to show isotopic depletion of δ37Cl values and enrichment in δ81Br because of evaporation (Frape, Stotler, and Harvey Citation2013; Magenheim et al. Citation1995; Wood, Sanford, and Frape Citation2005). Two trends in the δ37Cl-δ18O isotopic results are indicated on . The first trend (1) describes the evaporative enrichment of δ18O, which is correlated with the depletion of δ37Cl. The second group (2) of samples includes two saline and one dilute lake (L41, SS4, and 1122), maintaining a marine δ37Cl isotopic signature (0 ‰) despite evaporative enrichment of δ18O. However, lakes L41, SS4, and 1122 do have enriched δ81Br values, indicative of salt deflation ().
Figure 3. (A) δ37Cl compared to δ18O isotopic composition. δ81Br values compared to (B) δ18O isotopic values and (C) δ37Cl isotopic values. Analytical error corresponds to symbol size except where otherwise indicated by error bars
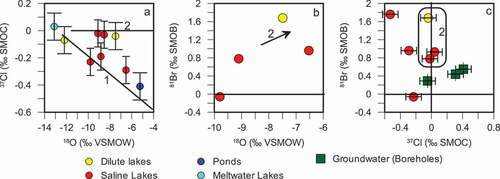
The majority of lake samples analyzed for δ81Br are positive, as predicted (see . However, a comparison plot of the δ37Cl and δ81Br results shows a poor correlation. That is, the most depleted δ37Cl values do not necessarily correspond to the most enriched δ81Br values (). It is clear that the impact of evaporation on δ37Cl and δ81Br signatures is complex and the limited data provided here are only a first attempt at understanding δ37Cl and δ81Br processes in Arctic lake environments.
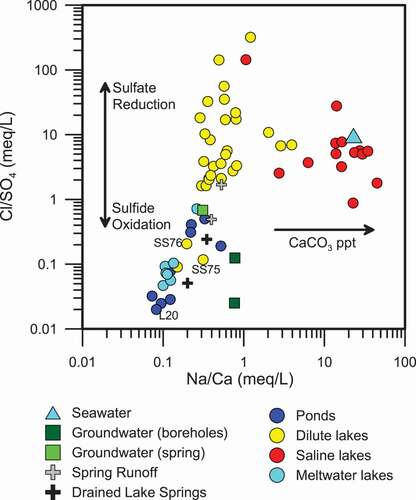
Saline lakes tend to have excess Na+ versus Ca2+ when compared to dilute lakes (). As lake salinity increases through evaporation or by salt exclusion during winter ice formation, CaCO3 saturation may be reached and CaCO3 will precipitate, removing Ca2+ and increasing the Na/Ca ratio. CaCO3-rich layers can be observed in lake cores around the fjord Kangerlussuaq (Anderson et al. Citation2002, Citation2001; Bennike Citation2000; McGowan, Ryves, and Anderson Citation2003), supporting the precipitation of CaCO3 as a mechanism for Ca2+ depletion in saline lakes. Anderson et al. (Citation2002) also suggest that CaCO3 precipitation may occur during the summer months when pH is increased because of photosynthesis.
Weathering and water rock interaction (strontium isotopes)
The 87Sr/86Sr isotopic ratios can be a useful tool for tracing the sources of salinity, because the isotopes do not fractionate during mineral dissolution and precipitation (Frape et al. Citation2014; McNutt et al. Citation1990). Thus, the 87Sr/86Sr isotopic ratio in water will directly reflect the 87Sr/86Sr isotopic ratios of the sources of strontium, such as weathering, sea-salt aerosols, or mixing with another water source (i.e., groundwater discharge). In Antarctica, 87Sr/86Sr isotopic ratios in lake waters were often observed to be similar to the 87Sr/86Sr isotopic ratios found in soil salts, derived from the weathering of silicates in the nearby bedrock and regolith (Jones and Faure Citation1978). Green and Canfield (Citation1984) demonstrated that the Onyx River derived a significant fraction of its salts from interaction with soils in the Wright Valley, Antarctica.
Research concerning strontium in recently exposed glacial and proglacial environments suggests that biotite weathering may have a strong influence on 87Sr/86Sr ratios (Anderson, Drever, and Humphrey Citation1997; Blum and Erel Citation1997; Sharp, Creaser, and Skidmore Citation2002). Generally, during chemical weathering, feldspars, specifically plagioclase and K-feldspar, are initially weathered (Grant Citation1963; Nesbitt and Young Citation1996). However, in glacial environments, where biotite is enriched in fine-grained sediment, biotite weathering occurs rapidly and then decreases during a relatively short time scale (10 ky) because of the loss of reactive mineral surfaces (Anderson, Drever, and Humphrey Citation1997; Blum and Erel Citation1995, Citation1997; Eggleston, Hochella, and Parks Citation1989; Nesbitt and Young Citation1996; Taylor and Blum Citation1995). The large range of 87Sr/86Sr isotopic ratios covered by ice-marginal lakes represents the relative influence of various mineral controls, such as biotite weathering versus feldspar (Blum and Erel Citation1997; McNutt et al. Citation1990).
A plot comparing the 87Sr/86Sr isotopic ratio in lake waters with increasing distance from the coast (shown as Eastings, which represents the distance east from the coast toward the ice margin) reveals that lakes become increasingly radiogenic with distance from the coast (). The regional geology in the Kangerlussuaq area is dominated by felsic and intermediate gneisses and amphibolite facies mafic intrusions. These rock types are rich in biotite and feldspars and provide a potential source for a radiogenic strontium signature. The 87Sr/86Sr isotopic signature of the ice-marginal lakes exceeded 0.73 and is dominated by weathering of soil and rock material recently released from the melting ice. Many of these lakes, surrounded by relatively fresh glacial sediment, can have a highly radiogenic strontium isotope signature approaching 0.76 (). For example, lakes L12, L13, and L14 are located in nonvegetated till in front of Leverett Glacier () and have 87Sr/86Sr ratios of 0.7533 and 0.7576, respectively. The input of recently thawed, fine sediments transported as aeolian dust into many lakes near the ice margin would also rapidly increase the radiogenic signature.
Figure 5. The 87Sr/86Sr signature of lakes increases toward the ice sheet where weathering of biotite becomes increasingly important
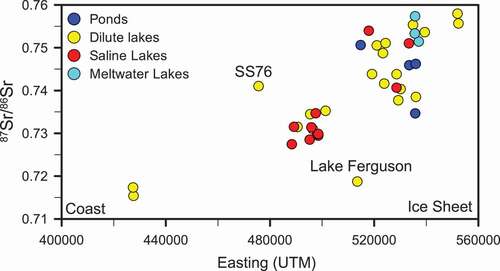
Aeolian dust can provide a significant chemical flux, especially in glacial environments where a large amount of sediment with small particle size is available (Lamoureux, Gilbert, and Lewis Citation2002; Lawrence and Neff Citation2009). The rate of dust deposition has been shown to be greatest during glacial maxima (Lambert et al. Citation2008), although the Kangerlussuaq region would have been covered in ice at this time. The mineralogical composition of dust can be variable, depending on local geology, but generally contains silicate minerals such as quartz, feldspars, and phyllosilicates (Schütz and Sebert Citation1987). Winds in the Kangerlussuaq area are dominantly katabatic winds from the east that come off the ice sheet; however, coastal storms from the west do occur (Aebly and Fritz Citation2009). Easterly winds coming from the ice sheet pick up dust from the exposed glacial sediments, the ice surface, and large sand flats present in front of the ice sheet (personal observation). Aolian deposits were found to overlay glacial till over much of the L26 lake catchment (Johansson et al. Citation2015). It is likely that there will be increased inputs of aeolian dust to lakes in the ice-marginal area as glaciers retreat, leaving more extensive areas of unvegetated glacial deposits, and a corresponding increase in the input of radiogenic strontium to lakes.
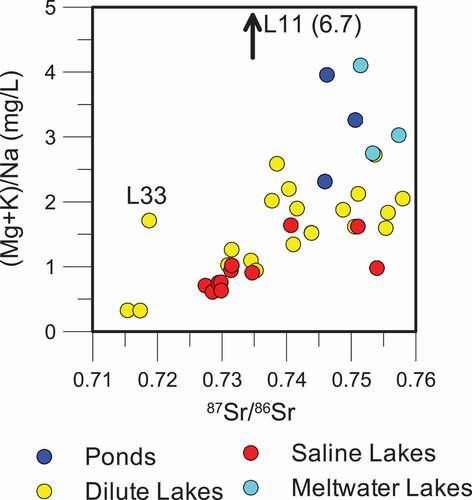
In the upper fjord and coastal areas, highly radiogenic 87Sr/86Sr ratios are not observed. In these older, more weathered or geologically mature landscapes, the influence of feldspar weathering, marine aerosols, and/or deflated marine sediments, with much less radiogenic 87Sr/86Sr isotopic signatures, becomes more significant (). Overall, a trend of decreasing 87Sr/86Sr can be observed with distance from the ice sheet (), reflecting the reduced impact of radiogenic mineral input and weathering with time since deglaciation (Blum and Erel Citation1997), and an increased marine input closer to the coast. The influence of biotite weathering on 87Sr/86Sr ratios can also be observed on a plot of (Mg2+ + K+)/Na+ (). Biotite weathering will contribute Mg2+ and K+ to the lake chemistry, while feldspar weathering and marine inputs will contribute Na+. As a result, a pattern of increasing Mg2+ and K+ relative to Na+ with increasingly radiogenic 87Sr/86Sr values is observed. Ponds and meltwater lakes show a large range of high (Mg2+ + K+)/Na+ ratios and radiogenic 87Sr/86Sr isotopic ratios, suggesting that biotite weathering is a key process for these water bodies. Two outliers exist in : L33 (Lake Ferguson) is impacted by marine 87Sr/86Sr, as discussed previously. Lake L11 had high (Mg2+ + K+)/Na+, possibly a result of removal of Na+ through halite precipitation or because of local geology (mafic material in the surrounding rock).
Figure 6. Comparison of the ratio of (Mg + K)/Na to 87Sr/86Sr isotope ratios for all lake types. The ratio of (Mg + K) to Na increases with increasingly radiogenic 87Sr/86Sr isotopic ratios
It has been proposed that increased weathering of glacially comminuted sediments during continental scale glaciations may have affected the marine 87Sr/86Sr record during short and longer time periods (Anderson, Drever, and Humphrey Citation1997; Hodell, Mueller, and Garrido Citation1991; Zachos et al. Citation1999). With an increase in glacial melting because of climate warming and the potential for enhanced geochemical loading to the oceans, further understanding of processes that affect 87Sr/86Sr in this proglacial environment will aid in the study of the interaction between the marine 87Sr/86Sr signature and glaciation.
Sulfur oxidation and reduction
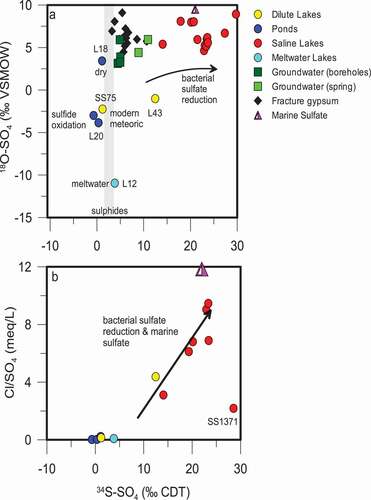
The variability in δ34S and δ18O of sulfate () in the lakes of the Kangerlussuaq region could be indicative of several processes: sulfide oxidation, sulfate reduction, and mixing with marine sulfate. Sulfide oxidation may cause a slight depletion of sulfur-34 (approximately 2–5.5‰) in the resulting sulfate (Toran and Harris Citation1989), or may result in no discernible fractionation between sulfide and sulfate (Gavelin, Parwel, and Ryhage Citation1960; Nakai and Jensen Citation1964; Seal, Alpers, and Rye Citation2000). Sulfate reduction that is mediated by sulfate-reducing bacteria causes enrichment in the remaining δ34S (SO4), because the bacteria preferentially use the lighter sulfur isotopes because of their lower activation energy, which maximizes the energy yield for bacterial metabolic processes (Clark and Fritz Citation1997; Kaplan and Rittenberg Citation1963). Finally, marine sulfate may be a significant source of sulfate close to the coastal regions (area 3) and the head of the fjord (area 2).
Figure 7. (A) the isotopic composition (δ34S and δ18O) of sulfate in lakes and groundwaters as well as gypsum found as a fracture mineral in the bedrock. δ34S of sulfide minerals found in the bedrock are indicated by the stippled area. (B) A comparison of the δ34S of sulfate in lakes to the Cl/SO4 ratio shows that as δ34S is enriched during bacterial sulfate reduction, sulfate is removed and the Cl/SO4 ratio increases
Oxidation of sulfides
The δ18O of the sulfate produced during sulfide oxidation is a mixture of atmosphere δ18O (+23‰) and the δ18O of the water present during oxidation. The relative importance of atmospheric δ18O versus water δ18O depends on the degree of water saturation. With increasing saturation, the δ18O isotopic signature of the sulfate will more closely resemble that of the water, while in relatively dryer conditions the δ18O isotopic signature of the sulfate will more closely resemble that of the atmospheric O2 (Clark and Fritz Citation1997; van Everdingen and Krouse Citation1985).
Sulfide minerals, including pyrite and chalcopyrite, from the bedrock core obtained during drilling of the research borehole DH-GAP04 (), have a δ34S of from 2.3‰ to 3.7‰ (Figure 7A; ). A dilute lake (lake SS75) and a large, acidic (pH 3.4) pond (lake L20) have δ34S-δ18O signatures, indicative of sulfate produced during the oxidation of sulfide in the presence of modern meteoric water and atmosphere (). That is, the δ34S (SO4) values of lake 20 and lake SS75 (−3.0‰ and −3.9‰, respectively) are slightly depleted relative to that of the sulfides (2.4‰ and 3.7‰). The δ18O (SO4) of lake 20 and lake SS75 (−0.7‰ and 1.2‰, respectively) reflects the oxidation of sulfide in the presence of meteoric water (~ −19‰) and atmospheric oxygen (+23.5‰; van Everdingen and Krouse Citation1985). Lakes L20, SS75, and SS76 have low Cl/SO4 ratios (), high SO42- as percentage of anions, and low alkalinity. Local geology is likely responsible for these anomalous lakes. Anderson et al. (Citation2001) observed highly weathered and friable orange-reddish rocks (gossans) on the slopes around lakes SS75 and SS76, which are presumed to be similar to the weathered sulfur-rich rocks that were observed around lake L20. Sulfide oxidation generates H+, resulting in low alkalinity and low pH (lake L20 had a pH of 3.5). Weathering of these sulfide-rich rocks would contribute increased sulfate concentrations (lower Cl/SO4 ratios; see . The mineral jarosite (KFe3(OH)6(SO4)2) was also observed in gossans in the Kangerlussuaq region (Pratt and Peng Citation2014), which may also impact the isotopic signature of sulfate in adjacent lakes.
A thaw lake located within the till at the front of Leverett Glacier (L12) has a similar δ34S (SO4) and depleted δ18O (SO4) to the lakes indicated earlier, where sulfide oxidation occurred in the presence of modern meteoric water (). In the case of L12, the δ18O (SO4) is a mixture between glacial meltwater or melt from frozen till, which has highly depleted δ18O and atmospheric oxygen, resulting in sulfate with depleted δ18O relative to sulfate produced from modern precipitation (Clark and Fritz Citation1997; van Everdingen and Krouse Citation1985). In other cases, such as L18 (δ18O (SO4) = 3.4‰) where high evaporation rates enrich the δ18O of the water (δ18O = −5.2‰) and sulfide oxidation may occur under relatively dry conditions, an enriched δ18O (SO4) signature was observed ().
A similar effect on the δ34S and δ18O of SO42- as that produced by the oxidation of inorganic sulfide minerals may be caused by oxidation of organic sulfur compounds (Canfield Citation2001; Clark and Fritz Citation1997). However, the extent of SO42- derived from organic sulfur may be limited in the ice-marginal area where vegetation is fairly sparse, and cold, dry conditions limit productivity.
Sulfate reduction and marine sulfate
Sulfide oxidation is the main process affecting lakes in close proximity to the ice sheet (area 1). Further from the ice sheet, in the upper fjord area (area 2), sulfate-reducing bacteria and sea spray have a significant effect on lake chemistry and the isotopic composition of sulfate. Sulfate-reducing bacteria (SRB) have been observed in other cold-climate areas, such as Antarctica, where SRB were observed in lakes in the McMurdo Dry Valleys (Green, Angle, and Chave Citation1988) as well as in the Canadian Arctic (Burton and Barker Citation1979). Leng et al. (Citation2012) theorized that in a coastal lake (area 3), sulfur was derived from both the catchment (oxidation of pyrite) and sea-salt aerosols, and sulfate reduction was identified as an important process.
The effects of both marine sulfate and sulfate-reducing bacteria can be seen on a plot comparing the Cl/SO4 ratio and δ34S (SO4; . Bacterial sulfate reduction removes sulfate from lakes by reducing SO42- to H2S, increasing the Cl/SO4 ratio and enriching the remaining sulfate in δ34S (SO4). The H2S may then react with metal ions to precipitate metal sulfides such as FeS. H2S may also be utilized by purple sulfur bacteria, observed in the Kellyville Basin lakes (), which oxidize the sulfide to elemental sulfur (Fry et al. Citation1988). The addition of marine sulfate creates a similar effect because of the higher Cl/SO4 ratio of seawater (). The enrichment of δ34S (SO4) beyond that of seawater indicates that sulfate reduction is occurring along with mixing with marine sulfate. The saline lakes around the head of the fjord are the most impacted by SRB enrichment of δ34S (SO4). Surface-water environments become less hostile to microbial communities with distance from the ice sheet because of warmer temperatures and increased time for the evolution and development of communities. For example, cooler temperatures at about 8,200 years BP caused a definitive decrease in productivity in a lake close to the head of the fjord near Kangerlussuaq, based on isotopic and fossil records from a lake core (Anderson et al. Citation2008). The cooler, dryer conditions at approximately 8,200 years BP and the reduction in productivity may be related both to the close proximity of the ice sheet to the lake at that time (Anderson et al. Citation2008) and to thermohaline slow down documented in the North Atlantic (e.g., Alley and Ágústsdóttir Citation2005).
Marine influence
Marine aerosols have been found to impact lake chemistry in both the Arctic and Antarctic (Green and Canfield Citation1984; Harris et al. Citation2007; Jones and Faure Citation1978; Keys and Williams Citation1981; Kokelj, Zijdlik, and Thompson Citation2009; Lyons et al. Citation2005). Anderson et al. (Citation2001) found that the percentage of Cl− in lakes increased toward the west coast of Greenland. In southeast Greenland, an increased influence of marine-derived Na+ and Cl− was found in shallow groundwater toward the coast (Kristiansen et al. Citation2013). Kristiansen et al. (Citation2013) found that both present-day salt spray and Holocene marine sediment deposits contributed to the marine Na+ and Cl− in the shallow groundwater. Several lines of evidence indicate that marine aerosols affect lake chemistry in the Kangerlussuaq region:
Chloride from marine aerosols has a Na:Cl equivalent ratio of 1 and an initial δ37Cl signature of 0‰. Many of the sampled lakes have Na:Cl ratios that fall along or close to the 1:1 line (). Some excess Na+ is observed, likely because of weathering or aeolian inputs (Anderson et al. Citation2001). Many of the dilute lakes as well as several saline lakes analyzed for stable chlorine isotopes have a δ37Cl isotopic value that is within analytical error of 0‰, providing further evidence of marine Cl− input () throughout the study area.
The enriched δ34S-δ18O of SO42- found in many of the upper fjord lakes, which trend toward a marine signature, indicate mixing with marine sulfate (); however, it is difficult to separate this effect from enrichment because of microbial sulfate reduction.
87Sr/86Sr ratios show a pattern of decreasing radiogenic Sr toward the coast (). While some of the decrease in 87Sr/86Sr ratios may be explained by a shift from biotite-dominated weathering with distance from the ice sheet, it is likely that marine aerosols (with a 87Sr/86Sr signature of ~0.7092) are also a factor.
The majority of lakes in the study area are above the marine limit (approximately 100 m above modern sea level; Anderson et al. Citation2001; Funder and Hansen Citation1996). The marine limit is the maximum elevation at which relict marine shorelines are observed, and thus lakes above this elevation are unlikely to contain relict seawater. A prominent exception is Lake Ferguson (L33; , which is located below the marine limit. The 87Sr/86Sr ratio of Lake Ferguson (0.7187) is atypical for its distance from the coast (Easting; and is closer to that of seawater (0.7092). Lake L45 () is also below the marine limit at 96 m elevation. The 87Sr/86Sr isotopic ratio of lake L45 (0.7267) is typical of lakes at similar distance from the coast (), suggesting that the lake salinity is derived from local weathering processes and aeolian activity rather than relic seawater. Lake L45 is unique in its high concentration of Cl− relative to Na+ () and has a Na/Cl ratio of 0.73. The Na/Cl ratio of lake L45 is less than that of seawater (0.86), suggesting either an additional removal mechanism for Na+ or an additional source of Cl−.
The majority of ponds and dilute lakes fall along a trend of increasing Cl− compared to SO42- (). Ponds, meltwater lakes, and runoff tend to have higher SO42- relative to Cl−. The ponds and meltwater lakes sampled were located in the ice-marginal area and, as such, most likely have decreased input of marine Cl− and limited or no removal of SO42- by sulfate reduction. Winds in the ice-marginal area are dominantly katabatic winds from the ice sheet (Aebly and Fritz Citation2009), which may limit marine Cl− inputs.
Evidence for deep groundwater discharge
It has been previously assumed that groundwater discharge into lakes in the Kangerlussuaq region is insignificant (e.g., Anderson et al. Citation2001). However, a number of conceptual and numerical models for groundwater flow under and adjacent to continental-scale ice sheets predict significant groundwater discharge at the front of the ice sheet (Boulton et al. Citation1996; Lemieux et al. Citation2008), with the potential for highly saline groundwater discharge (Starinsky and Katz Citation2003). Determining the extent of groundwater discharge into lakes in the study area was an important objective of surface-water studies. van Tatenhove and Olesen (Citation1994) estimate that in the Kangerlussuaq area, lakes with diameters greater than 30 m do not freeze completely during the winter and, thus, are capable of supporting a closed talik. Thermal modeling may be used to predict the size of lake necessary to create a through or closed talik (e.g., Burn Citation2002). Thermal modeling performed as part of the GAP indicated that lakes with a width greater than 200 m have the potential to form a through talik, while lakes that are at least 100 m wide may support closed taliks (Harper et al. Citation2011). Thermal modeling also indicated that taliks may form during only a few 100 years (Harper et al. Citation2011). The relatively short time scale on which taliks may form implies that the majority of lakes capable of supporting a through talik have had sufficient time to form the talik.
Deep (subpermafrost) groundwaters measured by the GAP boreholes tend to have δ18O/δ2H isotopic signatures that fall along the GMWL and are depleted relative to surface waters. The subpermafrost groundwaters are similar in composition to glacial meltwaters (). These groundwaters have high sulfate concentrations with a characteristic δ34S/δ18O of SO4 (). Groundwaters do not contain tritium and have low strontium isotopic signatures (<0.71). Significant discharge of groundwater into lakes via a through talik would cause the lake chemistry and isotopic signature to shift toward the groundwater end member; that is, anomalously low δ18O, δ2H, 3H, and 87Sr/86Sr isotopic values and higher concentrations of SO4. None of the lakes sampled in the ice-marginal area (area 1) show evidence of groundwater discharge (e.g., and ) and have 87Sr/86Sr isotopic ratios greater than 0.73.
In area 2 (head of the fjord), the geochemical and isotopic composition of the Kellyville Basin lakes bear some similarities to groundwater, such as high chloride concentrations, less radiogenic 87Sr/86Sr ratios, and enriched δ18O of sulfate; however, alternative explanations have been presented for these characteristics earlier in the article. The most compelling argument against significant groundwater discharge into lakes in the region is the lack of a mixing trend with a more depleted (δ18O/δ2H) groundwater source (). If groundwater discharge is occurring, volumes are too small to affect the lake isotopic composition or other geochemical parameters of the lake waters discussed in this article.
Conclusions
The use of a variety of established and novel environmental isotopes provided new insight into a number of key processes in the Kangerlussuaq region. The impact of evaporation, weathering, transport of marine aerosols, and microbial activity on lakes was found to relate to distance from the coast and ice margin, providing insight into the role that climatic gradients and glacial processes play in lake geochemical evolution.
Evaporation is one of the main processes affecting the geochemical evolution of lakes in this study, especially in the inland areas close to the ice sheet. Evaporation in closed-basin lakes is interpreted to be responsible for the higher, though still brackish, salinities (>800 μS/cm) observed in a number of the Kangerlussuaq-region lakes. Both δ37Cl and δ81Br provide new insight into the importance of salt precipitation and recycling in soils and shallow lake areas. Chlorine and bromine isotopes provide further evidence that evaporation and salt precipitation are important processes to lake geochemistry.
Highly radiogenic 87Sr/86Sr ratios indicate that biotite weathering is enhanced in more recently deglaciated catchments. Marine aerosols and feldspar weathering become increasingly important with distance from the ice sheet and with proximity to the coast. These findings support previous work (Anderson, Drever, and Humphrey Citation1997; Blum and Erel Citation1995, Citation1997; Sharp, Creaser, and Skidmore Citation2002) on enhanced biotite weathering in proglacial environments and indicate that these theories are applicable to deglaciated areas of Greenland. The findings from the Kangerlussaq study area may have implications for the impact of glaciation and climate warming on the potential changes to the marine 87Sr/86Sr signature (Anderson, Drever, and Humphrey Citation1997; Hodell, Mueller, and Garrido Citation1991; Zachos et al. Citation1999). With the increased observed (e.g., van As et al. Citation2012) and predicted melting of the ice cap, increased runoff rates, and the potential increase in solute loadings to the ocean (Anderson, Drever, and Humphrey Citation1997; Hasholt et al. Citation2012), increased radiogenic strontium from glaciated and recently deglaciated terrain will enter the ocean, potentially shifting the marine 87Sr/86Sr signature.
In more recently deglaciated areas, sulfide oxidation is the main source of sulfate in lakes, while the influence of marine aerosols increases around the fjord. The impact of sulfide oxidation under varying conditions (saturation, water source) is apparent in the δ18O signature of the resulting sulfate. Bacterial sulfate reduction does not appear to be an important process in most of the lakes in close proximity to the ice sheet, but, in the warmer, more productive lakes around the head of the fjord, significant δ34S enrichment because of microbial sulfate reduction is observed. However, it is difficult to separate the impact of sulfate reduction from mixing with marine aerosols. This study provides new information on the extent of bacterial sulfate reduction in lakes in the Kangerlussuaq area and the relationship between SRB and landscape evolution after deglaciation.
An important objective of the GAP surface-water studies was to determine the role of taliks in the groundwater system and the extent of groundwater–surface water interaction. Characterizing groundwater surface interaction is important for understanding lake hydrology, water balance, and chemistry. Previously, studies of lakes in the area had assumed little interaction between groundwater and surface water (e.g., Leng and Anderson Citation2003). Providing direct evidence for this assumption, a comparison of surface and groundwater isotopic (δ18O, δ2H, 3H, δ34S, and δ18O) and geochemical data collected as part of this study found no evidence for significant groundwater discharge within the sampled lakes. The lack of observable groundwater discharge may indicate low discharge volumes and little groundwater–surface water interaction or recharge conditions, such as those observed between L26 and the DH-GAP01 borehole.
Acknowledgments
NWMO, Posiva, and SKB for providing the financial support for the Greenland Analogue Project. NSERC for their financial support. Lillemor Claesson Liljedahl and Anne Kontula for their support of this research, their leg work, and their company in the field. All my other GAP colleagues.
References
- Aebly, F. A., and S. C. Fritz. 2009. Palaeohydrology of Kangerlussuaq (Søndre Strømfjord), West Greenland during the last ~ 8000 years. The Holocene 1:1–20. doi:https://doi.org/10.1177/0959683608096601.
- Alley, R. B., and A. M. Ágústsdóttir. 2005. The 8k event: Cause and consequence of a major Holocene abrupt climate change. Quaternary Science Reviews 24:1123–49. doi:https://doi.org/10.1016/j.quascirev.2004.12.004.
- Anderson, N. J. 2014. Landscape disturbance and lake response: Temporal and spatial perspectives. Freshwater Reviews 7:77–120. doi:https://doi.org/10.1608/FRJ-7.2.811.
- Anderson, N. J., and K. P. Brodersen. 2001. Determining the date of ice-melt for low Arctic lakes along Søndre Strømfjord, southern West Greenland. Geology of Greenland Survey Bulletin 189:54–58.
- Anderson, N. J., K. P. Brodersen, D. B. Ryves, S. McGowan, L. S. Johansson, E. Jeppesen, and M. J. Leng. 2008. Climate versus in-lake processes as controls on the development of community structure in a low-arctic lake (South-West Greenland). Ecosystems (New York, N.Y.) 11:307–24. doi:https://doi.org/10.1007/s10021-007-9123-y.
- Anderson, N. J., S. C. Fritz, C. E. Gibson, B. Hasholt, and M. J. Leng. 2002. Lake-catchment interactions with climate in the low Arctic of southern West Greenland. Geology of Greenland Survey Bulletin 191:144–49.
- Anderson, N. J., R. Harriman, D. Ryves, and S. Patrick. 2001. Dominant factors controlling variability in the ionic composition of West Greenland lakes. Arctic, Antarctic, and Alpine Research 33:418–25. doi:https://doi.org/10.2307/1552551.
- Anderson, N. J., and M. J. Leng. 2004. Increased aridity during the early Holocene in West Greenland inferred from stable isotopes in laminated-lake sediments. Quaternary Science Reviews 23:841–49. doi:https://doi.org/10.1016/j.quascirev.2003.06.013.
- Anderson, N. J., A. C. Liversidge, S. McGowan, and M. D. Jones. 2012. Lake and catchment response to Holocene environmental change: Spatial variability along a climate gradient in southwest Greenland. Journal of Paleolimnology 48:209–22. doi:https://doi.org/10.1007/s10933-012-9616-3.
- Anderson, N. J., J. E. Saros, J. E. Bullard, S. M. Cahoon, S. McGowan, E. A. Bagshaw, C. D. Barry, R. Bindler, B. T. Burpee, J. L. Carrivick, et al. 2017. The Arctic in the twenty-first century: Changing biogeochemical linkages across a paraglacial landscape of Greenland. BioScience 67 (2):118–33.
- Anderson, N. J., and C. A. Stedman. 2007. The effect of evaporation on dissolved organic carbon concentration and quality in lakes of SW Greenland. Freshwater Biology 52:280–89. doi:https://doi.org/10.1111/j.1365-2427.2006.01688.x.
- Anderson, S. P., J. I. Drever, and N. F. Humphrey. 1997. Chemical weathering in glacial environments. Geology 25:399–402. doi:https://doi.org/10.1130/0091-7613(1997)025<0399:CWIGE>2.3.CO;2.
- Bennike, O. 2000. Palaeoecological studies of Holocene lake sediments from west Greenland. Palaeogeography, Palaeoclimatology, Palaeoecology 155:285–304. doi:https://doi.org/10.1016/S0031-0182(99)00121-2.
- Birks, H., V. Jones, and N. Rose. 2004. Recent environmental change and atmospheric contamination on Svalbard as recorded in lake sediments: Synthesis and general conclusions. Journal of Paleolimnology 31:531–46. doi:https://doi.org/10.1023/B:JOPL.0000022550.81129.1a.
- Blum, J. D., and Y. Erel. 1995. A silicate weathering mechanism linking increases in marine 87Sr/86Sr with global glaciation. Nature 373:415–18. doi:https://doi.org/10.1038/373415a0.
- Blum, J. D., and Y. Erel. 1997. Rb/Sr isotope systematics of a granitic soil chronosequence: The importance of biotite weathering. Geochimica Et Cosmochimica Acta 61:3193–204. doi:https://doi.org/10.1016/S0016-7037(97)00148-8.
- Bocher, T. W. 1949. Climate, soil and lakes in continental west Greenland. Meddelelser om Grønland 147:1–63.
- Bohlke, J. K., and J. M. Denver. 1995. Combined use of groundwater dating, chemical, and isotoppic analyses to resolve the history and fate of nitrate contamination in two agricultural watersheds, Atlantic coast plain, Maryland. Water Resources Research 31 (9):2319–39. doi:https://doi.org/10.1029/95WR01584.
- Boulton, G. S., P. E. Caban, K. van Gijssel, A. Leijnse, M. Punkari, and F. H. A. van Weert. 1996. The impact of glaciation on the groundwater regime of Northwest Europe. Global and Planetary Change 12:397–413. doi:https://doi.org/10.1016/0921-8181(95)00030-5.
- Bullen, T. D., and C. Kendall. 1998. Tracing weathering reactions and water flowpaths: A multi-isotope approach. In Isotope tracers in catchment hydrology, eds. C. Kendall and J. J. McDonnell, 611–43. Elsevier, Amsterdam.
- Burn, C. 2002. Tundra lakes and permafrost, Richards Island, western Arctic coast, Canada. Canadian Journal of Earth Sciences 39:1281–98. doi:https://doi.org/10.1139/e02-035.
- Burton, H. R., and R. J. Barker. 1979. Sulfur chemistry and microbiological fractionation of sulfur isotopes in a saline Antarctic lake. Geomicrobiology Journal 1:329–40. doi:https://doi.org/10.1080/01490457909377739.
- Canfield, D. E. 2001. Biogeochemistry of sulfur isotopes. Reviews in Mineralogy and Geochemistry 43:607–36. doi:https://doi.org/10.2138/gsrmg.43.1.607.
- Cappelen, J., ed. 2012: Weather and climate data from Greenland 1958–2011: Observational data with description. DMI Technical Report 12-15. Copenhagen, Denmark.
- Cartwright, K., and H. J. H. Harris. 1981. Hydrogeology of the dry valley region, antarctica. In Antarctic research series volume 33: Dry Valley drilling project, ed. L. D. McGinnis, vol. 33, 193–214. Washington DC: American Geophysical Union.
- Claesson Liljedahl, L., A. Kontula, J. Harper, J.-O. Näslund, J.-O. Selroos, P. Pitkänen, I. Puigdomenech, M. Hobbs, S. Follin, S. Hirschorn et al. 2016. Greenland Analogue Project final report. SKB Report TR-14-13. Stockholm, Sweden.
- Clark, I., and P. Fritz. 1997. Environmental isotopes in hydrogeology, ed. Groundwater Quality; Sub section ; Sulphate, Sulphide and the Suphur Cycle p. 137 to 147 J. Stein and A. W. Starkweather. New York: Lewis Publishers.
- DeFoor, W., M. Person, H. C. Larsen, D. Lizarralde, D. Cohen, and B. Dugan. 2011. Ice sheet-derived submarine groundwater discharge on Greenland’s continental shelf. Water Resources Research 47:1–14. doi:https://doi.org/10.1029/2011WR010536.
- Dicken, A. P. 2000. Radiogenic isotopic geology, “the Rb-Sr-Method.” New York: Cambridge University Press.
- Eggenkamp, H. 2014. The geochemistry of stable chlorine and bromine isotopes. In Advances in isotope geochemistry, ed. J. Hoefs. BerlIn: Springer.
- Eggenkamp, H. G. M. 1994. The geochemistry of chlorine isotopes. Utrecht, The Netherlands: University of Utrecht.
- Eggenkamp, H. G. M., R. Kreulen, and A. F. Koster van Groos. 1995. Chlorine stable isotope fractionation in evaporites. Geochimica Et Cosmochimica Acta 59:5169–75. doi:https://doi.org/10.1016/0016-7037(95)00353-3.
- Eggleston, C., M. J. Hochella, and G. Parks. 1989. Sample preparation and aging effects on the dissolution rate and surface composition of diopside. Geochimica Et Cosmochimica Acta 53:797–804. doi:https://doi.org/10.1016/0016-7037(89)90026-4.
- Eisner, W., T. Törnqvist, E. Koster, O. Bennike, and J. F. N. van Leeuwen. 1995. Paleoecological studies of a Holocene lacustrine record from the Kangerlussuaq (Søndre Strømfjord) region of West Greenland. Quaternary Research 43:55–66. doi:https://doi.org/10.1006/qres.1995.1006.
- Engstrom, D., S. Fritz, J. Almendinger, and S. Juggins. 2000. Chemical and biological lake evolution in recently deglaciated terrain. Nature 408:161–66. doi:https://doi.org/10.1038/35041500.
- Engström, J., and K. E. S. Klint. 2014. Continental collision structures and post-orogenic geological history of the kangerlussuaq area in the Southern part of the Nagssugtoqidian Orogen, Central West Greenland. Geosciences 4:316–34. doi:https://doi.org/10.3390/geosciences4040316.
- Epstein, S., and T. K. Mayeda. 1953. Variations of the 18O content of waters from natural sources. Geochimica Et Cosmochimica Acta 4:213–24. doi:https://doi.org/10.1016/0016-7037(53)90051-9.
- Frape, S. K., A. Blyth, R. L. Stotler, T. Ruskeeniemi, R. Blomqvist, R. H. McNutt, and M. Gascoyne. 2014. Deep fluids in the continents. In Treatise on geochemistry, ed. J. I. Drever, vol. 7, 2nd ed., 517–62. Amsterdam, the Netherlands: Elsevier.
- Frape, S. K., R. Stotler, and F. E. Harvey. 2013. The isotopic distribution of 37Cl and 81Br in highly evaporated alkaline lakes of the Sand Hills, Nebraska, U.S.A. 10th International Symposium on Applied Isotope Geochemistry, Budapest, Hungary.
- Fredskild, B. 1977. The development of the Greenland lakes since the last glaciation. Folia Limnol Scandinavian. 17:101–6.
- Fritz, S. C., and N. J. Anderson. 2013. The relative influences of climate and catchment processes on Holocene lake development in glaciated regions. Journal of Paleolimnology 49:349–62. doi:https://doi.org/10.1007/s10933-013-9684-z.
- Fry, B., W. Ruf, H. Gest, and J. M. Hayes. 1988. Sulphur isotope effects associated with oxidation of sulphide by O2 in aqueous solution. Chemical Geology 73:205–10.
- Funder, S., and L. Hansen. 1996. The Greenland ice sheet: A model for its culmination and decay during and after the last glacial maximum. Bulletin of the Geological Society of Denmark 42:137–52.
- Gavelin, S., A. Parwel, and R. Ryhage. 1960. Sulfur isotope fractionation in sulfide mineralization. Economic Geology1 55:510–30. doi:https://doi.org/10.2113/gsecongeo.55.3.510.
- Gibson, J. J., S. J. Birks, and Y. Yi. 2016. Stable isotope mass balance of lakes: A contemporary perspective. Quaternary Science Reviews 131:316–28. doi:https://doi.org/10.1016/j.quascirev.2015.04.013.
- Gibson, J. J., and T. W. D. Edwards. 2002. Regional water balance trends and evaporation-transpiration partitioning from a stable isotope survey of lakes in northern Canada. Global Biogeochemical Cycles 16 (2):10–14. doi:https://doi.org/10.1029/2001GB001839.
- Gibson, J. J., T. W. D. Edwards, S. J. Birks, N. A. St Amour, W. M. Buhay, P. McEachern, B. B. Wolfe, and D. L. Peters. 2005. Progress in isotope tracer hydrology in Canada. Hydrological Processes 19:303–27. doi:https://doi.org/10.1002/(ISSN)1099-1085.
- Gooseff, M. N., W. B. Lyons, D. M. McKnight, B. H. Vaughn, A. G. Fountain, and C. Dowling. 2006. A stable isotopic investigation of a Polar Desert Hydrologic System, McMurdo Dry Valleys, Antarctica. Arctic, Antarctic, and Alpine Research 38:60–71. doi:https://doi.org/10.1657/1523-0430(2006)038[0060:ASIIOA]2.0.CO;2.
- Grant, W. H. 1963. Weathering of Stone Mountain granite. In Clays and clay minerals, ed. B. Ingersol, 65–73. Oxford: Pergamon.
- Green, W., and D. Canfield. 1984. Geochemistry of the Onyx River (Wright Valley, Antarctica) and its role in the chemical evolution of Lake Vanda. Geochimica Et Cosmochimica Acta 48:2457–67. doi:https://doi.org/10.1016/0016-7037(84)90297-7.
- Green, W. J., M. P. Angle, and K. E. Chave. 1988. The geochemistry of Antarctic streams and their role in the evolution of four lakes of the McMurdo Dry Valleys. Geochimica Et Cosmochimica Acta 52:1265–74. doi:https://doi.org/10.1016/0016-7037(88)90280-3.
- Hagerty, S. K., and J. A. Webb. 2008. Aquifer interactions and groundwater discharge into streams identified using 87Sr/86Sr isotope ratios in the Upper Loddon catchment, central Victoria. Water down under, 2008. Adelaide, Australia
- Hanlon, C. 2015: A characterization of bromine and chlorine stable isotopes in the Sand Hills Region of Nebraska, USA ( MSc. Thesis). University of Waterloo, Canada.
- Hanlon, C., R. Stotler, S. K. Frape, and R. Gwynne. 2017. Comparison of δ81Br and δ37Cl composition of volatiles, salt precipitates and associated waters terrestrial evaporative saline systems. Isotopes in Environmental and Health Studies 53 (5):446–65. doi:https://doi.org/10.1080/10256016.2017.1324856.
- Harper, J., A. Hubbard, T. Ruskeeniemi, L. Claesson Liljedahl, A. Lehtinen, A. Booth, and D. van As 2011. The Greenland Analogue Project yearly report 2010. Stockholm, Sweden: SKB.
- Harper, J., A. Hubbard, T. Ruskeeniemi, L. Claesson Liljedahl, A. Lehtinen, M. Bougamont, and D. van As. 2016. GAP data, processes and conceptual understanding. SKB-Report-14-13. Stockholm, Sweden: SKB.
- Harris, K. J., E. Carey, W. B. Lyons, K. Welch, and A. G. Fountain. 2007. Solute and isotope geochemistry of subsurface ice melt seeps in Taylor Valley, Antarctica. Geological Society of America Bulletin 119:548–55. doi:https://doi.org/10.1130/B25913.1.
- Hasholt, B., A. B. Mikkelsen, M. H. Nielsen, and M. A. D. Larsen. 2012. Observations of runoff and sediment and dissolved loads from the Greenland ice sheet at Kangerlussuaq, West Greenland. Zeitschrift Für Geomorphologie supplement:3–27.
- Henkemans, E. 2016. Geochemical characterization of groundwaters, surface waters and water-rock interaction in an area of continuous permafrost adjacent to the Greenland ice sheet, Kangerlussuaq, southwest Greenland ( PhD Thesis). University of Waterloo.
- Hodell, D., P. Mueller, and J. Garrido. 1991. Variations in the strontium isotopic composition of seawater during the Neogene. Geology 19:24–27. doi:https://doi.org/10.1130/0091-7613(1991)019<0024:VITSIC>2.3.CO;2.
- Jansson, M., L. Persson, A. M. De Roos, R. I. Jones, and L. J. Tranvik. 2007. Terrestrial carbon and intraspecific size-variation shape lake ecosystems. Trends in Ecology & Evolution 22:316–22. doi:https://doi.org/10.1016/j.tree.2007.02.015.
- Jensen, I. A. 1889. Undersegelse af Gronlands Vestkyst fra 64-68 n.b. Meddelelser om Grønland 8:33–130.
- Johansson, E., L.-G. Gustafsson, S. Berglund, T. Lindborg, J.-O. Selroos, L. Claesson Liljedahl, and G. Destouni. 2015. Data evaluation and numerical modeling of hydrological interactions between active layer, lake and talik in a permafrost catchment, Western Greenland. Journal of Hydrology 527:688–703. doi:https://doi.org/10.1016/j.jhydrol.2015.05.026.
- Johnson, T. M., R. C. Roback, T. L. McLing, T. D. Bullen, D. J. DePaolo, C. Doughty, R. J. Hunt, R. W. SMith, L. D. Cecil, and M. T. Murrell. 2000. Groundwater fast paths in the Snake River Plain aquifer: Radiogenic isotope ratios as natural groundwater tracers. Geology 28:871–74. doi:https://doi.org/10.1130/0091-7613(2000)28<871:GFPITS>2.0.CO;2.
- Jones, L. M., and G. Faure. 1978. A study of strontium isotopes in lakes and surficial deposits of the ice-free valleys, Southern Victoria Land, Antarctica. Chemical Geology 22:107–20. doi:https://doi.org/10.1016/0009-2541(78)90027-X.
- Kaplan, I. R., and S. C. Rittenberg. 1963. Microbiological fractionation of sulphur isotopes. Journal of General Microbiology 34:195–212. doi:https://doi.org/10.1099/00221287-34-2-195.
- Kaufmann, R., A. Long, H. Bentley, and S. Davis. 1984. Natural chlorine isotope variations. Nature 309:338–40.
- Kendall, C. 1998. Tracing nitrogen sources and cycles in catchments. In Isotope tracers in catchment hydrology, ed. C. Kendall and J. J. McDonnell, 519–69. Amsterdam: Elsevier.
- Kendall, C., and J. J. McDonnell. 1998. Isotope tracers in catchment hydrology. Amsterdam: Elsevier.
- Kern Hansen, C. 1990: Data basis for permafrost studies in Greenland. In Polartech ’90: International Conference on Development and Commerical Utilization of Technologies in Polar Regions. Copenhagen, Denmark.
- Keys, J. R., and K. Williams. 1981. Origin of crystalline, cold desert salts in the McMurdo region, Antarctica. Geochimica Et Cosmochimica Acta 45:2299–309. doi:https://doi.org/10.1016/0016-7037(81)90084-3.
- Kling, G. W., G. W. Kipphut, M. M. Miller, and W. J. O’Brient. 2000. Integration of lakes and streams in a landscape perspective: The importance of material processing on spatial patterns and temporal coherence. Freshwater Biology 43:477–97. doi:https://doi.org/10.1046/j.1365-2427.2000.00515.x.
- Kokelj, S. V., B. Zijdlik, and M. S. Thompson. 2009. The impacts of thawing permafrost on the chemistry of lakes across the subarctic boreal-tundra transition, Mackenzie Delta Region Canada. Permafrost and Periglacial Processes 20:185–99. doi:https://doi.org/10.1002/ppp.641.
- Kristiansen, S. M., J. C. Yde, T. G. Bárcena, B. H. Jakobsen, J. Olsen, and N. T. Knudsen. 2013. Geochemistry of groundwater in front of a warm-based glacier in Southeast Greenland. Geografiska Annaler: Series A, Physical Geography 95:97–108. doi:https://doi.org/10.1111/geoa.12003.
- Krieger, R.A. 1964. The chemistry of saline waters. Groundwater 2:51. doi:https://doi.org/10.1111/j.1745-6584.1964.tb01747.x.
- Lambert, F., B. Delmonte, J. Petit, M. Bigler, P. R. Kaufmann, M. A. Hutterli, T.F. Stocker, U. Ruth, J.P. Steffensen, and V. Maggi. 2008. Dust-climate couplings over the past 800,000 years from the EPICA Dome C ice core. Nature 452:616–19. doi:https://doi.org/10.1038/nature06763.
- Lamoureux, S., R. Gilbert, and T. Lewis. 2002. Lacustrine sedimentary environments in High Arctic proglacial Bear Lake, Devon Island, Nunavut, Canada. Arctic, Antarctic, and Alpine Research 34:130–41. doi:https://doi.org/10.2307/1552464.
- Lawrence, C. R., and J. C. Neff. 2009. The contemporary physical and chemical flux of aeolian dust: A synthesis of direct measurements of dust deposition. Chemical Geology 267:46–63. doi:https://doi.org/10.1016/j.chemgeo.2009.02.005.
- Lemieux, J.-M., E. A. Sudicky, W. R. Peltier, and L. Tarasov. 2008. Simulating the impact of glaciations on continental groundwater flow systems: 2. Model application to the Wisconsinian glaciation over the Canadian landscape. Journal of Geophysical Research 113: F03018.
- Leng, M. J., and N. J. Anderson. 2003. Isotopic variation in modern lake waters from western Greenland. The Holocene 13:605–11. doi:https://doi.org/10.1191/0959683603hl620rr.
- Leng, M. J., B. Wagner, N. J. Anderson, O. Bennike, E. Woodley, and S. J. Kemp. 2012. Deglaciation and catchment ontogeny in coastal south-west Greenland: Implications for terrestrial and aquatic carbon cycling. Journal of Quaternary Science 27:575–84. doi:https://doi.org/10.1002/jqs.v27.6.
- Lyons, W., K. Welch, A. Carey, P. Doran, D. Wall, R. Virginia, A.G. Fountain, B.M. Csatho, and C. M. Tremper. 2005. Groundwater seeps in Taylor Valley Antarctica: An example of a subsurface melt event. Annals of Glaciology 40:200–6. doi:https://doi.org/10.3189/172756405781813609.
- Lyons, W. B., C. A. Nezat, L. V. Benson, T. D. Bullen, E. Y. Graham, J. Kidd, K.A. Welch, and J. M. Thomas. 2002. Strontium isotopic signatures of the streams and lakes of Taylor Valley, Southern Victoria Land, Antarctica: Chemical weathering in a polar climate. Aquatic Geochemistry. 8: 75–95.
- Lyons, W. B., S. W. Tyler, H. E. Gaudette, and D. T. Long. 1995. The use of strontium isotopes in determining groundwater mixing and brine fingers in a playa spring zone, Lake Tyrrell, Australia. Journal of Hydrology 167:225–39. doi:https://doi.org/10.1016/0022-1694(94)02601-7.
- Magenheim, A., A. Spivack, P. Michael, and J. M. Gieskes. 1995. Chlorine stable isotope composition of the oceanic crust: Implications for Earth’s distribution of chlorine. Earth and Planetary Science Letters 131:427–32. doi:https://doi.org/10.1016/0012-821X(95)00017-7.
- Matsubaya, O., H. Sakai, T. Toril, H. Burton, and K. Kerry. 1979. Antarctic saline lakes-stable isotopic ratios, chemical compositions and evolution. Geochimica Et Cosmochimica Acta 43:7–25. doi:https://doi.org/10.1016/0016-7037(79)90042-5.
- McArthur, J. M., J. Turner, W. B. Lyons, and M. F. Thirlwall. 1989. Salt sources and water-rock interaction on the Yilgarn Block Australia: Isotope and major element tracers. Applied Geochemistry 4:79–92. doi:https://doi.org/10.1016/0883-2927(89)90060-7.
- McGowan, S., D. B. Ryves, and N. J. Anderson. 2003. Holocene records of effective precipitation in West Greenland. The Holocene 13:239–49. doi:https://doi.org/10.1191/0959683603hl610rp.
- McNutt, R. H., S. K. Frape, P. Fritz, M. G. Jones, and I. A. N. M. Macdonald. 1990. The 87Sr/86Sr values of Canadian Shield brines and fracture minerals with applications to groundwater mixing, fracture history, and geochronology. Geochimica Et Cosmochimica Acta 54:205–15. doi:https://doi.org/10.1016/0016-7037(90)90208-3.
- Morrison, J. 1997. Inorganic oxygen isotope analysis by EA-pyrolysis-IRMS. Presented at 4th Canadian Continuous Flow: IRMS Conference, Waterloo, Ontario.
- Morrison, J., T. Fallick, T. Donelly, M. Leossen, G. St. Jean, and R. J. Drimmie. 1996 δ34S measurements of standards from several laboratories by continuous flow isotope ratio mass spectrometry (CF-IRMS). Micromass UK Ltd.,Wilmslow, UK. Technical Report.
- Morrison, J. T., T. Brockwell, T. Merren, F. Fourel, and A. M. Phillips. 2001. On-line high precision stable hydrogen isotopic analyses on nanoliter water samples. Analytical Chemistry 73:3570–75. doi:https://doi.org/10.1021/ac001447t.
- Moser, H. 1977: Internal report of the Institute for Radiohydrometrie. GSF Munich 169: 70–71.
- Nakai, N., and M. L. Jensen. 1964. The kinetic isotope effect in the bacterial reduction and oxidation of sulfur. Geochimica Et Cosmochimica Acta 28:1893–912. doi:https://doi.org/10.1016/0016-7037(64)90136-X.
- Negrel, P., E. Petelet-Giraud, J. Barbier, and E. Gautier. 2003. Surface water-groundwater interactions in an aluvial plain: Chemical and isotopic systematics. Journal of Hydrology 277:248–67. doi:https://doi.org/10.1016/S0022-1694(03)00125-2.
- Negrel, P., and S. Roy. 1998. Chemistry of rainwater in the Massif Central (France): A strontium isotope and major element study. Applied Geochemistry 13 (8):941–52. doi:https://doi.org/10.1016/S0883-2927(98)00029-8.
- Nesbitt, H., and G. Young. 1996. Petrogenesis of sediments in the absence of chemical weathering: Effects of abrasion and sorting on bulk composition and mineralogy. Sedimentology 43:341–58. doi:https://doi.org/10.1046/j.1365-3091.1996.d01-12.x.
- Olsen, J., N. J. Anderson, and M. J. Leng. 2013. Limnological controls on stable isotope records of late-Holocene palaeoenvironment change in SW Greenland: A paired lake study. Quaternary Science Reviews 66:85–95. doi:https://doi.org/10.1016/j.quascirev.2012.10.043.
- Ouellet, M., M. Dickman, and P. Page. 1989. Physico-chemical characteristics and origin of hypersaline meromictic Lake Garrow in the Canadian High Arctic. Hydrobiologia 172:215–34. doi:https://doi.org/10.1007/BF00031624.
- Pere, T. 2014. Geological logging of the Greenland Analogue Project drill cores DH-GAP01, 03 and 04. Posiva Working Report 2013-59. Posiva Oy, Olkiluoto, Finland.
- Pratt, L., and Y. Peng. 2014. Isotopic study of jarosite in a gossan on the western margin of the Greenland Ice Sheet. GSA annual meeting, Vancouver, BC.
- Schütz, L., and M. Sebert. 1987. Mineral aerosols and source identification. Journal of Aerosol Science 18:1–10. doi:https://doi.org/10.1016/0021-8502(87)90002-4.
- Seal, R. R., C. N. Alpers, and R. O. Rye. 2000. Stable isotope systematics of sulfate minerals. Reviews in Mineralogy and Geochemistry 40:541–602. doi:https://doi.org/10.2138/rmg.2000.40.12.
- Shand, P., F. D. P. Darbyshire, D. Gooddy, and A. H. Haria. 2007. 87Sr/86Sr as an indicator of flow paths and weathering rates in the Plynlimon experimental catchments, Wales, U.K. Chemical Geology 236:247–365. doi:https://doi.org/10.1016/j.chemgeo.2006.09.012.
- Sharp, M., R. A. Creaser, and M. Skidmore. 2002. Strontium isotope composition of runoff from a glaciated carbonate terrain. Geochimica Et Cosmochimica Acta 66:595–614. doi:https://doi.org/10.1016/S0016-7037(01)00798-0.
- Shouakar-Stash, O., R. J. Drimmie, and S. K. Frape. 2005a. Determination of inorganic chlorine stable isotopes by continuous flow isotope ratio mass spectrometry. Rapid Communications in Mass Spectrometry 19:121–27. doi:https://doi.org/10.1002/(ISSN)1097-0231.
- Shouakar-Stash, O., S. K. Frape, and R. J. Drimmie. 2005b. Determination of bromine stable isotopes using continuous-flow isotope ratio mass spectrometry. Analytical Chemistry 77:4027–33. doi:https://doi.org/10.1021/ac048318n.
- Starinsky, A., and A. Katz. 2003. The formation of natural cryogenic brines. Geochimica Et Cosmochimica Acta 67:1475–84. doi:https://doi.org/10.1016/S0016-7037(02)01295-4.
- Taylor, A., and J. D. Blum. 1995. Relation between soil age and silicate weathering rates determined from the chemical evolution of a glacial chronosequence. Geology 23:979–82. doi:https://doi.org/10.1130/0091-7613(1995)023<0979:RBSAAS>2.3.CO;2.
- Taylor, C. B. 1977. Tritium enrichment of environmental waters by electrolysis: Development of cathodes exhibiting high isotopic separation and precise measurements of tritium enrichment factors. Proceedings of the International Conference of Low-Radioactivity Measurements and Applications, Bratislava.
- Toran, L., and R. F. Harris. 1989. Interpretation of sulfur and oxygen isotopes in biological and abiological sulfide oxidation. Geochimica Et Cosmochimica Acta 53:2341–48. doi:https://doi.org/10.1016/0016-7037(89)90356-6.
- Turner, K. W., B. B. Wolfe, T. W. D. Edwards, T. C. Lantz, R. I. Hall, and G. Larocque. 2014. Controls on water balance of shallow thermokarst lakes and their relations with catchment characteristics: A multi-year, landscape-scale assessment based on water isotope tracers and remote sensing in Old Crow Flats, Yukon (Canada). Global Change Biology 20:1585–603. doi:https://doi.org/10.1111/gcb.12465.
- van As, D., A. L. Hubbard, B. Hasholt, A. B. Mikkelsen, M. R. van den Broeke, and R. S. Fausto. 2012. Large surface meltwater discharge from the Kangerlussuaq sector of the Greenland ice sheet during the record-warm year 2010 explained by detailed energy balance observations. The Cryosphere 6:199–209. doi:https://doi.org/10.5194/tc-6-199-2012.
- van Everdingen, R. O., and H. R. Krouse. 1985. Isotope composition of sulphates generated by bacterial and abiological oxidation. Letters to Nature 315:395–96. doi:https://doi.org/10.1038/315395a0.
- van Tatenhove, F. G. M., and O. Olesen. 1994. Ground temperature and related permafrost characteristics in West Greenland. Permafrost and Periglacial Processes 5:199–215. doi:https://doi.org/10.1002/ppp.3430050402.
- Wagner, T., P. A. Soranno, K. E. Webster, and K. S. Cheruvelil. 2011. Landscape drivers of regional variation in the relationship between total phosphorus and chlorophyll in lakes. Freshwater Biology 56:1811–24. doi:https://doi.org/10.1111/fwb.2011.56.issue-9.
- Webster, K. E., T. K. Kratz, C. J. Bowser, and J. J. Magnuson. 1996. The influence of landscape position on lake chemical responses to drough in northern Wisconsin. Limnology and Oceanography 41 (5):977–84. doi:https://doi.org/10.4319/lo.1996.41.5.0977.
- Webster, K. E., P. A. Soranno, S. B. Baines, T. K. Kratz, C. J. Bowser, P. J. Dillon, P. Campbell, and R. E. Hecky. 2000. Structuring features of lake districts: Landscape controls on lake chemical responses to drought. Freshwater Biology 43:499–515. doi:https://doi.org/10.1046/j.1365-2427.2000.00571.x.
- Weidick, A. 1975. A review of quaternary investigations in Greenland. Institute of Polar Studies Report 55. Geological Survey of Greenland, Copenhagen, Denmark.
- Whiteford, E. J., S. McGowan, C. D. Barry, and N. J. Anderson. 2016. Seasonal and regional controls of phytoplankton production along a climate gradient in South-West Greenland during ice-cover and ice-free conditions. Arctic, Antarctic, and Alpine Research 48:139–59. doi:https://doi.org/10.1657/AAAR0015-003.
- Williams, W. D. 1991. Comments on the so-called salt lakes of Greenland. Hydrobiologia 210:67–74. doi:https://doi.org/10.1007/BF00014323.
- Wood, W. W., and W. E. Sanford. 1995. Eolian transport, saline lake basins and groundwater solutes. Water Resources Research 31 (12):3121–29. doi:https://doi.org/10.1029/95WR02572.
- Wood, W. W., W. E. Sanford, and S. K. Frape. 2005. Chemical openness and potential for misinterpretation of the solute environment of coastal sabkhat. Chemical Geology 215:361–72. doi:https://doi.org/10.1016/j.chemgeo.2004.06.043.
- Zachos, J., B. Opdyke, T. Quinn, C. E. Jones, and A. Halliday. 1999. Early cenozoic glaciation, antarctic weathering, and seawater 87Sr/86Sr: Is there a link? Chemical Geology 161:165–80. doi:https://doi.org/10.1016/S0009-2541(99)00085-6.
- Zhang, M., S. K. Frape, A. J. Love, A. L. Herczeg, B. E. Lehmann, U. Beyerle, and R. Purtschert. 2007. Chlorine stable isotope studies of old groundwater, southwestern Great Artesian Basin, Australia. Applied Geochemistry 22:557–74. doi:https://doi.org/10.1016/j.apgeochem.2006.12.004.