ABSTRACT
Alpine ecosystems are vulnerable to ever-changing environmental conditions, leading to shifts in vegetation distribution and composition with implications on soil functionality and carbon (C) turnover. Although deadwood represents an important global C stock, scarce information is available on how slope exposure influences the wood-inhabiting microbiota throughout the decomposition process in an Alpine setting. We therefore evaluated the impact of slope exposure (north- vs. south-facing sites) on physicochemical and microbiological properties (microbial abundance based on real-time PCR: fungal 18S rRNA, dinitrogen reductase [nifH]; microbial biomass: double strand DNA; and microbial activity: hydrolytic enzyme activities of the main nutrient cycles) of Picea abies wood blocks and the underlying soil in a field experiment in the Italian Alps during a three-year period. Overall, a higher abundance of fungi and nitrogen-fixing bacteria was recorded in the soil at the north-facing site where cooler and moister conditions were observed. In contrast, no exposure effects were found for these two microbial groups in the wood blocks, while their abundance increased over time, accompanied by more acidic conditions with progressing decay. The impact of exposure was also enzyme specific and time dependent for both the P. abies wood blocks and the underlying soil.
Introduction
Mountain Alpine ecosystems have gained increasing attention during the past several years because they are particularly sensitive to ever-changing climatic conditions (Egli et al. Citation2006, Citation2009). Indeed, an increase of about 2°C has been recorded in the annual minimum temperature in the European Alps during the twentieth century (Beniston, Diaz, and Bradley Citation1997). As a consequence, previous studies have reported the occurrence of an upward migration of tree and shrub species in alpine environments owing to rising temperatures (Dullinger, Dirnböck, and Grabherr Citation2003; Motta and Nola Citation2001). Furthermore, changes in land use and management also constitute a dominant factor affecting soil biodiversity in alpine ecosystems (Meyer et al. Citation2013). Altogether, this can ultimately entail changes in the quality and quantity of soil organic matter (SOM), as well as in the vegetation composition and/or activities of soil organisms (Djukic et al. Citation2010; Myers et al. Citation2001; Siles et al. Citation2017), with implications for both carbon (C) dynamics and soil functionality (A’Bear et al. Citation2014; Allison et al. Citation2010; Theurillat and Guisan Citation2001).
Among the organic C reservoirs, deadwood represents a global C store estimated to be in the range of 73 ± 6 Pg (Pan et al. Citation2011). This is mainly because of its high lignin content resulting in a slow C turnover rate, making its decomposition dynamics determinant for the soil C balance (Floudas et al. Citation2012; Moroni et al. Citation2015). As woody material decays, its physicochemical properties gradually change over time, which can lead to a succession of microbial communities in wood according to their biochemical requirements and nutrient availability (Rajala et al. Citation2012). Fungi (brown rot, soft rot, and white rot) are considered the main wood decomposers, having different wood-degradation strategies. In particular, white-rot fungi are capable of degrading all components of the wood cell wall (including lignin) by secreting a plethora of extracellular lignocellulolytic enzymes (Kielak et al. Citation2016; van der Wal et al. Citation2007). Evidence also exists of complex fungal-bacterial interactions, both positive and negative, within the deadwood environment as reviewed by Johnston, Boddy, and Weightman (Citation2016), even though the identity and ecology of bacterial communities in decomposing wood remained underexplored compared to fungi. For instance, one assumes that fungi might be able to meet the nitrogen requirements for their vegetative and generative growth through the associations with nitrogen-fixing bacteria. Accordingly, Hoppe et al. (Citation2014) found positive correlations between fungal sporocarps and the richness of nifH (dinitrogen reductase) genes in deadwood logs from Fagus sylvatica and Picea abies. This is also in line with recent findings from Gómez-Brandón et al. (Citation2017), who observed that fungal abundance (qPCR-based) was strongly correlated with nifH abundance in the coarse woody debris of P. abies at different stages of natural decay.
Several factors were found to act as drivers for the changes in wood-inhabiting microbiota, such as the soil type (Sun et al. Citation2013); wood physicochemical properties, particularly density; pH; moisture content; and total lignin and cellulose (Hoppe et al. Citation2016; Purahong et al. Citation2014). The wood decay stage, with an increase in fungal and bacterial abundance as wood decomposes (Gómez-Brandón et al. Citation2017), together with the host tree species (Hoppe et al. Citation2016) are also of importance. In addition, topographic features, particularly the slope exposure, may influence the deadwood decay dynamics in subalpine environments (Fravolini et al. Citation2016; Petrillo et al. Citation2015). However, there is still a paucity of information about how slope exposure and climate, in general, affect deadwood-inhabiting microbiota in alpine environments (Gómez-Brandón et al. Citation2017).
Therefore, we performed a field mesocosm experiment with equally sized wood blocks of P. abies to evaluate the effect of exposure (north- vs. south-facing slope) on the abundance of nitrogen-fixing bacteria (nifH gene) and fungi assessed by real-time PCR. In addition, several potential enzymatic activities that are involved in the main nutrient cycles were determined during the early stage of decomposition (0–156 weeks) of deadwood in an alpine setting. Furthermore, we assessed which physicochemical parameters were the most important drivers shaping the microbial communities in wood and in the underlying soil.
We hypothesised that: (1) the microbial biomass and activity will be more favored at the south- than at the north-facing slope during P. abies wood decomposition in a high alpine setting and that such exposure effects will be time dependent, and (2) the changes in fungal and nifH gene abundances as a function of exposure will be more evident at the end of the monitoring owing to increased nutrient availability as woody decay progresses.
Material and methods
Experimental setup
Two study sites were selected at an altitude of 2,400 m above sea level (a.s.l.) in Val di Rabbi (Trentino, Italy) on a north- (N5) and south-facing (S10) slope, respectively. We selected natural grasslands close to the border of the potential tree line in order to minimize the influence of human activities and livestock grazing, and to assess how such sites might be affected by the input of wood as trees advance in elevation. Both alpine sites belong to an already well-known climosequence (Bardelli et al. Citation2017; Egli et al. Citation2006; Fravolini et al. Citation2016), and they were located in catchments with acidic paragneiss (Bardelli et al. Citation2017). The soils were classified as Podzol (north-facing site) and Cambisols (south-facing site; Egli et al. Citation2006), and are sandy to silty (N5: sand 53%, silt 28%, clay 19%; S10: sand 51%, silt 27%, clay 21%) according to Bardelli et al. (Citation2017). Mean annual precipitation is approximately 1,300 mm, mean annual air temperature is approximately −1.0°C (Sboarina and Cescatti Citation2004), and mean annual soil temperature ranges from 2.2°C to 4.5°C (Egli et al. Citation2016) at north and south exposure, respectively.
A field experiment using open mesocosms was set up at both study sites with the purpose of monitoring the early stage of P. abies (L.) Karst deadwood decomposition as a function of slope exposure and time in wood blocks and the topsoil layer (0–5 cm) that is in intimate contact with the wood blocks. Mesocosms (PVC tubes, 10.2 cm and 25 cm diameter and height, respectively) were installed into the natural soil in summer 2012, prior to the addition of the wood blocks of P. abies at a distance of greater than 0.5 m from the adjacent mesocosms, leaving at the surface a border of about 1 cm. From the mesocosm set-up (August 2012) to the placement of the wood blocks (June 2013), one year passed in order to permit normal conditions to be restored and, as such, the decay monitoring study would be performed under undisturbed conditions. Considering that the size and geometry of deadwood may have a strong influence on the decay processes (van der Wal et al. Citation2007), equal-sized (5 cm × 5 cm × 2 cm) wood blocks of P. abies were directly placed on the soil surface in each of the mesocosm tubes. Three replicate mesocosms for each time point were installed in each of the study sites. The wood blocks and the topsoil layer (0–5 cm) were collected (using lab gloves) in July 2014 (t1; 52 weeks), in July 2015 (t2; 104 weeks), and in July 2016 (t3; 156 weeks), resulting in a total of eighteen samples for each substrate (two sites × three times × three replicates), with three wood blocks kept as controls (t0). Prior to the placement of the wood blocks into the mesocosms, five soil subsamples (t0) were collected in the surrounding area of the mesocosm. All samples were kept in cooling boxes and transported to the laboratory. The soil samples were sieved (<2 mm), the wood blocks were air dried at room temperature, cut milled (4 mm; Retsch mill), and aliquoted into 50-ml sterile conical centrifuge tubes. The samples were then stored at 4°C and −20°C for physicochemical and (micro)biological analyses, respectively.
Wood and soil physicochemical analyses
The fresh and dry weight of the wood blocks were determined to assess the wood mass as a function of progressing decay as described in Fravolini et al. (Citation2016) and Petrillo et al. (Citation2016). Wood (1 g fresh weight [fw]) and soil (5 g, fw) samples were oven dried (105°C) for 24 h to determine their dry weight. The volatile solid (VS) concentration was determined from the weight loss following ignition in a muffle furnace (Carbolite, CWF 1000) at 550°C for 5 h. Total C and nitrogen contents of the oven-dried samples were analyzed using a CN analyzer (TruSpec CHN; LECO, Michigan, USA). Electrical conductivity (EC) and pH were determined in water extracts (1:10 and 1:20, w/v for soil and wood, respectively) using a conductivity Meter LF 330 (WTW, Weilheim, Germany) and a pH meter Metrohm 744, respectively. Inorganic nitrogen (NH4+, NO3−) was measured in 0.0125 M CaCl2 soil extracts (Kandeler Citation1993a, Citation1993b). Both the total and available P contents in soil samples were determined according to the ascorbic acid method (Kuo Citation1996).
Potential enzyme activities
A heteromolecular exchange procedure, using a 4 percent solution of lysozyme as desorbant and a bead-beating agent (Fornasier and Margon Citation2007), was performed to assess the following hydrolases in wood (0.1 g, fw) and soil (0.2 g, fw) samples—(1) C-cycle: β-glucosidase (gluc), xylosidase (xylo), and cellulase (cell); (2) N-cycle: chitinase (chit) and leucine-aminopeptidase (leu); and (3) P-cycle: alkaline and acid phosphomonoesterases (alkP and acP). In order to perform the enzymatic multiple assay for wood extracts, some modifications were required as described by Gómez-Brandón et al. (Citation2017). All measurements were performed in duplicate for each field replicate, and the activities were expressed as nanomoles of 4-methyl-umbelliferyl (MUF) min−1 g−1 dry (wood/soil).
Molecular analyses
Wood and soil microbial biomass index (dsDNA)
Direct extraction of total wood (0.1 g, fw) and soil (0.2 g, fw) DNA followed by PicoGreen-based quantification of crude (not purified) double-stranded DNA (dsDNA) was performed to estimate wood and soil microbial biomass (Fornasier et al. Citation2014). Some modifications were required to determine the dsDNA content in wood as described in Gómez-Brandón et al. (Citation2017).
DNA extraction
The whole-community DNA was extracted from wood (0.1 g, fw) and soil samples (0.2 g, fw) and was purified using a commercial kit (FastDNA Kit for Soil, MP-Biomedicals) as described in Ascher et al. (Citation2009) for further downstream analyses. For wood samples, one ceramic sphere (Lysing Matrix E, MP, Biomedicals) was added to the lysing tubes so as to guarantee accurate cell disruption of the woody tissue. DNA extracts were qualitatively and quantitatively characterized as described in Bardelli et al. (Citation2017).
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was performed to determine the 18S rRNA gene copy number of fungi, the abundance of the functional gene nifH in the wood and the soil samples. qPCR was carried out using a Rotorgene 6000 Real Time Thermal Cycler (Corbett Research, Sydney, Australia) in combination with the Rotorgene Series Software 1.7. To build the standard curves we used as templates the purified PCR products of known concentrations of the following pure cultures: Fusarium solani (DSMZ 10696), fungi, and Azospirillum irakense (DSMZ 11586), nifH gene. The primer pairs were FF390/FR1 (fungi; Prévost-Bouré et al. Citation2011) and nifHf/nifHr (nifH gene; Töwe et al. Citation2010). Stock concentration (gene copies µL−1) was determined via PicoGreen measurement and was freshly prepared for each run. Tenfold dilutions ranging from 109 to 102 copies µL−1 were used for the standard curve construction. Quantitative PCR was performed in 20-µL volumes. Each reaction mix contained 1X Sensimix™ SYBR® Hi-rox (Bioline, USA), forward and reverse primers (200 nM each primer), 0.4 mg mL−1 BSA, distilled water (RNase/DNase free, Gibco™, UK), and 2 µL of either 1:10 diluted DNA extract or tenfold diluted standard DNA. All the standards and samples were run in duplicate. After an initial denaturation at 95°C for 10 min, thermal cycling comprised forty cycles of 15 s at 95°C, 30 s at 50°C, 30 s at 72°C for fungi, and 45 s at 95°C, 45 s at 55°C, 45 s at 72°C for nifH gene. To check for product specificity and potential primer dimer formation, runs were completed with a melting analysis starting from 65°C to 95°C with temperature increments of 0.25°C (0.5°C for nifH gene) and a transition rate of 5 s. The purity of the amplicons was checked by the presence of a single band of the expected length on a 1 percent agarose gel stained with the DNA stain Midori Green (Nippon Genetics, Germany) and visualized by UV transillumination (Vilber Lourmat Deutschland GmbH).
Statistical analyses
A factorial analysis of variance (ANOVA) was done to evaluate the effect of exposure (north vs. south exposure) and time (t0, t1, t2, t3) on wood and soil physicochemical and microbiological parameters. Normality and variance homogeneity of the data were tested prior to ANOVA using the Shapiro-Wilks and Levene tests, respectively. Prior to analysis, data were log- or square root–transformed to meet the assumptions for ANOVA (when it was necessary). Significant differences (p ≤ 0.05) were analyzed by paired comparisons with the Tukey HSD test. A nonparametric test (Kruskal-Wallis test) was performed when the data did not meet the normality condition. Associations between the potential enzymatic activities and the main chemical and microbiological variables were explored by Pearson’s correlation. These analyses were performed using Statistica 9 (StatSoft, USA). Nonmetric multidimensional scaling (NMDS) based on Bray-Curtis distance was performed using R 3.1.2 (open source software) to map the wood and soil physicochemical parameters to the shifts in microbial biomass (dsDNA), microbial abundance (qPCR), and hydrolytic enzyme activities as a function of slope exposure and time. The lengths of the arrows indicate the direction of maximum correlation of the physicochemical parameters, and the significance level was assessed with a permutation test implemented in the envfit function of vegan library (Oksanen et al. Citation2008).
Results
An overview of the wood physicochemical and (micro)biological parameters as a function of exposure (north- vs. south-facing slope) and time (0–156 weeks) is given in and and those for the soil in and . The statistical results are shown in .
Table 1. Physicochemical properties of the wood samples collected in August 2013 (t0), July 2014 (t1; 52 weeks), July 2015 (t2; 104 weeks), and July 2016 (t3; 156 weeks) in the in-field mesocosm experiment at north- and south-facing sites (N5 and S10, respectively) located at the same elevation (2,400 m a.s.l.). Values are means (n = 3) with standard deviations in brackets. Data are expressed on a dry weight (dw) basis
Table 2. Physicochemical properties of the soil samples collected in August 2013 (t0), July 2014 (t1; 52 weeks), July 2015 (t2; 104 weeks), and July 2016 (t3; 156 weeks) in the in-field mesocosm experiment at north- and south-facing sites (N5 and S10, respectively) located at the same elevation (2,400 m a.s.l.). Values are means (n = 3) with the standard deviations in brackets. Data are expressed on a dry weight (dw) basis
Table 3. Microbiological properties of the wood and soil samples collected in August 2013 (t0), July 2014 (t1; 52 weeks), July 2015 (t2; 104 weeks), and July 2016 (t3; 156 weeks) in the in-field mesocosm experiment at north- and south-facing sites (N5 and S10, respectively) located at the same elevation (2,400 m a.s.l.). Values are means (n = 3) with the standard deviations in brackets. Data are expressed on a dry weight (dw) basis
Table 4. Factorial ANOVA of the soil and wood physicochemical and microbiological parameters as a function of slope exposure and time at the north- and south-facing sites (N5 and S10, respectively) located at the same elevation (2,400 m a.s.l.)
Wood physicochemical parameters
Exposure had a significant impact on the wood moisture content, with higher values at the north- than at the south-facing slope ( and ). Moreover, a sevenfold increase in moisture levels was recorded after fifty-two weeks compared to the beginning of the experiment at both slopes, followed by a decrease until the end of the monitoring period (156 weeks). The wood VS concentration was not significantly influenced by the slope exposure, and neither exposure nor time significantly affected the wood mass ( and ). In addition, the lowest pH value was measured at the end of the monitoring period (156 weeks) at both the north- and the south-facing slopes ( and ). The exposure effect on the EC of the wood was only evident after 156 weeks, with higher values (three times higher) at the south- than at the north-facing slope. A significant decrease in EC was found after fifty-two weeks (three to five times lower than at t0), followed by an increase until the end of the monitoring at both slopes ( and ). Exposure did not have a significant impact on the wood total C content ( and ). The nitrogen content in the wood blocks was below the detection limit irrespective of the exposure and time.
Soil physicochemical parameters
Soil moisture levels were significantly higher at the north- than at the south-facing slope, and the lowest values were found after 104 weeks at both slopes ( and ). The VS concentration was almost twice as high at the north- than at the south-facing site, whereas no significant changes were found over time ( and ). Slope exposure had a significant impact on the soil pH after fifty-two weeks, with an average value of 5.0 at the north- and 5.5 at the south-facing site ( and ). A significant decrease in soil pH was recorded after fifty-two weeks at the north-facing slope, but it remained stable afterward until the end of the monitoring period. No significant differences in soil pH with time were observed at the south-facing slope ( and ). The soil EC at the south-facing site was twice as high as at the north-facing site at both the beginning and the end of the monitoring period (156 weeks), while no exposure effects were found at the intermediate sampling times. Additionally, the highest EC value was recorded after 156 weeks at both slopes ( and ). In general, the north-facing slope showed higher total C and nitrogen contents compared to the south-facing slope, regardless of the duration of the experiment ( and ). There was no significant exposure effect on the NH4+ and NO3− contents. Both parameters, however, significantly varied over time. Indeed, a very distinct increase in NH4+ was measured after fifty-two weeks at both slopes, followed by a continuous increase (two times) at the end of the monitoring period at the north-facing slope ( and ). Concerning the NO3− content, the lowest values were generally detected after 104 and 156 weeks at both north- and south-facing slopes ( and ). The total P content was influenced by neither slope exposure nor time ( and ). Overall, higher available P levels were observed at the south- than at the north-facing slope irrespective of time, except for the last sampling point (no exposure effect). Moreover, the highest available P levels were recorded after 104 weeks at both slopes, followed by a significant decrease after 156 weeks at the south-facing slope ( and ).
Wood (micro)biological parameters
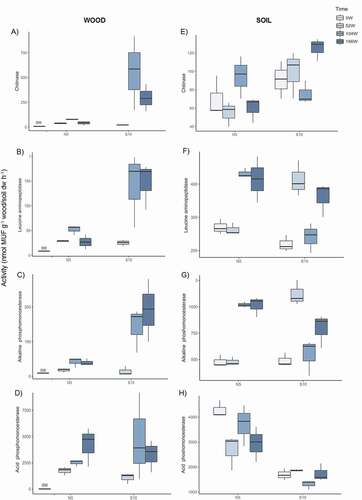
Wood microbial biomass assessed as double-stranded DNA yield (dsDNA) was as much as three times higher at the south- than at the north-facing slope irrespective of time ( and ). Moreover, a significant increase in the dsDNA content was found over time at both slopes (). Fungal and nifH gene abundances in wood were not significantly affected by the slope exposure; however, significant differences were recorded in both microbial groups over time ( and ). The highest nifH abundance was recorded after 104 and 156 weeks at the north- and the south-facing slopes, respectively. A reduction in the fungal abundance was registered after fifty-two weeks at both slopes, followed by an increase after 104 weeks, reaching similar values to those observed in the initial wood blocks (). β-glucosidase, xylosidase, and cellulase activities did not vary significantly in terms of exposure (); however, a sharp increase (approximately sixteen times higher for β-glucosidase and sixtyfold higher for xylosidase and cellulase activities) was observed after 104 weeks compared to the beginning of the experiment at both slopes (–). Afterward, a decrease in these three enzyme activities was recorded after 156 weeks at the north-facing slope. The exposure effect on the chitinase, leucine-aminopeptidase, and alkaline phosphomonoesterase activities was time dependent (–, ). Higher activities (between four and six times higher) were recorded at the south- than at the north-facing slope after 104 and 156 weeks, while no exposure effect was found after fifty-two weeks (–). Moreover, the highest activity of the three aforementioned enzymes was registered at the end of the monitoring at both slopes. Although the acid phosphomonoesterase activity was not significantly influenced by slope exposure (), a pronounced increase was found over time compared to the beginning of the monitoring period ().
Figure 1. Potential activities of β-glucosidase (A, D), xylosidase (B, E), and cellulase (C, F) of the wood and soil samples collected in August 2013 (0 weeks), July 2014 (52 weeks), July 2015 (104 weeks), and July 2016 (156 weeks) at the north- and south-facing sites (N5 and S10, respectively). Values are means (n = 3) with standard deviation
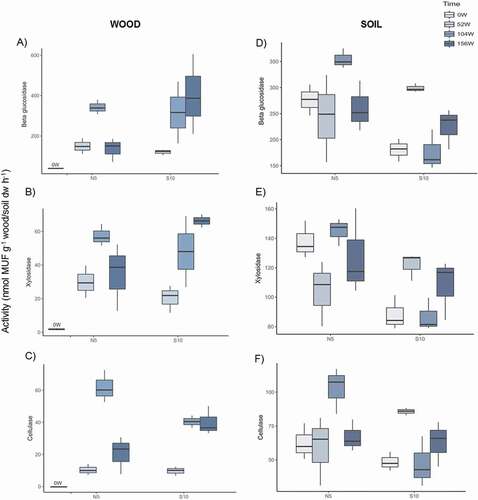
Figure 2. Potential activities of chitinase (A,E), leucine aminopeptidase (B,F), alkaline phosphomonoesterase (C, G), and acid phosphomonoesterase (D, H) of the wood and soil samples collected in August 2013 (0 weeks), July 2014 (52 weeks), July 2015 (104 weeks), and July 2016 (156 weeks) at the north- and south-facing sites (N5 and S10, respectively). Values are means (n = 3) with standard deviation
Soil (micro)biological properties
Soil microbial biomass (dsDNA) was influenced by neither slope exposure nor time ( and ). Nonetheless, slope exposure had a significant impact on soil fungal and nifH gene abundances (), being approximately three times higher at the north- than at the south-facing slope irrespective of the time (). Overall, the β-glucosidase, xylosidase, and cellulase activities were two times higher at the north- than at the south-facing slope after 104 weeks (–), whereas no exposure effect was observed for the other sampling times, except for the xylosidase activity at the beginning of the monitoring period (N > S; ). However, no significant differences over time were recorded for these enzyme activities (–, ),except for the β-glucosidase at the south-facing site (52 > 104 weeks; two times higher). Chitinase activity significantly varied in terms of exposure only after 156 weeks (S > N; , ), while no significant differences were detected over time irrespective of the slope exposure (). The exposure effect on the leucine-aminopeptidase and alkaline phosphomonoesterase activities was also time dependent (). A higher activity was recorded at the south- than at the north-facing slope after fifty-two weeks, whereas the opposite trend (N > S) was found after 104 weeks ( and ). Furthermore, both enzymes showed higher activities at the last two sampling times (104 and 156 weeks) at the north-facing site, while a higher activity was recorded after fifty-two weeks at the south-facing site. Nevertheless, acid phosphomonoesterase activity was significantly higher at the north- than at the south-facing slope regardless of the sampling time (, ).
Nonmetric multidimensional scaling (NMDS) analysis
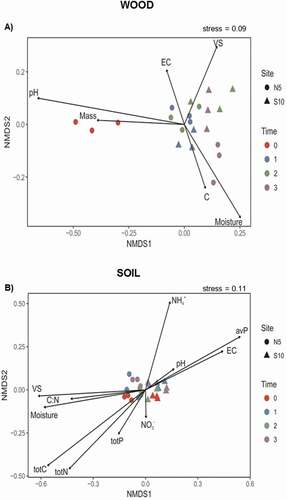
Overall, the P. abies wood blocks collected at the beginning of the monitoring (t0) clustered in the negative side of the first ordination axis (), indicating that they represent a specific microhabitat. All the other wood samples were located on the positive side of the first axis (), with wood pH (R2 = 0.45, p ≤ 0.01) being the major determinant for this differentiation. Additionally, along the second axis a separation as a function of exposure was mainly observed for the wood samples collected at the end of the monitoring period (i.e., 156 weeks; t3). Wood moisture appeared to be the most determinant factor for this differentiation (R2 = 0.31, p = 0.06). Furthermore, a clear separation in terms of slope exposure was recorded for the soil samples collected at the north- and south-facing slopes (negative and positive sides, respectively) along the first ordination axis (). The major physicochemical parameters responsible for this differentiation were total C (R2 = 0.51, p ≤ 0.001), total nitrogen (R2 = 0.40, p ≤ 0.01), and available P (R2 = 0.40, p ≤ 0.01), followed by VS (R2 = 0.38, p ≤ 0.05) and soil moisture (R2 = 0.35, p ≤ 0.05).
Figure 3. Nonmetric multidimensional scaling (NMDS) ordination was performed to map the physicochemical parameters to the shifts in wood (A) and soil (B) microbiological properties (microbial abundances and enzyme activities) as a function of time (t0 = control, August 2013; t1 = after 52 weeks, July 2014; t2 = after 104 weeks, July 2015; t3 = after 156 weeks, July 2016) and slope exposure (north-facing slope = point symbol; south-facing slope = triangle symbol)
Discussion
In line with previous studies performed in the Trentino area (Egli et al. Citation2006, Citation2009; Fravolini et al. Citation2016; Bardelli et al. Citation2017), the soils located at the north-facing slope were moister and richer in total C and nitrogen contents compared to those at the south-facing slope. Along a climosequence, Fravolini et al. (Citation2016) observed faster decay rates of the P. abies wood blocks at the north- than at the south-facing slopes up to an elevation of 1,800 m a.s.l., probably because of the higher soil moisture and clay content along with a lower soil pH. These are favorable conditions, especially for fungi, which can better develop on north-facing sites. Nevertheless, we did not observe distinct changes in the P. abies wood-block mass during the three-year observation period. In general, this fits well with the findings of Fravolini et al. (Citation2016) and Petrillo et al. (Citation2016), who measured very slow and sometimes almost barely measurable decay rates of P. abies in alpine environments with wood-mass decay rates often between 0.018 and 0.040 y−1. These authors explained the low decay rates to be the result of climatic conditions and the extremely slow decomposition rate of lignin.
In this study, the higher soil moisture and OM levels recorded at the northern slope were accompanied by higher soil fungal and nifH gene abundances in comparison with the southern slope. Bardelli et al. (Citation2017) did not observe an exposure effect on soil fungal communities (qPCR-based) up to an altitude of 1,800 m a.s.l. in the same study area. These discrepancies in terms of exposure might be related to the higher proportion of grassland and a colder climate registered at 2,400 m a.s.l. compared to the other study sites surveyed by Bardelli et al. (Citation2017). Indeed, complex interactions between local-scale factors, such as soil properties and vegetation composition, are known to largely affect soil microbial communities and their spatial distribution in mountain environments (Ascher et al. Citation2012; Regan et al. Citation2017; Siles et al. Citation2017).
In contrast to soil, exposure did not have a significant impact on the two previously mentioned microbial groups (fungi and nifH) in the P. abies wood blocks. This is in disagreement with our second hypothesis. However, a general increase in their microbial abundance was observed over time. This phenomenon could be ascribed to the release of nutrients with progressing wood decay, which provide a source of nutrients for new colonizing microorganisms—microbial succession (Hoppe et al. Citation2015). Moreover, more acidic conditions (lower pH values) were detected in the P. abies wood blocks at the end of the monitoring period, which might lead to favorable conditions for fungal growth and their activity. In accordance with Baldrian et al. (Citation2016) and Gómez-Brandón et al. (Citation2017), we also found that pH was one of the most influential driving factors shaping wood microbial abundance and activity during the early stages of decomposition. Hoppe et al. (Citation2015) reported, however, that variations in the abundances of bacterial phyla (using pyrosequencing analysis) are, rather, determined by a combination of several wood properties (i.e., C and nitrogen contents and wood moisture) than by single parameters such as pH alone.
Although previous studies pointed to an existing association between fungi and diazotrophic bacteria in wood (Gómez-Brandón et al. Citation2017; Hoppe et al. Citation2014; Johnston, Boddy, and Weightman Citation2016), we did not find a significant interaction between these two microbial groups in terms of abundance. This might be because of the fact that we focused on the early stages of decomposition of Norway spruce deadwood. Indeed, Hoppe et al. (Citation2014) found a stronger positive correlation between fungal fructification and nifH diversity during the intermediate stage of decay of P. abies and Fagus sylvatica logs. Another plausible explanation could be related to the moisture content, because it is known to be one of the limiting factors of the biological nitrogen fixation (Rinne et al. Citation2017). In fact, a decrease in wood moisture level was found at the end of the monitoring period in our study.
Furthermore, enzyme-specific exposure effects were observed for the P. abies wood blocks as previously shown by Gómez-Brandón et al. (Citation2017). On the one hand, the C-related enzyme activities were not affected in terms of exposure, while, on the other hand, those activities involved in the N cycle (leucine-aminopeptidase and chitinase) and P cycle (alkaline phosphomonoesterase) showed higher activities at the southern slope. This thermal signal (S > N) was time dependent (i.e., after 104 and 156 weeks), partially corroborating our first hypothesis. This is in line with the fact that the wood samples at the south-facing slope were characterized by a higher microbial biomass (based on dsDNA). Accordingly, the dsDNA yields were positively correlated with most of the previously mentioned enzymes (data not shown) at the end of the monitoring period, probably because of more suitable conditions (i.e., higher nutrient availability) for microbial growth as wood decay progresses (Gonzalez-Polo, Fernandez-Souto, and Austin Citation2013; Pastorelli et al. Citation2017; Petrillo et al. Citation2015, Citation2016). In fact, the increase in fungal abundance after 104 weeks was also accompanied by an increase in the C-related enzyme activities in the P. abies wood blocks, suggesting the higher activity of the wood-inhabiting fungi at this stage of decay.
As occurred with wood, the exposure effect on soil enzyme activities related to C and N cycles was also time dependent. Nonetheless, soil microbial biomass (dsDNA) did not vary in terms of exposure, which is in agreement with Bardelli et al. (Citation2017). These authors, however, found higher levels of microbial biomass on northern slopes by using the substrate-induced respiration approach. The different effect of exposure on soil microbial biomass could be interpreted in light of the so-called method-result effect (Nannipieri et al. Citation2003, Citation2017). Furthermore, we observed higher soil acid phosphomonoesterase activity at the northern slope over time probably because of the lower soil P availability at this slope than in the comparable south-facing one. Indeed, an increase in P-acquiring enzyme activities is expected under conditions of soil P deficiency (Fraser et al. Citation2015).
Conclusion
Our field mesocosm experiment enabled us to observe how different thermal and moisture conditions (due to exposure effects) affected wood- and soil-inhabiting microbiota in terms of their abundance and activity in the studied alpine setting. A higher abundance of fungi and nitrogen-fixing bacteria was recorded in the topsoil layer at the north-facing site, characterized by cooler and moister conditions. In the P. abies wood blocks, however, the abundance of these two microbial groups did not respond to exposure, while an increase in their abundance was observed with progressing wood decay along with more acidic conditions. This points to wood pH as a crucial driving factor of the deadwood microbiota during the early decay stages. The impact of exposure was also enzyme specific and time dependent for both the P. abies wood blocks and the underlying soil. Altogether, this indicates the importance of using multiple methods that include a broad array of enzymes to avoid potential misinterpretation of effects on specific nutrient cycles (Nannipieri et al. Citation2012) caused by environmental changes in alpine ecosystems.
Acknowledgments
We acknowledge Paul Fraiz for his highly valuable help in language editing. We are also indebted to Dr. Fabio Angeli of the Ufficio distrettuale forestale–Malé (Trento, Italy) and his team for their support in the field. We would like to thank Sabina Vrečko, Sebastian Waldhuber, and Ljubica Begovic for their help in the laboratory.
Additional information
Funding
References
- A’Bear, A. D., T. H. Jones, E. Kandeler, and L. Boddy. 2014. Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biology and Biochemistry 70:1–13.
- Allison, S. D., M. D. Wallenstein, and M. A. Bradford. 2010. Soil-carbon response to warming dependent on microbial physiology. Nature Geoscience 3:336–40.
- Ascher, J., M. T. Ceccherini, O. L. Pantani, A. Agnelli, F. Borgogni, G. Guerri, P. Nannipieri, and G. Pietramellara. 2009. Sequential extraction and genetic fingerprinting of a forest soil metagenome. Apply Soil Ecology 42:176–81.
- Ascher, J., G. Sartori, U. Graefe, B. Thornton, M. T. Ceccherini, G. Pietramellara, and M. Egli. 2012. Are humus forms, mesofauna and microflora in subalpine forest soils sensitive to thermal conditions? Biology and Fertility of Soils 48:709–25.
- Baldrian, P., P. Zrustova, V. Tláskal, A. Davidova, V. Merhautová, and T. Vrška. 2016. Fungi associated with decomposing deadwood in a natural beech-dominated forest. Fungal Ecology 23:109–22.
- Bardelli, T., M. Gómez-Brandón, J. Ascher-Jenull, F. Fornasier, P. Arfaioli, D. Francioli, M. Egli, G. Sartori, H. Insam, and G. Pietramellara. 2017. Effects of slope exposure on soil physico-chemical and microbiological properties along an altitudinal climosequence in the Italian Alps. Science of the Total Environment 575:1041–55.
- Beniston, M., H. F. Diaz, and R. S. Bradley. 1997. Climatic change at high elevation sites: An overview. Climatic Change 36:233–51.
- Djukic, I., F. Zehetner, A. Mentler, and M. H. Gerzabek. 2010. Microbial community composition and activity in different Alpine vegetation zones. Soil Biology and Biochemistry 42:155–61.
- Dullinger, S., T. Dirnböck, and G. Grabherr. 2003. Patterns of shrub invasion into high mountain grasslands of the northern calcareous Alps, Austria. Arctic, Antarctic and Alpine Research 35:434–41.
- Egli, M., S. Hafner, C. Derungs, J. Ascher-Jenull, F. Camin, G. Sartori, G. Raab, M. Paolini, L. Bontempo, L. Ziller, et al. 2016. Decomposition and stabilisation of Norway spruce needle-derived material in Alpine soils using a 13C-labelling approach in the field. Biogeochemistry 13:321–38.
- Egli, M., A. Mirabella, G. Sartori, R. Zanelli, and S. Bischof. 2006. Effect of north and south exposure on weathering rates and clay mineral formation in Alpine soils. Catena 67:155–74.
- Egli, M., G. Sartori, A. Mirabella, and F. Favilli. 2009. Effect of north and south exposure on organic matter in high Alpine soils. Geoderma 149:124–36.
- Floudas, D., M. Binder, R. Riley, K. Barry, R. A. Blanchette, B. Henrissat, A. T. Martínez, R. Otillar, J. W. Spatafora, J. S. Yadav, et al. 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–19.
- Fornasier, F., J. Ascher, M. T. Ceccherini, E. Tomat, and G. Pietramellara. 2014. A simplified rapid, low-cost and versatile DNA-based assessment of soil microbial biomass. Ecological Indicators 45:75–82.
- Fornasier, F., and A. Margon. 2007. Bovine serum albumin and Triton X-100 greatly increase phosphomonoesterases and arylsulphatase extraction yield from soil. Soil Biology and Biochemistry 39:2682–84.
- Fraser, T. D., D. H. Lynch, E. Bent, M. H. Entz, and K. E. Dunfield. 2015. Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biology and Biochemistry 88:137–47.
- Fravolini, G., M. Egli, C. Derungs, P. Cherubini, J. Ascher-Jenull, M. Gómez Brandón, T. Bardelli, R. Tognetti, F. Lombardi, and M. Marchetti. 2016. Soil attributes and microclimate are important drivers of initial deadwood decay in sub-alpine Norway spruce forests. Science of the Total Environment 569-570:1064–76.
- Gómez-Brandón, M., J. Ascher-Jenull, T. Bardelli, F. Fornasier, G. Fravolini, P. Arfaioli, M. T. Ceccherini, G. Pietramellara, K. Lamorski, C. Slawiński, et al. 2017. Physico-chemical and microbiological evidence of exposure effects on Picea abies: Coarse woody debris at different stages of decay. Forest Ecology and Management 391:376–89.
- Gonzalez-Polo, M., A. Fernandez-Souto, and A. T. Austin. 2013. Coarse woody debris stimulates soil enzymatic activity and litter decomposition in an old-growth temperate forest of Patagonia, Argentina. Ecosystems 16:1025–38.
- Hoppe, B., T. Kahl, P. Karasch, T. Wubet, J. Bauhus, F. Buscot, and D. Krüger. 2014. Network analysis reveals ecological links between N-fixing bacteria and wood-decaying fungi. PLoS ONE 9:e88141.
- Hoppe, B., D. Krüger, T. Kahl, T. Arnstadt, F. Buscot, F. Bauhus, and T. Wubet. 2015. A pyrosequencing insight into sprawling bacterial diversity and community dynamics in decaying deadwood logs of Fagus sylvatica and Picea Abies. Scientific Reports 5:9456.
- Hoppe, B., W. Purahong, T. Wubet, T. Kahl, J. Bauhus, T. Arnstadt, M. Hofrichter, F. Buscot, and D. KrüGer. 2016. Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Diversity 77:367–79.
- Johnston, S. R., L. Boddy, and A. J. Weightman. 2016. Bacteria in decomposing wood and their interactions with wood-decay fungi. FEMS Microbiolgy Ecology 92:1–12.
- Kandeler, E. 1993a. Bestimmung von Ammonium. In Bodenbiologische Arbeitsmethoden, ed. F. Schinner, R. Öhlinger, E. Kandeler, and R. Margesin, 366–68. Berlin and Heidelberg: Springer.
- Kandeler, E. 1993b. Bestimmung von Nitrat. In Bodenbiologische Arbeitsmethoden, ed. F. Schinner, R. Öhlinger, E. Kandeler, and R. Margesin, 369–71. Berlin and Heidelberg: Springer.
- Kielak, A. M., T. R. Scheublin, L. W. Mendes, J. A. van Veen, and E. E. Kuramae. 2016. Bacterial community succession in pine-wood decomposition. Frontiers in Microbiology 7:1–12. doi:https://doi.org/10.3389/fmicb.2016.00231.
- Kuo, S. 1996. Phosphorus. In Methods of soil analysis. Part 3: Chemical methods. SSSA Book Series, ed D. L. Sparks, vol. 5, 869–919. Madison, WI: Soil Science Society of America.
- Meyer, A., A. Focks, V. Radl, D. Keil, G. Welzl, I. Schöning, S. Boch, S. Marhan, E. Kandeler, and M. Schloter. 2013. Different land use intensities in grassland ecosystems drive ecology of microbial communities involved in nitrogen turnover in soil. PLoS ONE 8:e73536.
- Moroni, M. T., D. M. Morris, C. Shaw, J. N. Stokland, M. E. Harmon, N. J. Fenton, K. Merganičová, J. Merganič, K. Okabe, and U. Hagemann. 2015. Buried wood: A common yet poorly documented form of deadwood. Ecosystems 18:605–28.
- Motta, R., and P. Nola. 2001. Growth trends and dynamics in sub-alpine forest stands in the Varaita Valley (Piedmont, Italy) and their relationships with human activities and global change. Journal of Vegetation Science 12:219–30.
- Myers, R. T., D. R. Zak, D. C. White, and A. Peacock. 2001. Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Science Society of American Journal 65:359–67.
- Nannipieri, P., J. Ascher, M. T. Ceccherini, L. Landi, G. Pietramellara, and G. Renella. 2003. Microbial diversity and soil functions. European Journal of Soil Science 54:655–70.
- Nannipieri, P., J. Ascher-Jenull, M. T. Ceccherini, L. Giagnoni, G. Pietramellara, and G. Renella. 2017. Nannipieri, P., Ascher, J., Ceccherini, M.T., Landi, L., Pietramellara, G. & Renella, G., 2003: Microbial diversity and soil functions. European Journal of Soil Science, 54: 655–670. European Journal of Soil Science 68:2–5. doi:https://doi.org/10.1111/ejss.2_12398.
- Nannipieri, P., L. Giagnoni, G. Renella, E. Puglisi, B. Ceccanti, G. Masciandaro, F. Fornasier, M. C. Moscatelli, and S. Marinari. 2012. Soil enzymology: Classical and molecular approaches. Biology and Fertility of Soils 48:743–62.
- Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, G.L. Simpson, P. Sólymos, M. Henry, H. Stevens, and H. Wagner. 2008. Vegan: Community ecology package – R package version 1. 15–2. Accessed December 19, 2013. http://cran.r-project.org.
- Pan, Y., R. A. Birdsey, J. Fang, R. Houghton, P. E. Kauppi, W. A. Kurz, O. L. Phillips, A. Shvidenko, S. L. Lewis, J. G. Canadell, et al. 2011. A large and persistent carbon sink in the world’s forests. Science 333:988–93.
- Pastorelli, R., A. E. Agnelli, I. De Meo, A. Graziani, A. Paletto, and A. Lagomarsino. 2017. Analysis of microbial diversity and greenhouse gas production of decaying pine logs. Forests 8:224. doi:https://doi.org/10.3390/f8070224.
- Petrillo, M., P. Cherubini, G. Fravolini, M. Marchetti, J. Ascher-Jenull, M. Schärer, H. A. Synal, D. Bertoldi, F. Camin, R. Larcher, et al. 2016. Time since death and decay rate constants of Norway spruce and European larch deadwood in subalpine forests determined using dendrochronology and radiocarbon dating. Biogeosciences 13:1537–52.
- Petrillo, M., P. Cherubini, G. Sartori, S. Abiven, J. Ascher, D. Bertoldi, F. Camin, A. Barbero, R. Larcher, and M. Egli. 2015. Decomposition of Norway spruce and European larch coarse woody debris (CWD) in relation to different elevation and exposure in an Alpine setting. iForest 9:154–64.
- Prévost-Bouré, N. C., R. Christen, S. Dequiedt, C. Mougel, M. Lelièvre, C. Jolivet, H. R. Shahbazkia, L. Guillou, D. Arrouays, and L. Ranjard. 2011. Validation and application of a PCR primer set to quantify fungal communities in the soil environment by real-time PCR. PLoS One 6:e24166.
- Purahong, W., B. Hoppe, T. Kahl, M. Schloter, E. D. Schulze, J. Bauhus, F. Buscot, and D. Krüger. 2014. Changes within a single land-use category alter microbial diversity and community structure: Molecular evidence from wood-inhabiting fungi in forest ecosystems. Journal of Environmental Management 139:109–19.
- Rajala, T., M. Peltoniemi, T. Pennanen, and R. Mäkipää. 2012. Fungal community dynamics in relation to susbstrate quality of decaying Norway spruce (Picea abies [L.] Karst.) logs in boreal forests. FEMS Microbiology Ecology 81:494–505.
- Regan, K., B. Stempfhuber, M. Schloter, F. Rasche, D. Prati, L. Philippot, R. S. Boeddinghaus, E. Kandeler, and S. Marhan. 2017. Spatial and temporal dynamics of nitrogen fixing, nitrifying and denitrifying microbes in an unfertilized grassland soil. Soil Biology and Biochemistry 109:214–26.
- Rinne, K. T., T. Rajala, K. Peltoniemi, J. Chen, A. Smolander, and R. Mäkipää. 2017. Accumulation rates and sources of external nitrogen in decaying wood in a Norway spruce dominated forest. Functional Ecology 31:530–41.
- Sboarina, C., and A. Cescatti, 2004: Il clima del Trentino – Distribuzione spaziale delle principali variabili climatiche [The climate of Trentino: Spatial distribution of the principal climatic variables]. Report 33. Centro di Ecologia Alpina Monte Bondone, Trento, Italy, p. 20.
- Siles, J. A., T. Cajthaml, A. Filipová, S. Minerbi, and R. Margesin. 2017. Altitudinal, seasonal and interannual shifts in microbial communities and chemical composition of soil organic matter in Alpine forest soils. Soil Biology and Biochemistry 112:1–13.
- Sun, B., X. Y. Wang, F. Wang, Y. J. Jiang, and X. X. Zhang. 2013. Assessing the relative effects of geographic location and soil type on microbial communities associated with straw decomposition. Applied and Environmental Microbiology 79:3327–35.
- Theurillat, J. P., and A. Guisan. 2001. Potential impact of climate change on vegetation in the European Alps: A review. Climatic Change 50:77–109.
- Töwe, S., A. Albert, K. Kleineidam, R. Brankatschk, A. Dümig, G. Welzl, J. C. Munch, J. Zeyer, and M. Schloter. 2010. Abundance of microbes involved in nitrogen transformation in the rhizosphere of Leucanthemopsis alpina (L.) heavy wood grown in soils from different sites of the Damma glacier forefield. Microbial Ecology 60:762–70.
- van der Wal, A., W. de Boer, W. Smant, and J. A. van Veen. 2007. Initial decay of woody fragments in soil is influenced by size, vertical position, nitrogen availability and soil origin. Plant and Soil 301:189–201.
