ABSTRACT
Body size is one of the most important individual traits, determining various other life-history traits, including fitness. Both evolutionary and ecological factors shape the body size in arthropods, but the relative contribution of abiotic drivers acting at different spatial scales has been little investigated. We aimed to identify the importance of two broad-scale variables (study region and elevation) in shaping body size of the free-running and locally abundant wolf spider Pardosa palustris (Linnaeus 1758), in contrast to the fine-scaled variable topographic position. Therefore, we set up transects along environmental gradients in the arctic-alpine ecosystems of Norway, which we analyzed using a random forest approach to identify the relative importance of topographic position, elevation, and study region on body size of P. palustris. Our approach revealed that research region was the best explanatory variable, followed by elevation and topographic position. Differences in body size were most likely a consequence of the pronounced differences in season length and the ability of P. palustris to avoid local unfavorable environmental conditions due to its high mobility.
Introduction
The identification of spatial patterns in life-history traits, as well as the mechanisms that generate and maintain them, is mandatory in ecological field studies. This will, consequently, result in a better understanding of spatio-temporal patterns of species distribution and adaptations in response to changing environments. Consequently, the driving principles of phenotypic variation in individual–environment interactions are in the focus of current research (e.g., Chevin, Lande, and Mace Citation2010; Ameline et al. Citation2018; Hein et al. Citation2018). However, knowledge about specific spatial and temporal variations of life history traits remains scarce in invertebrates, especially in cold environments (Homburg et al. Citation2013; Høye and Sikes Citation2013; Ameline et al. Citation2017). Body size is one of the key features of life history traits research in ectotherms (Angiletta et al. Citation2004; Chown and Gaston Citation2010; Høye and Hammel Citation2010), because it is considered a proxy for fecundity, body condition, and survival (e.g., Hodkinson Citation2005; Bowden, Høye, and Buddle Citation2013; Penell, Raub, and Höfer Citation2018). In general, larger individuals show higher fecundity and lower mortality rates (Smith and Fretwell Citation1974; Fox and Czesak Citation2000).
Body size is shaped by both evolutionary and ecological factors acting at different scales. Still, few studies have investigated both its fine- and broad-scale drivers (but see Lowe et al. Citation2014). The main local ecological drivers of body size in terrestrial arthropods are season length, the concomitant resource availability and quality, and temperature (Chown and Gaston Citation2010; Shelomi Citation2012). Univoltine alpine species are often limited by season length with respect to completion of their life cycles (Strathdee and Bale Citation1998). Consequently, variations of the onset of the growing season lead to an increase in the size of the arctic wolf spider Pardosa glacialis (Thorell 1872) in Greenland. A longer summer season length induced by the earlier onset of the growing season leads to bigger individuals (Høye et al. Citation2009). This finding corresponds to a converse Bergmann’s cline, where warmer temperatures and prolonged summer season result in larger specimens, whereas a Bergmann’s cline describes larger specimens in response to colder temperatures (e.g., Mousseau Citation1997; Blanckenhorn and Demont Citation2004). In this context, the characteristic snow-cover patterns and the concomitant environmental drivers in the alpine environments of the Scandes formed by topography and the dominant wind direction (Gjærevoll Citation1956; Löffler Citation2002) might also determine the life-history traits of spiders. At a local scale, we found the thickest snow layers and thus the shortest seasons on the southern exposed lee-slopes, and the least snow-cover duration on ridges with little or no snow cover during winter (Löffler Citation2005; Löffler and Finch Citation2005), resulting in specific distribution and abundance patterns of ectothermic species in arctic-alpine ecosystems (Hein et al. Citation2014; Beckers et al. Citation2018).
Commonly, higher temperatures lead to faster development and thus earlier fertility in ectothermic species (Willmer, Stone, and Johnston Citation2004; Blanckenhorn and Demont Citation2004). This results in the largest body sizes at the lowest temperatures (Chown and Gaston Citation2010), which is also known as the temperature-size rule (reviewed by Atkinson and Sibly Citation1997). Increasing body size in colder environments is assumed to be the result of prolonged life-cycles (slower growth) in response to cold temperatures (Angiletta et al. Citation2004). Here, the disadvantage of delayed maturation is compensated for by an increase in fecundity. Larger females have an increased fecundity and they often produce a larger number of eggs (e.g., Simpson Citation1995; Prenter, Elwood, and Montgomery Citation1999; Fox and Czesak Citation2000; Puzin et al. Citation2011). Spiders are suitable model organisms when focusing on individual–environment interactions (e.g., Wise Citation1993; Hendrickx et al. Citation2003; Renault et al. Citation2016), because prosoma width is fixed at each life stage and not, or only slightly, influenced by starvation (Miyashita Citation1968). Lycosid spiders are especially suitable for such a study for several reasons: (1) lycosid spiders are highly abundant in many ecosystems; (2) they can be easily sampled with pitfall traps; (3) they are known to show a strong relationship to micro-climate and vegetation structure. Lycosid spiders, like Araneae in general, are known to have well-defined habitat preferences (Schaefer Citation1970; Frick, Nentwig, and Kropf Citation2007; Muff et al. Citation2009), which are expressed at very fine scales in the heterogenous arctic-alpine ecosystems of Scandinavia (Finch and Löffler Citation2010; Hein et al. Citation2014). Prosoma width indeed proved to be a reliable proxy for body size in lycosid spiders in previous studies (e.g., Hagstrum Citation1971; Jakob, Marshall, and Uetz Citation1996; Hendrickx and Maelfait Citation2003; Pétillon et al. Citation2009).
The aim of this study was to compare the importance of broad-scaled (study region and elevation) versus fine-scaled (topographic position) environmental variables in order to decipher their respective effect on the body size of the free-running and locally abundant wolf spider Pardosa palustris (Linnaeus 1758). We tested the hypothesis that the influence of these variables on body size decreases as the scale they act on increases. The local abiotic factor topographic position, acting at the fine scale, is thus expected to show a higher explanatory power on body size than the regional abiotic factors of study region and elevation, which act at the broad scale. Consequently, this hypothesis was tested using a study design from fine- to broad-scale in order to gain insights into how extent local and regional factors shape body size in the highly mobile and locally abundant spider Pardosa palustris (e.g., Hauge and Refseth Citation1979; Hein et al. Citation2014, Citation2015).
Material and methods
Study sites
The sampling was carried out in Vågå (Oppland, 61°53ʹ N; 9°15ʹ E; the highest peak here is Blåhø 1617 m.a.s.l.) and Geiranger (Møre og Romsdal, 62°03ʹ N; 7°15ʹ E; the highest peak here is Dalsnibba 1495 m.a.s.l.) in central Norway in 2009 (). Due to the north-south extension of the Scandinavian mountain chain, Norway is divided into a western oceanic and an eastern continental climate section (Moen Citation1998). Within a relatively short linear distance (approximately 100 km in our study), considerable climatic contrasts emerge in this part of Norway. According to Moen (Citation1998), the western study region is part of the weak oceanic section (o1), with relatively high winter temperatures, relatively low summer temperatures, and relatively high precipitation and humidity values. In contrary, the eastern study region is part of the weak continental section (c1), with relatively low winter temperatures, relatively high summer temperatures, and low precipitation values. The study region in Vågå is part of the most continental climate section in Norway and is characterized by low annual precipitation, about 300– 400 mm in the valleys. In contrast, the western study region is part of the inner fjords climate section with high annual precipitation of about 1500–2000 mm in the valleys (Löffler and Finch Citation2005). As such, the climatic contrasts are expressed mainly by differences in annual precipitation and the concomitant snow-cover duration, which is, on average over the last fifty years, about thirty days shorter at Vågå ().
Figure 1. Map of Norway with focus on the two study regions in the western suboceanic (Geiranger) and the eastern continental (Vågå) part of Norway. According to Moen (Citation1998), Geiranger is part of the weak oceanic section (o1) and Vågå part of the continental (c1) section.
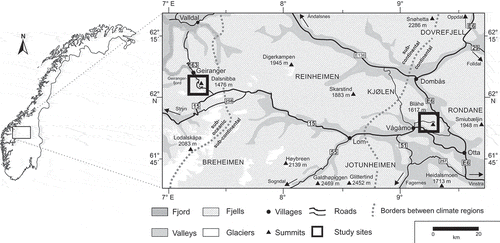
Figure 2. Long-term data on the snow water equivalent (upper) and the snow-free period (lower) in the western (left) and eastern (right) study areas. Data on the snow-free period are based on the core seasonal (i.e., continuous) snow cover, without consideration of short-term snow cover. The long-term mean is given in number of days, including the standard deviation.
Data source: MET Norway (Citation2018).
In both study regions, the low-alpine vegetation is dominated by shrub and heather communities, whereas the middle-alpine vegetation is dominated by graminoids (Dahl Citation1986). In Geiranger, the treeline is located at ~750 m.a.s.l., and in Vågå at ~1030 m.a.s.l. The upper limit of the low-alpine belt is found at ~1200 m.a.s.l. in Geiranger and at ~1350 m.a.s.l. in Vågå, respectively. The pronounced oceanity in the western oceanic section results in a depression of the elevational belts, which leads to similar and comparable ecological settings at different elevational levels in the western oceanic and the eastern continental section (Löffler Citation2003; Löffler and Finch Citation2005; Löffler, Pape, and Wundram Citation2006).
Sampling design
To address the general biogeographic problem of scale (Levin Citation1992), we implemented a multiscale instrumental monitoring network along the regional climate gradient, the alpine elevational gradient, and the microtopographic gradient (e.g., Löffler, Pape, and Wundram Citation2006; Hein et al. Citation2014). Pitfall traps were installed at ridges, depressions, and south-/north-facing slopes to account for the pronounced differences in snow-cover duration. Ridges remain more or less snow-free during the winter, whereas snow cover lasts longest at south-facing slopes as a result of snow redistribution by the strongest winds, which occur from northern directions. Ground-dwelling arthropods were sampled at sampling sites along two elevation transects from the tree line up to the highest peaks (western oceanic Norway—Geiranger n = 32; eastern continental Norway—Vågå n = 40). The transects reached from ~ 830–1422 m.a.s.l. in Geiranger, and from 1035–1534 m.a.s.l. in Vågå, respectively. Our microtopographic gradients along the elevation gradient were assumed to display the strongest environmental differences at short distances (Nagy and Grabherr Citation2009; Wundram, Pape, and Löffler Citation2010). The snow-cover duration results in specific fine-scaled vegetation patterns, where ridge positions are dominated by lichen species (Alectoria ochroleuca and Flavocetraria nivalis), slopes by dwarf shrubs (Betula nana, Calluna vulgaris, Vaccinium myrtillus), and depressions by mosses and sedges (Sphagnum ssp. and Carex ssp). At higher elevations, the vegetation becomes scarcer and graminoid species increase, with Carex bigelowii and Juncus trifidus being the most abundant species. For a more detailed description of the vegetation and the respective microclimate, see Löffler (Citation2003). The specific vegetation patterns enabled us to identify representative sites for topography-dependent snow-cover duration.
During the snow-free period, three pitfall traps with saturated salt solution as a preservative and Agepon© as a detergent were installed at each site, resulting in a total of 216 traps being used (Geiranger n = 96, Vågå n = 120). The pitfall trapping was implemented in 2009 as soon as the individual sites became snow-free. The pitfall traps consisted of a glass with a diameter of 5.5 cm at the rim and a depth of approximately 12 cm. By means of a plastic rim on top of the glass, a polycarbonate plate (13.7 cm x 13.7 cm) was fixed 5 cm above the opening as a rain shelter (e.g., Naujok and Finch Citation2004). The pitfall traps were emptied every two weeks and their contents were transferred to 70 percent ethanol for preservation. In order to reduce differences in phenological development in both research regions, three sampling periods were implemented after the first adult specimens of P. palustris were sampled in the respective study regions. Our three sampling periods reached from 20 May until 1 July (first sampling 12 June; second sampling 17 June; third sampling 1 July) in the western oceanic climate section, and from 26 May until 7 July (first sampling 9 June; second sampling 23 June; third sampling 7 July) in the eastern continental climate section, respectively. The difference in the beginning of the sampling in the respective study regions is a result of the timing of snow melt and the appearance of adult specimens in the pitfall traps.
Pitfall trapping, largely used to sample ground-active arthropods (Uetz and Unzicker Citation1976; Topping and Sunderland Citation1992; Woodcock Citation2005), was argued to suffer from a number of biases: (1) notably towards male specimens during times of reproduction; and (2) bigger and more mobile species and specimens (Merrett and Snazell Citation1983). In our study, however, we assume a limited overall bias of the sampling method, because we are focusing on one species only and we do not focus on counts per pitfall trap, but size per sampled individual. Additionally, the constraints noted earlier were systematically present at all sites, and thus should result in a comparable data set.
Pardosa palustris
Pardosa palustris is commonly found in open habitats and on both moist and dry meadows, and appears from the lowlands up to 2500 m.a.s.l. (Nentwig et al. Citation2019). Pardosa palustris has a holarctic distribution (World Spider Catalog Citation2017) and can be found from the northernmost parts of Scandinavia to southern Europe (Nentwig et al. Citation2019). In our two study regions, P. palustris is one of the most abundant lycosid species appearing from the tree line up to 1488 m.a.s.l. in the western oceanic climate section and up to 1534 m.a.s.l. in the eastern continental climate section (Hein et al. Citation2014). We took photographs of each specimen with a digital camera, by means of a 10× optical and a 100× digital magnification. From these photographs, we measured prosoma widths to the nearest 0.01 mm using ImageJ software (Rasband Citation2007).
Statistical analysis
Machine-learning techniques have proven to outperform more established statistical methods for many applications in ecology (Elith et al. Citation2006; Cutler et al. Citation2007; Mayr, Vanselow, and Samimi Citation2018). Furthermore, relationships between biotic and abiotic factors often behave nonlinearly. Based on these facts, we decided to use a non-parametric random forest regression approach for statistical analysis. This machine-learning approach is based on an ensemble of single regression trees (Breiman Citation2001). It generates several random subsets from the training data set using bootstrap resampling (Efron and Tibshirani Citation1993; Liaw and Wiener Citation2002). These subsets were then used to grow a high number of single regression trees (hence forest). The trees were gradually divided into smaller binary classes, utilizing the best-fitting predictor from a randomly chosen subset of the explanatory variables. Referring to this, a threshold value was chosen that maximizes the homogeneity of the two resulting classes with regard to the response variable (Prasad, Iverson, and Liaw Citation2006). Then, the resulting single trees were combined to a stable final regression (Brenning Citation2009). A particular advantage of random forests is the out-of-bag validation (Liaw and Wiener Citation2002; Brenning Citation2009). At each bootstrap replication, the data that were excluded from the bootstrap sample (i.e., out-of-bag or OOB) are used as the test data; i.e., they were predicted on the basis of the tree grown with the bootstrap sample. The predictions for all individual trees grown with the bootstrap samples were consolidated, and the overall error rate was calculated. As such, OOB accuracy estimates were efficiently cross-validated and unbiased, providing that a sufficient number of trees were fitted (Breiman Citation2001). Beyond the model fit, the algorithm calculates an importance score for each explanatory variable that is based on permutation. The respective variable was permuted, while the other variables remained unchanged. The larger the increase in the prediction error due to this permutation, the higher the variable importance (Liaw and Wiener Citation2002). We tested the significance of the random forest models using a permutation test according to Murphy, Evans, and Storfer (Citation2010), with 9999 permutations. In order to test for significant differences in body size (prosoma width in mm) in females and males between the two populations of P. palustris, we performed a two-sided Wilcoxon rank sum test. All statistics were implemented in R (R Core Team Citation2016) using the r-package randomForest (Liaw and Wiener Citation2015) for the regression analysis and the stats r-package (R Core Team Citation2016) for the Wilcox test.
The explanatory variables used in the sex-specific random forest approaches were topographic position, elevation, and study region. The sampling locations along the elevation gradient correspond to the typical conditions at the specific elevation and are included as a numerical variable in m.a.s.l. The four different topographical positions in focus are covering the main relief positions displaying sites with the most pronounced contrasts in snow-cover duration in arctic-alpine ecosystems. The four different topographical positions are A = ridges, B = depressions, C = south-facing slopes, and D = north-facing slopes, included as a categorical variable in the random forest model. A total number of n = 500 trees were grown in each of the respective models.
Results
In total, 163 adult female and 651 male specimens of P. palustris were sampled and measured during three trapping periods, corresponding to six weeks of sampling; juveniles were excluded in this study. In the western oceanic study region of Geiranger, a total of n = 50 female and n = 205 male specimens were sampled. Accordingly, in the eastern continental study region of Vågå, a total of n = 113 female and n = 446 male specimens of P. palustris were sampled. The largest female specimen in the western oceanic research region measured 2.335 mm, and the largest male was 2.45 mm (both sampled at 1333 m.a.s.l.). Mean prosoma width in female specimens was 2.05 mm (sd = 0.14), and 2.06 mm in males (sd = 0.10). In the eastern continental study region, the largest female specimen was 2.497 mm (sampled at 1035 m.a.s.l.), and the largest male was 2.515 mm (sampled at 1384 m.a.s.l.). Mean prosoma width in female specimens was 2.15 mm (sd = 0.13), and 2.15 mm (sd = 0.10) in males. The Wilcoxon test revealed significant differences in prosoma width between the western oceanic and the eastern continental study region in females (W = 1727, p < .001) and males (W = 23433, p < .001), respectively, with generally larger specimens in the continental region ().
Figure 3. Body size variation of P. palustris along the elevational gradient. Displayed is the mean prosoma width in females (a) and males (b) of P. palustris along the elevational gradient in the two study regions. Given are the sampling-site specific means and the standard deviations of prosoma width (mm). The values for prosoma width for the two study regions are marked as triangles (continental) or circles (oceanic).
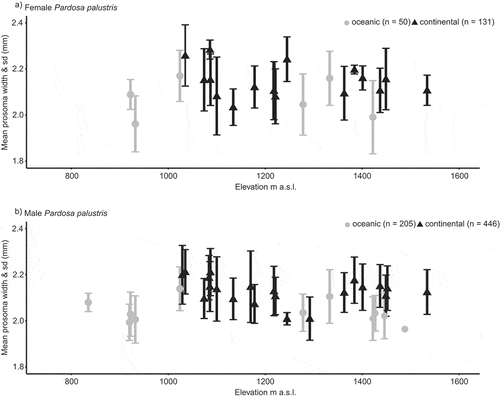
Beyond that, it is difficult to unravel clear patterns in body size in relation to elevation or topographic position. However, it is clear that there are obviously no linear trends along the elevational gradient; body size in P. palustris shows a somehow curvilinear pattern, probably related to the transition zones between the elevational belts.
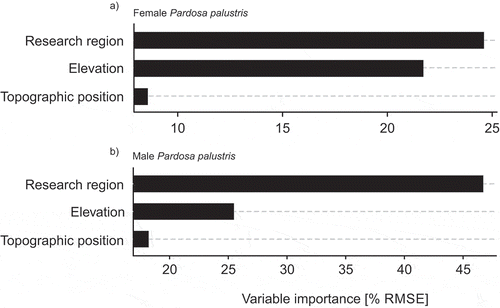
The results from the random forest approach showed a mean of squared residuals of 0.016 with an explained variation of 17 percent (p < .0001) in females of P. palustris, and a mean of squared residuals of 0.010 with an explained variation of 20 percent (p < .0001) in males of P. palustris. In both sexes, the explanatory variables sorted by their importance in decreasing order are: (1) study region; (2) elevation; (3) topographic position (). Here, the removal of topographic position from the models led to almost no loss in explanatory power of the variance in body size of P. palustris.
Figure 4. Importance scores of the explanatory variables used in the model. Importance is quantified as percent increase in mean square error of the random forest model when that explanatory variable is permuted. Sex-specific results for the importance of the explanatory variables are displayed for females (a) and males (b), respectively.
Discussion
Trying to disentangle the influence of fine- vs. broad-scale ecological factors on body size of a highly mobile spider, we found that the effects of the broad-scale factors of study region and elevation were both more important than the fine-scaled factor of topographic position. This was contrary to our expectations that local factors would explain more of the variance in body size than regional factors Although a relatively large part of the variance remains unexplained, we found individuals sampled in the eastern continental climate section to be significantly larger, maybe as a result of mean annual precipitation and concomitant season length. Additionally, this effect was probably due to increased mobility of the specimens at finer spatial scales, which might have homogenized effects on body size by the ability to avoid unfavorable micro-habitat conditions. In this context, lycosids display high mobility on the ground up to hundreds of meters a day (Richter Citation1970; Morse Citation1997, Citation2002), whereas ballooning is supposed to be less frequent in this family (Blandenier et al. Citation2013), but see exceptions later in the discussion. Furthermore, ballooning is sometimes reduced in specialist species (see, e.g., Edgar Citation1971; Bonte et al. Citation2003), and often results in random (passive) displacement of individuals (Bonte, Bossuyt, and Lens Citation2007). In a web-building spider, Lowe et al. (Citation2014) also found that body size was mostly influenced by broad-scale factors. Whether the differences in body size in our study were the result of direct (e.g., temperature, precipitation) or indirect climatic effects (e.g., resource availability, competition) could not be unraveled. We found indication for both direct and indirect effects, which we discuss in the following paragraphs at the scale of which they act the most.
Effects of study region
One has to keep in mind that our results regarding the effect of study region are unfortunately biased by the unbalanced sampling design, with the lack of sufficient replicates regarding the broader-scaled variables (e.g., Oksanen Citation2001). Comparison of the climatic conditions in the two study regions, however, revealed huge differences regarding the annual mean season length and snow-water equivalent from 1957–2017 (MET Norway Citation2018). Summer season length in the western oceanic research region is on average thirty days shorter. In this context, the differences in body size between our two populations could be related either to a Bergmann’s cline or to a converse Bergmann’s cline (e.g., Blanckenhorn and Demont Citation2004). Following Bergmann, a larger body size is supposed to be a form of adaptive plasticity in ectotherms to cold environments, where individual body size tends to be bigger because of slower growth (Atkinson and Sibly Citation1997; Angiletta et al. Citation2004). Consequently, this is a result of prolonged life cycles and thus of a delayed adulthood. This Bergmann trait is in concordance with some previous studies on spiders. Legault and Weis (Citation2013) showed that a lycosid spider species would react rapidly with an increase in body size to an artificial increase in snow-cover duration. Opell (Citation2010) found larger individuals of Amaurobioides maritima (O. P.-Cambridge 1883) along a latitudinal cline in New Zealand as a result of Bergmann’s rule. In our study, however, the increased body size is more likely a result of a converse Bergmann’s cline. Here, an increase in season length and thus in the ability to gain more resources might result in an increase in body size. This phenomenon has been described earlier for various other ectothermic species (Mousseau Citation1997; Ashton and Feldman Citation2003), including spiders (e.g., Høye et al. Citation2009; Puzin, Leroy, and Pétillon Citation2014).
Beyond that, there is also another possible explanation regarding the differences in body size. Entling et al. (Citation2010) and Fattorini et al. (Citation2014) found a larger body size in spiders and tenebrionid beetles in warm/dry habitats compared to cool/moist habitats. This is assumed to be most likely a result of a lower desiccation risk of bigger arthropods, and may apply to P. palustris in our study as well, resulting in larger specimens in our continental climate section. When looking only at the differences in precipitation between western oceanic and eastern continental climate sections, the risk of desiccation seems obvious; however, previous studies revealed continuously high soil moisture at all microtopographic sites in both study regions (Löffler Citation2002, Citation2007).
Effects of elevation
Elevation was a little less important than climate region on body size in our study. However, the elevational effect on body size in P. palustris was complex and difficult to unravel, due to the relatively large variation along the elevation gradient.
Even though various earlier studies have shown pronounced differences in body size of wolf spiders as a consequence of harsher conditions with elevation (e.g., Høye and Hammel Citation2010; Bowden, Høye, and Buddle Citation2013), this was not consistent for epigeal spiders in our study. Usually, spiders of the genus Pardosa have an annual life cycle in the lowlands that is prolonged in some species at higher elevations (Edgar Citation1972; Steigen Citation1975; Buddle Citation2000; Pickavance Citation2001). In this context, Steigen (Citation1975) has shown that P. palustris overwinters three times before reaching maturity at Hardangervidda in southern Norway. Moreover, a prolonged maturation commonly results in a larger body size in ectothermic species (Angiletta et al. Citation2004; Blanckenhorn and Demont Citation2004). However, Otto and Svensson (Citation1982) found a decrease in body size in several Araneae species with elevation and assumed that it is advantageous to be of smaller size at higher elevations, because a smaller size makes it easier to seek shelter in open habitats if predators appear. In previous studies, we found body size in different Pardosa species to decrease, but also to increase with elevation in central Norway (Hein et al. Citation2015), and there also seems to be a pronounced inter-annual variation in body size rather than a variation along the elevational gradient (Hein et al. Citation2018). This corresponds with findings of Lee, Somers, and Chown (Citation2011), who were not able to detect a linear trend in the size of the indigenous spider Myro kerguelensis (O.P.-Cambridge 1876) with elevational in a sub-antarctic environment. Additionally, a recent study in arctic and sub-arctic environments showed that body size and reproductive traits did not vary with elevation in a consistent manner in four different Pardosa species, including P. palustris (Ameline et al. Citation2018).
Toft (Citation1979) assumed that average temperatures, in combination with prey availability, affect life-history traits and determine whether species are annual or biennial in a certain habitats. A change in foraging strategies related to prey availability of P. palustris along the elevational gradient might thus also be a possible explanation for our observed patterns, which might reflect related site-specific variation in body size along the elevational gradient ().
There seems to be a curvilinear relationship between body size and elevation that might have biological relevance. The transition zone, where the low-alpine and middle-alpine belts overlap, is where the highest inter-specific competition is to be expected (Marshall and Rypstra Citation1999). Here, the increase in competition might lead to a decrease in prosoma width due to the higher number of competitors, and would thus correspond with the mid-elevation effect (e.g., Rahbek Citation1995; Thaler Citation2003; Becker et al. Citation2007). This might be the reason for the observed decrease in male body size at approximately 1350 m.a.s.l. in our study as a consequence of the highest species numbers within the transition zones along the elevation gradient in the eastern continental study area (Hein et al. Citation2014).
Moreover, with elevation, less vegetation coverage and higher proportions of open ground result in higher daily maximum temperatures during summer (Löffler, Cypionka, and Löffler Citation2008; Wundram, Pape, and Löffler Citation2010). As a consequence, the conditions for P. palustris might be more favorable with elevation than expected. As such, the heterogeneity of microhabitat structures contrast with the overall assumption of unfavorable conditions with elevation.
Effects of topographic position
Our results show that topography-determined snow-cover distribution and its related constraints at finer scales influence body size of epigeal spiders only to a (very) limited extent. Consequently, if topographic position was removed from the model, it would explain a similar amount of variance in body size. This is most likely a result of the high variability of micro-climatic conditions within relatively short distances in arctic-alpine areas (Mani Citation1968; Wundram, Pape, and Löffler Citation2010; Scherrer and Körner Citation2011), and thus the ability of spiders to avoid unsuitable conditions by active selection of optimal micro-habitats (Goldsbrough, Hochuli, and Shine Citation2004). Wolf spiders in general are supposed to be highly mobile (Samu, Sziranyi, and Kiss Citation2003), and P. palustris is supposed to be one of the most active lycosid aeronauts (Richter Citation1970). Several studies have reported fine-scale differences in spider body size as a response to micro-habitat conditions (e.g., Hendrickx et al. Citation2003; Pétillon et al. Citation2009; Torres–Sánchez and Gasnier Citation2010). Consequently, migration, foraging, and movement behaviors of lycosid spiders have so far received little attention in response to body-size variation, because of great difficulties involved in following the relatively small and highly active specimens in heterogeneous environments (Samu, Sziranyi, and Kiss Citation2003). The inclusion of environmental data with finer spatial resolution, especially regarding the micro-topographical gradient and the concomitant changing conditions, might result in a higher explanatory power of the variable topographic position.
Conclusions
In our model, differences in environmental factors (i.e., snow-cover duration and related season length) along broad-scaled elevational and regional gradients showed higher explanatory power as drivers of body size than the fine-scaled topographic heterogeneity. Body size variations at finer scales—here topographic position—could not be explained sufficiently. This is probably a result of interactions between elevation and topographic position-related patterns of snow-cover duration, and the concomitant variation of resource availability, predation pressure, and mobility. Accordingly, fine-scale alterations in body size related to the timing of snowmelt seem to be overruled by the broad-scale regional climatic drivers. Our results might indicate that broad-scale patterns of the body size of P. palustris may be more predictable, whereas the fine-scale variation visible along the topographic and elevation gradient remains largely unclear. Consequently, further research in arthropods is needed regarding the reciprocal effects of phenotypic plasticity vs. mobility and dispersal in response to future climate variability.
Supplemental Material
Download Zip (658.5 KB)Acknowledgments
We would like to thank Annie Arbuthnot for help with the laboratory work and Yan Steil for help during fieldwork. Special thanks to our other coworkers within the LTAER-No in Norway.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary materials
Supplemental data for this article can be accessed here.
References
- Ameline, C., C. Puzin, J. J. Bowden, K. Lambeets, P. Vernon, and J. Pétillon. 2017. Habitat specialization and climate affect arthropod fitness: A comparison of generalist vs. specialist spider species in Arctic and temperate biomes. Biological Journal of the Linnean Society 121:592–99. doi:10.1093/biolinnean/blx014.
- Ameline, C., T. Høye, J. Bowden, R. Hansen, O. Liset Pryds Hansen, C. Puzin, P. Vernon, and J. Pétillon. 2018. Elevational variation of body size and reproductive traits in high-latitude wolf spiders (Araneae: Lycosidae). Polar Biology 41:2561–74. doi:10.1007/s00300-018-2391-5.
- Angiletta, M. J., Jr., H. P. Niewiarowski, A. E. Dunham, A. D. Leaché, and W. P. Porter. 2004. Bergmann’s clines in ectotherms: Illustrating a life-history perspective with Sceloporine Lizards. American Naturalist 164:168–83. doi:10.1086/425222.
- Ashton, K. G., and C. R. Feldman. 2003. Bergmann’s rule in non-avian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution 57:1151–63. doi:10.1111/j.0014-3820.2003.tb00324.x.
- Atkinson, D., and R. M. Sibly. 1997. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends in Ecology & Evolution 12:235–39. doi:10.1016/s0169-5347(97)01058-6.
- Becker, A., C. Körner, J.-J. Brun, A. Guisan, and U. Tappeiner. 2007. Ecological and land use studies along elevational gradients. Mountain Research and Development 27:58–65. doi:10.1659/0276-4741(2007)27[58:EALUSA]2.0.CO;2.
- Beckers, N., N. Hein, K. A. Vanselow, and J. Löffler. 2018. Effects of microclimatic thresholds on the activity-abundance and distribution patterns of alpine Carabidae species. Annales Zoologici Fennici 55:25–44. (in press). doi:10.5735/086.055.0104.
- Blanckenhorn, W. U., and M. Demont. 2004. Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum? Integrative and Comparative Biology 44:413–24. doi:10.1093/icb/44.6.413.
- Blandenier, G., O. T. Bruggisser, O. P. Rohr, and L. F. Bersier. 2013. Are phenological patterns of ballooning spiders linked to habitat characteristics? Journal of Arachnology 41:126–32. doi:10.1636/P12-48.
- Bonte, D., B. Bossuyt, and L. Lens. 2007. Aerial dispersal plasticity under different wind velocities in a salt marsh wolf spider. Behavioral Ecology 18:438–43. doi:10.1093/beheco/arl103.
- Bonte, D., N. Vandenbroecke, L. Lens, and J. P. Maelfait. 2003. Low propensity for aerial dispersal in specialist spiders from fragmented landscapes. Proceedings: Biological Sciences 270:1601–07. doi:10.1098/rspb.2003.2432.
- Bowden, J. J., T. T. Høye, and C. M. Buddle. 2013. Fecundity and sexual size dimorphism of wolf spiders (Araneae: Lycosidae) along an elevational gradient in the Arctic. Polar Biology 36:831–36. doi:10.1007/s00300-013-1308-6.
- Breiman, L. 2001. Random forests. Machine Learning. 45:5–32. doi:10.1023/A:1010933404324.
- Brenning, A. 2009. Benchmarking classifiers to optimally integrate terrain analysis and multispectral remote sensing in automatic rock glacier detection. Remote Sensing of Environment 113:239–47. doi:10.1016/j.rse.2008.09.005.
- Buddle, C. M. 2000. Life history of Pardosa moesta and Pardosa mackenziana (Araneae, Lycosidae) in central Alberta, Canada. Journal of Arachnology 28:319–28. doi:10.1636/0161-8202(2000)028[0319:LHOPMA]2.0.CO;2.
- Chevin, L.-M., R. Lande, and G. M. Mace. 2010. Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biology 8 (4):e1000357. doi:10.1371/journal.pbio.1000357.
- Chown, S. L., and K. J. Gaston. 2010. Body size variation in insects: A macroecological perspective. Biological Reviews 85:139–69. doi:10.1111/j.1469-185X.2009.00097.x.
- Cutler, D. R., Edwards, T. C., Jr., Beard, K. H., Cutler, A., Hess, K. T., Gibson, J., and J. J. Lawler 2007. Random forests for classification in ecology. Ecology 88:2783–92. doi:10.1890/07-0539.1.
- Dahl, E. 1986. Zonation in Arctic and alpine tundra and fellfield ecobiomes. In Ecosystem theory and application, ed. N. Polunin, 35–62. Chichester: Wiley.
- Edgar, W. D. 1971. Seasonal weight changes, age structure, natality and mortality in the wolf spider Pardosa lugubris Walck in central Scotland. Oikos 22:84–92. doi:10.2307/3543365.
- Edgar, W. D. 1972. The life-cycle of the wolf spider Pardosa lugubris in Holland. Journal of Zoology 168:1–7. doi:10.1111/j.1469-7998.1972.tb01336.x.
- Efron, B., and R. J. Tibshirani. 1993. An introduction to the bootstrap. New York: Chapman & Hall.
- Elith, J., C. H. Graham, R. P. Anderson, M. Dudík, S. Ferrier, A. Guisan, R. J. Hijmans, F. Huettmann, J. R. Leathwick, A. Lehmann, et al. 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–51. doi:10.1111/j.2006.0906-7590.04596.x.
- Entling, W., M. H. Schmidt-Entling, S. Bacher, R. Brandl, and W. Nentwig. 2010. Body size-climate relationships of European spiders. Journal of Biogeography 37:477–85. doi:10.1111/j.1365-2699.2009.02216.x.
- Fattorini, S., R. Lo Monaco, A. Di Giulio, and W. Ulrich. 2014. Climatic correlates of body size in European tenebrionid beetles (Coleoptera: Tenebrionidae). Organisms Diversity & Evolution 14:215–24. doi:10.1007/s13127-013-0164-0.
- Finch, O.-D., and J. Löffler. 2010. Indicators of species richness at the local scale in an alpine region: A comparative approach between plant and invertebrate taxa. Biodiversity and Conservation 19:1341–52. doi:10.1007/s10531-009-9765-5.
- Fox, C. W., and M. E. Czesak. 2000. Evolutionary ecology of progeny size in arthropods. Annual Review of Entomology 45:341–69. doi:10.1146/annurev.ento.45.1.341.
- Frick, H., W. Nentwig, and C. Kropf. 2007. Influence of stand-alone trees on epigeic spiders (Araneae) at the alpine timberline. Annales Zoologici Fennici 44:43–57.
- Gjærevoll, O. 1956. The plant communities of the Scandinavian Alpine snow-beds. Trondheim: Kongelige Norske Videnskabers Selskabs Skrifter.
- Goldsbrough, C. L., D. F. Hochuli, and R. Shine. 2004. Fitness benefits of retreat-site selection: Spiders, rocks, and thermal cues. Ecology 85:1635–41. doi:10.1890/02-0770.
- Hagstrum, D. W. 1971. Carapace width as a tool for evaluating the rate of development of spiders in the laboratory and the field. Annals of the Entomological Society of America 64:757–60. doi:10.1093/aesa/64.4.757.
- Hauge, E., and D. Refseth. 1979. The spider fauna of 5 alpine and subalpine habitats in the Jotunheimen area, Southern Norway. Norwegian Journal of Entomology 26:84–90.
- Hein, N., H. Feilhauer, J. Löffler, and O.-D. Finch. 2015. Elevational variation of reproductive traits in five Pardosa (Lycosidae) species. Arctic, Antarctic, and Alpine Research 47:67–73. doi:10.1657/AAAR0013-111.
- Hein, N., H. Feilhauer, O.-D. Finch, S. Schmidtlein, and J. Löffler. 2014. Snow cover determines the ecology and biogeography of spiders (Araneae) in alpine tundra ecosystems. Erdkunde 68:157–72. doi:10.3112/erdkunde.2014.03.01.
- Hein, N., M. R. Brendel, H. Feilhauer, O.-D. Finch, and J. Löffler. 2018. Egg size versus egg number trade-off in the alpine-tundra wolf spider, Pardosa palustris (Araneae: Lycosidae). Polar Biology 41:1607–17. doi:10.1007/s00300-018-2301-x.
- Hendrickx, F., and J. P. Maelfait. 2003. Life cycle, reproductive patterns and their year-to-year variation in field population of the wolf spider Pirata piraticus (Araneae, Lycosidae). Journal of Arachnology 31:331–39. doi:10.1636/m01-98.
- Hendrickx, F., J. P. Maelfait, M. Speelmans, and N. M. Van Straalen. 2003. Adaptive reproductive variation along a pollution gradient in a wolf spider. Oecologia 134:189–94. doi:10.1007/s00442-002-1031-4.
- Hodkinson, I. D. 2005. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biological Reviews 80:489–513. doi:10.1017/s1464793105006767.
- Homburg, K., A. Schuldt, C. Drees, and T. Assmann. 2013. Broad-scale geographic patterns in body size and hind wing development of western Palaearctic carabid beetles (Coleoptera: Carabidae). Ecography 36:166–77. doi:10.1111/j.1600-0587.2012.07488.x.
- Høye, T. T., and D. S. Sikes. 2013. Arctic entomology in the 21st century. The Canadian Entomologist 145:125–30. doi:10.4039/tce.2013.14.
- Høye, T. T., and J. U. Hammel. 2010. Climate change and altitudinal variation in sexual size dimorphism of arctic wolf spiders. Climate Research 41:259–65. doi:10.3354/cr00855.
- Høye, T. T., J. U. Hammel, T. Fuchs, and S. Toft. 2009. Climate change and sexual size dimorphism in an Arctic spider. Biology Letters 5:542–44. doi:10.1098/rsbl.2009.0169.
- Jakob, E. M., S. D. Marshall, and G. W. Uetz. 1996. Estimating fitness: A comparison of body condition indices. Oikos 77:61–67. doi:10.2307/3545585.
- Lee, J. E., M. J. Somers, and S. L. Chown. 2011. Density, body size and sex ratio of an indigenous spider along an altitudinal gradient in the sub-Antarctic. Antarctic Science 24:15–22. doi:10.1017/s0954102011000629.
- Legault, G., and A. E. Weis. 2013. The impact of snow accumulation on a heath spider community in a sub-Arctic landscape. Polar Biology 36:885–94. doi:10.1007/s00300-013-1313-9.
- Levin, S. A. 1992. The problem of pattern and scale in ecology. Ecology 73:1943–67. doi:10.2307/1941447.
- Liaw, A., and M. Wiener. 2002. Classification and regression by randomforest. R News 2:18–22.
- Liaw, A., and M. Wiener. 2015. Randomforest: Breiman and Cutler’s random forests for classification and regression, version 4.6-12. Accessed May 30, 2017. http://cran.r-project.org/web/packages/randomForest/randomForest.pdf.
- Löffler, J. 2002. Altitudinal changes of ecosystem dynamics in the central Norwegian high mountains. Die Erde 133:227–58.
- Löffler, J. 2003. Micro-climatic determination of vegetation patterns along topographical, altitudinal, and oceanic-continental gradients in the high mountains of Norway. Erdkunde 57:232–49. doi:10.3112/erdkunde.
- Löffler, J. 2005. Snow cover dynamics, soil moisture variability and vegetation ecology in central Norwegian high mountain catchments. Hydrological Processes 19:2385–405. doi:10.1002/hyp.5891.
- Löffler, J. 2007. The influence of micro-climate, snow cover, and soil moisture on ecosystem functioning in high mountains. Journal of Geographical Science 17:3–19. doi:10.1007/s11442-007-0003-3.
- Löffler, J., and O.-D. Finch. 2005. Spatio-temporal gradients between high mountain ecosystems of central Norway. Arctic, Antarctic, and Alpine Research 37:499–513. doi:10.1657/1523-0430(2005)037[0499:SGBHME]2.0.CO;2.
- Löffler, J., R. Pape, and D. Wundram. 2006. The climatologic significance of topography, altitude and region in high mountains – A survey of oceanic-continental differentiations of the Scandes. Erdkunde 60:15–24. doi:10.3112/erdkunde.2006.01.02.
- Löffler, U. C. M., H. Cypionka, and J. Löffler. 2008. Soil microbial activity along an arctic-alpine altitudinal gradient from a seasonal perspective. European Journal of Soil Science 59:842–54. doi:10.1111/j.1365-2389.2008.01054.x.
- Lowe, E. C., S. M. Wilder, D. F. Hochuli, and M. G. (Gee) Chapman. 2014. Urbanisation at multiple scales is associated with larger size and higher fecundity of an orb-weaving spider. PLoS ONE 9 (8):e105480. doi:10.1371/journal.pone.0105480.
- Mani, M. S. 1968. Ecology and biogeography of high altitude insects. The Hague: W. Junk. doi:10.1007/978-94-017-1339-9.
- Marshall, S. D., and A. L. Rypstra. 1999. Spider competition in structurally simple ecosystems. Journal of Arachnology 27:343–50.
- Mayr, M., K. A. Vanselow, and C. Samimi. 2018. Fire regimes at the arid fringe: A 16-year remote sensing perspective (2000-2016) on the controls of fire activity in Namibia from spatial predictive models. Ecological Indicators 91:324–37. doi:10.1016/j.ecolind.2018.04.022.
- Merrett, P., and R. Snazell. 1983. A comparison of pitfall trapping and vacuum sampling for assessing spider faunas on heathland at Ashdown Forest, south-east England. Bulletin - British Arachnological Society 6:1–13.
- MET Norway. 2018. The norwegian meteorological institute. www.senorge.no.
- Miyashita, K. 1968. Growth and development of Lycosa T-insignita BOES. et STR. (Araneae: Lycosidae) under different feeding conditions. Applied Entomology and Zoology 3:81–88. doi:10.1303/aez.3.81.
- Moen, A. 1998. Nasjonalatlas for Norge: Vegetasjon. Hønefoss: Statens Kartverk.
- Morse, D. H. 1997. Distribution, movement, and activity patterns of an intertidal wolf spider Pardosa lapidicina population (Araneae, Lycosidae). Journal of Arachnology 25:1–10.
- Morse, D. H. 2002. Orientation and movement of wolf spiders Pardosa lapidicina (Araneae, Lycosidae) in the intertidal zone. Journal of Arachnology 30:601–09. doi:10.1636/0161-8202(2002)030[0601:OAMOWS]2.0.CO;2.
- Mousseau, T. A. 1997. Ectotherms follow the converse to Bergman’s rule. Evolution 51:630–32. doi:10.1111/j.1558-5646.1997.tb02453.x.
- Muff, P., C. Kropf, H. Frick, W. Nentwig, and M. H. Schmidt-Entling. 2009. Co-existence of divergent communities at natural boundaries: Spider (Arachnida: Araneae) diversity across an alpine timberline. Insect Conservation and Diversity 2:36–44. doi:10.1111/j.1752-4598.2008.00037.x.
- Murphy, M. A., J. S. Evans, and A. Storfer. 2010. Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology 91:252–61.
- Nagy, L., and G. Grabherr. 2009. The biology of Alpine habitats. New York: Oxford University Press.
- Naujok, J., and O.-D. Finch. 2004. Communities and spatio-temporal patterns of epigeic beetles (Coleoptera) in high mountain habitats of the Central Norwegian Scandes, with special emphasis on carabid beetles (Carabidae). Norwegian Journal of Entomology 51:31–56.
- Nentwig, W., T. Blick, D. Gloor, A. Hänggi, and C. Kropf. 2019. Spinnen Europas. Araneae Version 06.2019. https://www.araneae.nmbe.ch.
- Oksanen, L. 2001. Logic of experiments in ecology: Is pseudoreplication a pseudoissue? Oikos 94:27–38. doi:10.1034/j.1600-0706.2001.11311.x.
- Opell, B. D. 2010. Bergmanns’s size cline in New Zealand marine spray zone spiders (Araneae: Anyphaenidae: Amaurobioides). Biological Journal of the Linnean Society 101:78–92. doi:10.1111/j.1095-8312.2010.01480.x.
- Otto, C., and B. S. Svensson. 1982. Structure of communities of ground living spiders along altitudinal gradients. Holarctic Ecology 5:35–47.
- Penell, A., F. Raub, and H. Höfer. 2018. Estimating biomass from body size of European spiders based on regression models. The Journal of Arachnology 46:413–20. doi:10.1636/JoA-S-17-044.1.
- Pétillon, J., C. Puzin, A. Acou, and Y. Outreman. 2009. Plant invasion phenomenon enhances reproduction performance in an endangered spider. Naturwissenschaften 96:1241–46. doi:10.1007/s00114-009-0589-7.
- Pickavance, J. R. 2001. Life-cycles of four species of Pardosa (Araneae, Lycosidae) from the island of Newfoundland, Canada. Journal of Arachnology 29:367–77. doi:10.1636/0161-8202(2001)029[0367:LCOFSO]2.0.CO;2.
- Prasad, A., L. Iverson, and A. Liaw. 2006. Newer classification and regression tree techniques: Bagging and random forests for ecological prediction. Ecosystems 9:181–99. doi:10.1007/s10021-005-0054-1.
- Prenter, J., R. W. Elwood, and W. I. Montgomery. 1999. Sexual size dimorphism and reproductive investment by female spiders: A comparative analysis. Evolution 53:1987–94. doi:10.2307/2640458.
- Puzin, C., A. Acou, D. Bonte, and J. Pétillon. 2011. Comparison of reproductive traits between two salt-marsh wolf spiders (Araneae, Lycosidae) under different habitat suitability conditions. Animal Biology 61:127–38. doi:10.1163/157075511X566461.
- Puzin, C., B. Leroy, and J. Pétillon. 2014. Intra- and inter-specific variation in size and habitus of two sibling spider species (Araneae: Lycosidae): Taxonomic and biogeographic insights from sampling across Europe. Biological Journal of the Linnean Society 113:85–96. doi:10.1111/bij.12303.
- R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- Rahbek, C. 1995. The elevational gradient of species richness: A uniform pattern? Ecography 11:1551–66. doi:10.1111/j.1600-0587.1995.tb00341.x.
- Rasband, W. S. 2007. ImageJ. U.S. National Institutes of Health, Bethesda, Maryland, USA. http://imagej.nih.gov/ij/,1997–2012.
- Renault, D., C. Puzin, N. Foucreau, A. Bouchereau, and J. Pétillon. 2016. Chronic exposure to soil salinity in terrestrial species: Does plasticity and underlying physiology differ among specialized ground-dwelling spiders? Journal of Insect Physiology 90:49–58. doi:10.1016/j.jinsphys.2016.05.005.
- Richter, C. 1970. Aerial dispersal in relation to habitat in eight wolf spider species (Pardosa, Araneae, Lycosidae). Oecologia 5:200–14. doi:10.1007/bf00344884.
- Samu, F., A. Sziranyi, and B. Kiss. 2003. Foraging in agricultural fields: Local ‘sit-and-move’ strategy scales up to risk-averse habitat use in a wolf spider. Animal Behavior 66:939–47. doi:10.1006/anbe.2003.2265.
- Schaefer, M. 1970. Einfluss der Raumstruktur in Landschaften der Meeresküste auf das Verteilungsmuster der Tierwelt. Zoologische Jahrbücher, Abteilung Systematik, Ökologie Und Geographie Der Tiere 97:55–124.
- Scherrer, D., and C. Körner. 2011. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. Journal of Biogeography 38:406–16. doi:10.1111/j.1365-2699.2010.02407.x.
- Shelomi, M. 2012. Where are we now? Bergmann’s rule sensu lato in insects. The American Naturalist 180:511–19. doi:10.1086/667595.
- Simpson, M. R. 1995. Covariation of spider egg and clutch size: The influence of foraging and parental care. Ecology 76:795–800. doi:10.2307/1939345.
- Smith, C. C., and S. D. Fretwell. 1974. The optimal balance between size and number of offspring. The American Naturalist 108:499–506. doi:10.1086/282929.
- Steigen, A. L. 1975. Energetics in a population of Pardosa palustris L. (Araneae, Lycosidae) on Hardangervidda. Ecological Studies 17:129–44. doi:10.1007/978-3-642-66276-8_1.
- Strathdee, A. T., and J. S. Bale. 1998. Life on the edge: Insect ecology in arctic environments. Annual Review of Entomology 43:85–106. doi:10.1146/annurev.ento.43.1.85.
- Thaler, K. 2003. The diversity of high altitude arachnids (Araneae, Opiliones, Pseudoscorpiones) in the Alps. In Alpine biodiversity in Europe, ed. L. Nagy, G. Grabherr, C. Körner, and D. B. A. Thompson, 281–96. London: Springer.
- Toft, S. 1979. Life histories of eight Danish wetland spiders. Entmologiske Meddelelser 47:22–32.
- Topping, C. J., and K. D. Sunderland. 1992. Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. Journal of Applied Ecology 29:485–91. doi:10.2307/2404516.
- Torres–Sánchez, M. P., and T. R. Gasnier. 2010. Patterns of abundance, habitat use and body size structure of Phoneutria reidyi and P. fera (Araneae: Ctenidae) in a Central Amazonian rainforest. Journal of Arachnology 38:433–40. doi:10.1636/P08-93.1.
- Uetz, G. W., and J. D. Unzicker. 1976. Pitfall trapping in ecological studies of wandering spiders. Journal of Arachnology 3:101–11.
- Willmer, P., G. Stone, and I. Johnston. 2004. Environmental physiology of animals. Oxford: Blackwell-Science.
- Wise, D. H. 1993. Spiders in ecological webs. Cambridge: Cambridge University Press.
- Woodcock, B. A. 2005. Pitfall trapping in ecological studies. In Insect sampling in forest ecosystems, ed. S. R. Leather, 37–57. Oxford: Blackwell Science Ltd. doi:10.1002/9780470750513.ch3.
- World Spider Catalog. 2017. World spider catalog. Bern: Natural History Museum. Accessed June 1, 2017. http://wsc.nmbe.ch.
- Wundram, D., R. Pape, and J. Löffler. 2010. Alpine soil temperature variability at multiple scales. Arctic, Antarctic, and Alpine Research 42:117–28. doi:10.1657/1938-4246-42.1.117.
