ABSTRACT
Extreme abiotic conditions, geographic isolation, and low levels of disturbance have historically provided alpine, Arctic, and Antarctic regions with low input of and relative resistance to the introduction of new species. However, the climate is warming rapidly, concomitant with intense and diversified types of human influence in these cold environments. Consequently, many plant species, both native and nonnative, are now moving or expanding their ranges to higher elevations and latitudes, creating new species interactions and assemblages that challenge biodiversity conservation. Based on our synthesis, many of the same nonnative species invade multiple cold environments, and many more could move up or over from adjoining warmer areas. Transportation networks and the disturbances associated with burgeoning development are responsible for many movements. Prevention and monitoring for nonnative plant species is of paramount importance, and management should be directed toward species that negatively impact ecosystem function or human well-being. Management of native range shifters is more complicated; most movements will be desirable, but some may be locally undesirable. Overall, plant movements into alpine, arctic, and Antarctic areas are going to increase, and management will need to be adaptive because species movements and assemblages of the past will not reflect those of the future.
Introduction
Species dispersal and establishment processes under global warming can ultimately lead to directional changes in species distributions (Lenoir and Svenning Citation2013) and altered species interactions and assemblages (Walther et al. Citation2002; Alexander et al. Citation2018; Steinbauer et al. Citation2018). Disturbance can also play an important role in the success of species movements independent of climate. For instance, transportation networks enable some species to overcome biogeographical barriers and establish in new environments (Hulme Citation2009; Dainese et al. Citation2017). As human domination of the planet has increased, so too has the rate of movements and establishment of species into new areas (Williams et al. Citation2015) and this is set to continue and increase.
The average increase in global temperature has been 0.85°C since 1880 (Intergovernmental Panel on Climate Change Citation2013), but this is much more pronounced at high latitudes (Post et al. Citation2009) and elevations (Pepin et al. Citation2015), with strong evidence for an elevation-dependent warming (Pepin et al. Citation2015). Therefore, species are expected to redistribute to cold environments at high elevations and latitudes. Such movements have been observed since the beginning of the twentieth century (Chen et al. Citation2011; Lenoir and Svenning Citation2015; Steinbauer et al. Citation2018), though more quickly in marine than terrestrial systems (Antao et al. Citation2020; Lenoir et al. Citation2020), with important consequences for ecosystem functioning, the pattern and disparity of human well-being (e.g., food security) among regions, and the feedback on climate itself (Pecl et al. Citation2017). Thus, though effective dispersal is vital for the survival of many species in an era of global change, it can also create major disruptions to ecosystem services (Pecl et al. Citation2017). For instance, plant species movements to the highest habitable elevations and latitudes, and the increasing level of competition for space in these areas, can pose a threat to endemic, cold-adapted plants that already live at the edge of their regional niche (Pauli et al. Citation2007; Le Roux et al. Citation2019). For species living at the highest elevations or the highest latitudes on Earth, there is often nowhere else to go.
Dispersal and climatic barriers, particularly, have so far enabled cold environments of high elevations and latitudes to largely resist rapid colonization from nonnative species and climate-tracking native species (Pauchard et al. Citation2015). Now, climate warming, increases in transportation, the overall human footprint, and other factors related to globalization () are challenging the resilience of these so far relatively pristine ecosystems. However, the response of most species to global change is slow (Davis Citation1984). Species movements lag behind climate change (Bertrand et al. Citation2011), suggesting that less mobile species will be left behind (Alexander et al. Citation2018). These lagging dynamics for species moving into high elevation and latitude areas as conditions become more suitable may give conservation biologists and managers a short window of time to identify a gradient of potential responses for native species and communities. The responses may include creating refugia for species conservation, assisting with the colonization of new robust habitats, and, as a last resort, securing the long-term protection of plant species with nowhere to go (e.g., through seed banking).
Here we highlight plant species movements in alpine, Arctic, and Antarctic ecosystems (hereafter referred to as cold environments; ); the complexities of these movements; the challenges that conservation and land managers face in dealing with them; and the urgency of developing policy and management responses. We focus primarily on nonnative movements but also describe movements of climate-tracking native species (hereafter range shifters). We accept that range-shifting species share the same evolutionary history as the areas they expand into (Essl et al. Citation2019; Urban Citation2020; Wallingford et al. Citation2020). Urban (Citation2020) urges that the response to range shifters not be considered within the invasion ecology paradigm (i.e., that they should not be beaten back to preserve existing ecosystem structure), whereas Wallingford et al. (Citation2020) argue that some range shifters may have negative ecosystem impacts and this should be evaluated. We make no judgments about the impacts of range shifters in our synthesis, except where the authors of cited papers have done so. We discuss the dilemma of how to respond to range shifters in cold environments because these areas are potentially the last place left for many high-alpine and high-latitude species.
We built our review using two approaches, the inventories of nonnative plant species in alpine (Alexander et al. Citation2016), Arctic (Wasowicz et al. Citation2019), and Antarctic ecosystems and a literature review using Web of Science through June 2020. Our search terms were chosen to identify papers that focused on nonnative plant species and range-shifting native species in cold mountainous regions and high latitudes of the Arctic and Antarctic, with a focus on invasion pathways (including terms such as “invasion,” “nonnative,” “exotic,” “range shifter,” “range expansion,” “movement,” “plant species,” “high elevation,” “mountain,” “high latitude,” “alpine,” “arctic,” “Antarctic,” “global change,” “climate change”). We assessed the titles of the papers and read those that were performed on nonnative or range-shifting vascular plant species in cold alpine regions within the Arctic and Antarctic circles or the sub-Arctic and sub-Antarctic (the latter two regions mainly for reference) and were related to climate, global change, management, or conservation. Our article is a synopsis of the state of plant species movements in cold environments rather than a comprehensive review.
Movements in the alpine
Alexander et al. (Citation2016) completed a review of alpine surveys and literature and found 183 nonnative plant species in this ecosystem (i.e., above climatic tree line) globally, with 21 plant species found in multiple regions. Most of these 183 plant species are in the families Poaceae, Asteraceae, Caryophyllaceae, Fabaceae, and Brassicaceae. Transportation networks play a key role in moving plant species to new locations (Hulme Citation2009), with vehicles acting as effective long-distance dispersal agents (Taylor et al. Citation2012; Vakhlamova et al. Citation2016; Rew et al. Citation2018) and recreationalists moving seeds more locally on their clothing (Ansong and Pickering Citation2014) or equipment (Weiss, Brummer, and Pufal Citation2016). Consequently, most nonnative plant species reaching alpine areas spread from low elevations (Alexander et al. Citation2011). Nonnative species are spreading upslope at twice the rate of natives, with proximity to roads being a key driver (Dainese et al. Citation2017), but not all establish away from the roadside or have negative impacts on resident communities (Alexander et al. Citation2016). This means that the “typical” high-elevation nonnative plant is a generalist with a wide environmental tolerance, which allows that invader to deal with the low-elevation conditions first but then enables upward spread and establishment in a harsher environment (Kueffer et al. Citation2013; Steyn et al. Citation2017) or away from roads (McDougall, Wright, and Peach Citation2018). Many of the same species have also established at higher latitudes (Wasowicz, Przedpelska-Wasowicz, and Kristinsson Citation2013). Most introductions are accidental, but some species have been deliberately introduced for forage or to help soil stabilization along, for example, roadsides and ski slopes (Alexander et al. Citation2016). Both types of introduction demonstrate the role of humans in spreading nonnative organisms into mountain areas.
The effects of nonnative species on resident biota are often unknown and may not always be negative. Vilà et al. (Citation2011) performed a meta-analysis of nonnative species’ impacts globally, finding that less than half of the types of impacts assessed were significant and that the magnitude and direction of the responses were variable. As an example, in montane and alpine areas of New Zealand, experimental introduction of tussock hawkweed (Hieracium lepidulum) did not affect grassland species richness and diversity indices over a six-year period (Meffin et al. Citation2010), and pollinators were found to favor nonnative species but not adversely affect pollination of native species (Miller Citation2015). On the other hand, Muñoz and Cavieres (Citation2008) found a density-dependent effect of the nonnative dandelion (Taraxacum officinale) on pollination success of related native species in the Chilean Andes, and treeless communities in the Australian Alps heavily dominated by the nonnative ox-eye daisy (Leucanthemum vulgare: ) were found to have significantly fewer native plant species than adjoining vegetation less dominated by L. vulgare (McDougall, Wright, and Peach Citation2018).
Some native species, aided either by agricultural abandonment or climate change, are also expanding or shifting their ranges from lower elevations into alpine areas (Alexander et al. Citation2018; Steinbauer et al. Citation2018; Niu et al. Citation2019) and vice versa (Lembrechts et al. Citation2017). Transport networks are used by native species just as they are by nonnative species. Indeed, in a global survey of plant species on roads that connect lowlands with mountain tops (Kueffer et al. Citation2014), more native species were recorded on road verges than nonnative species (Seipel et al. Citation2012). Some of these native species are using the roads to move to higher elevations. In the Australian Alps, an endangered forb, hoary sunray (Leucochrysum albicans subsp. tricolor), has spread along a road to reach an elevation several hundred meters above its typical habitat (Doherty, Wright, and McDougall Citation2015). In a few cases, alpine species are using road verges to move downwards (Lembrechts et al. Citation2017). Timberline advances have been reported in many but not all regions (Harsch et al. Citation2009). Increases in shrub cover have been recorded (Hallinger, Manthey, and Wilmking Citation2010), as have changes in the cover of graminoids and forbs (Sebastià Citation2006). Increased shrubbiness in alpine areas has been linked to both climate change (Sanz-Elorza, Mateo, and Bernardo Citation2009) and changes in land use (especially abandonment of cutting and grazing in pastures; MacDonald et al. Citation2000). Kopp and Cleland (Citation2014) forecast a possible shift from alpine cushion communities to subalpine sagebrush after observing species shifts over a forty-nine-year period in the White Mountains of California. The authors emphasized the complexity of the range shifts and the need for a greater understanding of the multiple drivers that affect ecosystem change and native species expansion into the alpine, highlighting the need for improved policy on this issue.
In some cases, movements of nonalpine native species to alpine areas are regarded as having a negative impact on resident species. For instance, the treeline shrub dwarf pine (Pinus mugo) has not only expanded its range into alpine and open subalpine vegetation in Austria (Dullinger, Dirnböck, and Grabherr Citation2003) and the Czech Republic (Kašák et al. Citation2015), but it has also reduced the functional diversity of native carabid beetles, favoring only generalist species typical of forested vegetation (Kašák et al. Citation2015). In alpine areas of Japan, a native dwarf bamboo species, Sasa kurilensis, has invaded snow-meadow communities, leading to a more than 75 percent reduction in species richness (Kudo et al. Citation2011). In China, some native montane species are regarded as problematic because they lower grazing productivity in alpine areas by competing with palatable grasses and altering soil properties (Zong et al. Citation2016).
Movements in the Arctic
The Arctic has recorded 341 nonnative vascular plants, of which 188 are naturalized and 11 are considered invasive (Wasowicz et al. Citation2019). The genera with the most nonnative species in this group are Rumex (twelve), Poa (eight), Ranunculus (seven), Trifolium (seven), and Vicia (seven). Chenopodium album is the most widespread species found in thirteen of twenty-three subregions (classified mainly by flora), with Stellaria media and Fallopia convolvulus both in eleven regions (Wasowicz et al. Citation2019). On Svalbard (Norway), an archipelago in the Arctic, 96 nonnative plant species were recorded (Sandvik et al. Citation2019). Most are casual nonnatives but two are naturalized: Barbarea vulgaris and Anthriscus sylvestris. Many of the casual nonnatives, including Rumex acetosa found on both Svalbard and Jan Mayen Island, and the naturalized Anthriscus sylvestris likely spread from mainland Norway, where they are considered native (Sandvik et al. Citation2019).
Most nonnative populations in the Arctic are relatively small, limited in extent, or ephemeral. For example, the Data Portal of the Alaska Natural Heritage Program (AKEPIC Citation2020) listed forty-seven nonnative plant taxa from over 2,500 data records above the Arctic Circle (66.5° N) in Alaska (United States) and the northwestern Yukon (Canada), but the average area of introduction was only 0.18 ha. A study on Svalbard failed to relocate previously observed nonnative populations, suggesting that many introductions are ephemeral (Alsos, Ware, and Elven Citation2015). Similarly, Kent, Drezner, and Bello (Citation2018) found a large reduction in nonnative species richness in sub-Arctic Canada between 1989 and 2013 and attributed it to a reduction in human disturbances.
Human transportation networks and associated disturbances again play a strong role in the introduction of nonnative species. Seed contamination on vehicles has been identified as a major vector for the dispersal of nonnative species (Wasowicz et al. Citation2019). On Arctic islands, the footwear of tourists and returning residents are common introduction vectors. Ware et al. (Citation2012) recorded more than 1,000 seeds from a total of fifty-three species on the footwear of 259 travelers to Svalbard over one summer; 26 percent of the seeds germinated under local conditions. This suggests a locally intense propagule pressure of nonnative plants.
Although most nonnative plant species in the Arctic are naturalized or casual (Wasowicz et al. Citation2019) and perhaps cause little or no impact to native vegetation, the spread and dominance of eleven invasive species has triggered management concern. For instance, the nonnative forb Melilotus albus inhibits native seedling recruitment along rivers in the sub-Arctic (Spellman and Wurtz Citation2011) and variably affects pollination and reproductive capacity of two native arctic shrubs in Alaska (Spellman et al. Citation2015). Another example is Lupinus nootkatensis, a native of Canada and Alaska, that was introduced and is now widespread in Iceland, including above the Arctic Circle. Its presence has been shown to alter the composition of resident native species, facilitating late successional species and widespread nitrophilous ruderals to the disadvantage of some forbs, cushion plants, and small woody species (Vetter et al. Citation2018).
Climate change in concert with human disturbance is instrumental in enabling many species to move faster and further than they would from other dispersal vectors such as wind, animals, and water. With less snow in many areas, the network of transportation routes will expand, especially in the Arctic (Yang et al. Citation2018), accelerating the movement of undesired plant species and generating potential biosecurity issues. Introductions will place additional pressure on some areas of the Arctic where dispersal barriers have long provided protection. In Greenland, for instance, the climate is already suitable for a range of subarctic tree and shrub species, including some that occurred in Greenland during interglacial periods and are now used for amenity planting in settlements; with global warming and the removal of dispersal barriers, much of Greenland may be wooded by the end of the century (Normand et al. Citation2013), although there is no evidence of shrub expansion yet (Damgaard et al. Citation2018).
Spatial expansion and increases in abundances of native shrubs have been documented in various parts of the Arctic, where their greater growth rates have been attributed directly to climate warming (Olofsson et al. Citation2009) and indirectly to permafrost thaw (Blok et al. Citation2010). Increasing native shrub cover in the Arctic has been shown to lead to decreases in total species richness and bryophyte, lichen, dwarf shrub, and graminoid cover in some (Pajunen, Oksanen, and Virtanen Citation2011) but not all locations (Damgaard et al. Citation2018). This shrubification or greening of the Arctic (Sturm, Racine, and Tape Citation2001; Te Beest et al. Citation2016) through vegetation redistribution may in turn change the surface albedo (taller and darker tree canopies) as well as carbon sequestration rates and greenhouse gas release, thus affecting climate feedbacks (Pearson et al. Citation2013; Pecl et al. Citation2017). Such changes in ecosystem functioning are likely to lead to an increase in net radiation and atmospheric heating, thus amplifying high-latitude warming (Chapin et al. Citation2004). Additionally, the increase in fire frequency at high latitudes (Kasischke and Turetsky Citation2006) may further increase the positive feedback on climate warming. Extreme weather events, fire, and outbreaks of defoliating insects may temper the greening of the Arctic (Bjerke et al. Citation2014), highlighting the complexities of change in this ecosystem.
Ecological changes must not be viewed independently, because the response to global changes is complex and interwoven, impacting both social and ecological systems. For example, the expansion and increase in shrub cover and changes to snow and ice conditions resulting from climate warming are making it more difficult for indigenous people to utilize land in a traditional way (Forbes, Marcias Fauria, and Zetterberg Citation2010) by reducing the accessibility of forage for domesticated reindeer (Riseth et al. Citation2011) and increasing the likelihood of exotic disease transmission (Pauchard et al. Citation2015). The same patterns are not observed everywhere because of the spatial and temporal differences in climate warming and other global change drivers, making the inference of results between high-latititude areas difficult. In northern Fennoscandia and Siberia, grazing could be used to inhibit ongoing native shrub expansion (e.g., Olofsson et al. Citation2009) and prevent tree sapling establishment (Biuw et al. Citation2014) and thus slow down permafrost thawing. Similarly, on the Tibetan Plateau, grazing by domesticated stock reduced shrub expansion, increased primary productivity, and reduced the negative effect of climate warming on plant species richness (Klein, Harte, and Zhao Citation2004). In contrast, a twenty-eight-year study in southern Greenland found minimal long-term changes in functional group composition due to grazing or climate change (Damgaard et al. Citation2018), demonstrating the need for adaptive management tailored to local areas.
Movements to the Antarctic
The risk of plant invasions in the Antarctic region is relatively low. The areas of most concern are the Antarctic Peninsula and the Scotia Arc (including Deception Island and South Shetland Islands; Hughes et al. Citation2015) where it is warmer and anthropogenic pressure is highest (Hughes et al. Citation2020). Only two nonnative vascular plant species (Poa annua and Poa pratensis) have established and persist, although propagules of a third nonnative species, Juncus bufonius, were discovered during research on the continent’s two native species (Cuba-Díaz et al. Citation2012). Poa pratensis was introduced in soil used in transplant experiments of other species, none of which survived (Pertierra et al. Citation2017).
Eradication of nonnative species south of 60° S is encouraged as part of the Antarctic Treaty (Hughes and Convey Citation2012; Pertierra et al. Citation2017). Eradication efforts are ongoing for populations of P. annua on King George Island, and though spread is slow, controlled populations are reestablishing from the soil seed bank (Galera et al. Citation2019). A new population of P. annua was recently observed on Signy Island, demonstrating the potential for long-distance dispersal and the importance of monitoring (Malfasi et al. Citation2019) for new invasions, particularly around areas of high human activity (Fuentes-Lillo et al. Citation2017). Huiskes et al. (Citation2014) estimated that between 50,000 and 100,000 propagules were transported to one Antarctic station in a single year based on a sample of 853 visitors from Southern Hemisphere countries. The sample contained over 400 flowering plant morphotypes (representing at least 114 species) and included fragments of ferns, algae, diatoms, and fungi. People traveling to the Antarctic for employment dispersed most propagules, most of which were found on shoes and trousers (Huiskes et al. Citation2014), with an average of 9.5 seeds per person (Chown et al. Citation2012). Although most propagules do not establish successfully, increasing human activity (scientists, logistics personnel, and tourists) will provide continual long-distance transport of propagules to and within the Antarctic region, and implementing biosecurity measures to minimize such movement is a priority (Hughes et al. Citation2019).
The Antarctic has two native vascular plants (Deschampsia antarctica and Colobanthus quitensis). The main nonnative invader in Antarctica, P. annua, has been found to cause a decrease in biomass and photosynthetic performance when grown with these two native species (Molina-Montenegro et al. Citation2012) and has the potential to negatively impact them under future climate scenarios (Molina-Montenegro et al. Citation2019). However, with increasing space becoming available for plant colonization in Antarctica, it is uncertain whether nonnative species will be strictly the winners at the expense of native species. For instance, there is evidence from a sub-Antarctic island that nonnative plant species are utilizing newly available resources rather than competing for resources with resident native plant species (Chown et al. Citation2013).
What to manage now and into the future
Compared with many ecosystems, nonnative invasions and range-shifting expansions into alpine, arctic, and Antarctic areas have been few, although monitoring is generally limited. For example, the Hawaiian Islands alone have had about twice as many introductions (>1,000; Wagner, Herbst, and Sohmer Citation1999) as the known combined invasions into all alpine, arctic, and Antarctic areas. However, we expect invasions by nonnative species into alpine, arctic, and Antarctic areas to increase in coming decades, as climate change and land use pressure increase. The challenge for conservation and resource managers of these areas is to address what is problematic now and predict what will be problematic in the future, cognizant that the current impactful species may not be the same as those of the future. One way to approach this task is to search for commonalities in lists of invaders between cold environments. For instance, twenty-one nonnative plant species were observed in more than three alpine areas (Alexander et al. Citation2016), and sixteen of those are also found in more than four arctic areas (Wasowicz et al. Citation2019; ). Comparing the nonnative species lists of alpine and subalpine (McDougall et al. Citation2011; Alexander et al. Citation2016), the Arctic (Wasowicz et al. Citation2019), and sub-Antarctic islands (Frenot et al. Citation2005) shows that 6 percent (twenty-eight) of them are observed in all these ecosystems, and 20 percent (ninety-eight) of the almost 500 nonnative species are shared between at least two of the ecosystems (Supplemental Table 1). Many of these shared species are of little or no management concern in most areas because they are common ruderals of numerous ecosystems (Kueffer et al. Citation2013). However, management experiences and a lesson from one cold ecosystem may be valuable for another. For instance, Pilosella aurantiaca has been recorded in Arctic Iceland (Wasowicz et al. Citation2019) but is a species of considerable management concern in the high subalpine areas of Australasia (Kueffer et al. Citation2013) and is rated as highly invasive in Alaska, where it is known from southern coastal and boreal areas (Carlson et al. Citation2008). This species should be of concern wherever it establishes in the Arctic. Shared information on nonnative species could be invaluable for management of cold environments. McDougall et al. (Citation2011) found that if a nonnative plant species was of management concern in one alpine area, it was likely to be of management concern in many. Such lists could be used as the basis of risk assessments to identify the potentially most problematic species and could have been useful to the horizon scanning approach used to identify the species at highest risk to invade and negatively impact biodiversity and ecosystem functioning in Antarctica (Hughes et al. Citation2020). Similar information collation for range-shifting species that are having negative ecosystem impacts would also be valuable for managers of high-elevation and high-latitude cold environments.
A greater challenge for cold environment managers is identifying which species will be problematic in the future. They might do that in part by searching in neighboring ecosystems, from which many of the future invaders and range shifters will come. In many cases, the pool of potential invaders in these areas is great. In Kosciuszko National Park (Australia), for instance, 240 native and 25 nonnative vascular plants have been recorded in the alpine zone, but there are 564 native and 124 nonnative vascular plants in the surrounding subalpine zone (Doherty, Wright, and McDougall Citation2015). Similarly, in Alaska, though only 47 nonnative taxa have been recorded in the Arctic (above 66.5° latitude), 271 nonnative taxa have been recorded in adjoining sub-Arctic areas (between 60° N and 66.5° N (AKEPIC Citation2020). And, in Iceland, 16 nonnative species were observed in the arctic zone of the northern fringe and in the highlands, whereas 336 nonnative plant species were observed overall, most close to settlements in the southern lowlands (Wasowicz, Przedpelska-Wasowicz, and Kristinsson Citation2013). The changing climate and an increase in connectivity because of tourism raise concerns of escalating invasions into the coldest regions of Iceland in the future (Wasowicz Citation2016). Antarctica, with two persistent nonnative plant species, is less at risk of proximal invasions because of its isolation, but 108 nonnative plant species have been recorded on sub-Antarctic islands (Frenot et al. Citation2005), an indication that Antarctica is more at risk as the climate continues to change (Chown et al. Citation2012).
Deciding which range shifters to respond to is far more challenging. Urban (Citation2020) argues against resisting native movements. Wallingford et al. (Citation2020) suggest being cognizant that some range shifters will cause negative ecosystem impacts and that such species can be identified through an understanding of invasion theory and risk assessment tools and managed accordingly. Our synthesis shows that some native movements are being resisted. In some cases, this is for economic reasons (e.g., the impact of shrubification on traditional grazing practices) and in some for protecting the resident biota. However, overall, we should not hold back the tide of range shifters and need to accept that this is inevitable under global change. We also need to accept that some future communities will have no current analogue (Wallingford et al. Citation2020) and that within certain ecosystems and land uses some new species and assemblages may be performing desirable ecosystem services or functions (Walther et al. Citation2009; Wallingford et al. Citation2020), and our management practices should reflect such changes. However, there will be some cases where it is perhaps worth slowing down specific range shifters if the short-term gain is regarded as being cost-effective or essential in a utilitarian sense.
Planning for alpine, arctic, and Antarctic futures
Species, both native and nonnative, are moving into alpine and arctic areas as a result of climate, land use, and other global change factors (), and these movements are likely to increase in the coming decades; movements into the Antarctic are currently relatively small, but the risk of future invasions is clear. Recent movements of species across a wide spectrum of alpine and arctic environments are having substantial economic and environmental effects, which are often cascading and multitrophic and need time to completely unfold (Essl et al. Citation2015). Antarctic experts used horizon scanning (systematic examination) to identify species and areas of the Antarctic peninsula most likely to be invaded as the climate warms (Hughes et al. Citation2020), and this approach is recommended by the Arctic Invasive Alien Species group (CAFF and PAME Citation2017).
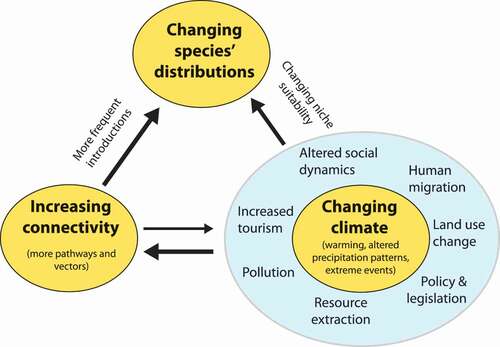
Figure 1. More species are moving into higher elevations of mountains and higher latitudes because the changing climate is altering the availability of resources and shifting the fundamental niche space of plant species, which are responding by changing their distributions. Simultaneously, anthropogenic drivers associated with climate change (blue circle; number and movement of people, changes in land use, etc.) are increasing the connectivity to other systems, which in turn means that a wider diversity of species and propagules are being introduced to these regions. This will alter species distributions and allow for new interactions between species and human society, causing challenges to conservation and management of high-elevation and high-latitude, cold environments. Climate change, increasing connectivity and changing species distributions (yellow circles), and associated anthropogenic drivers (blue circle) are key topics discussed in this article
Arctic countries and those with an interest in Antarctica have fostered high levels of intergovernmental cooperation in environmental protection. The Arctic Council (http://www.arctic-council.org/) is a cooperative organization comprising all eight Arctic nations concerned with changes to the environment, climate, biodiversity, oceans, and Arctic people and promotes improved health conditions for Arctic people. The Antarctic Treaty was signed in 1959 by the twelve countries then actively involved in Antarctic research and exploration. A “Protocol on Environmental Protection to the Antarctic Treaty” was adopted in 1991, which includes the clause “No species of animal or plant not native to the Antarctic Treaty area shall be introduced onto land or ice shelves, or into water in the Antarctic Treaty area except in accordance with a permit” (SAT Citation2020; Annex II). In the Arctic and Antarctic at least, the threats are recognized and there is cross-jurisdictional cooperation to achieve goals.
Figure 2. Alpine, Arctic, and Antarctic lands are experiencing plant species movements because of rapid climate change and increased human-mediated disturbance. We concentrate on the Arctic (dark blue; defined here as all land north of the Conservation of Arctic Flora and Fauna’s boundary line [dashed line]; CAFF and PAME Citation2017), the Antarctic (light blue; sub-Antarctic and Antarctic defined as all land south of the sub-Antarctic front [dashed line]; Orsi and Harris Citation2019; we concentrate on the Antarctic), and alpine areas (orange; depicted as elevations above 1,000 m in mountainous areas between the Arctic and Antarctic; UN-WCMC Citation2002)
![Figure 2. Alpine, Arctic, and Antarctic lands are experiencing plant species movements because of rapid climate change and increased human-mediated disturbance. We concentrate on the Arctic (dark blue; defined here as all land north of the Conservation of Arctic Flora and Fauna’s boundary line [dashed line]; CAFF and PAME Citation2017), the Antarctic (light blue; sub-Antarctic and Antarctic defined as all land south of the sub-Antarctic front [dashed line]; Orsi and Harris Citation2019; we concentrate on the Antarctic), and alpine areas (orange; depicted as elevations above 1,000 m in mountainous areas between the Arctic and Antarctic; UN-WCMC Citation2002)](/cms/asset/6e28fef2-d0af-4811-b5a4-f8e24c2f2493/uaar_a_1845919_f0002_oc.jpg)
Figure 3. Species are moving into high-elevation and high-latitude regions in response to the warming climate and other global change drivers. (Upper left) Ox-eye daisy invading subalpine grasslands in Kosciuszko National Park, Australia; the seed is dispersed by nonnative and native animals and humans. (Upper right) Nonnative pigs disturb native grasslands digging for tubers and bulbs, providing open sites for invasion, Kosciuszko National Park, Australia. (Lower left) Global climate change is increasing connectivity and recreation in arctic areas and the chance of undesired species movements. (Lower right) The warming climate and other global change drivers are intricately linked and impacting reindeer herding and human societies in the Arctic. Upper photos courtesy of Keith McDougall and lower photos by Jonas Lembrechts, University of Antwerp

These cooperative groups have now developed plans to manage non-native invasive species. The Arctic Invasive Alien Species Strategy and Action Plan is a framework that has four main strategies: prevention, early detection and rapid response, eradication, and control (CAFF and PAME Citation2017). The Antarctic also has a manual for nonnative species concentrating on prevention, monitoring, and response (Committee for Environmental Protection Citation2019). Greater cooperation between managers and policymakers in the Arctic and Antarctic will further improve management and reduce threats from climate change and its follow-on effects (Bennett et al. Citation2015). There is every reason to include alpine areas in this cooperative mix of cold environments because of similar pressures and threats and the overlap of many nonnative invaders ( and Supplemental Table 1), corroborating the benefits of an intensified exchange of knowledge and experiences between these groups of people and regions. If prioritization of management responses for invasive species is required in the Arctic and Antarctic, managers of alpine areas will often be in the best place to advise about the magnitude of threat and the optimal response. Many alpine areas already work collaboratively within regions, but greater national and international collaboration would be beneficial. This more collaborative and cooperative approach will be put to the test in coming decades in all cold environments as pressure mounts from exploitation of resources (e.g., Tolvanen et al. Citation2019) and large increases in tourism (e.g., Kaltenborn Citation2000), requiring innovative approaches to developing these areas sustainably (Trump, Kadenic, and Linkov Citation2018).
Table 1. Nonnative vascular plant species observed in three or more alpine and arctic areas, and the Antarctic
Prevention is the cornerstone of nonnative species management in little-invaded places because, if effective, it is much cheaper than the alternative of responding to invasions once they occur (McDougall et al. Citation2011). It comes in many forms and scales and is already being explicitly implemented in some cold environments. The State of Alaska provides one such example: a multi-agency invasive nonnative species working group (AKEPIC Citation2020); hosts annual invasive species workshops, has developed best management practices for road maintenance (Graziano, Seefeldt, and Clayton Citation2014), encourages professional and citizen science identification and mapping of all invasive nonnative species, and manages a data exchange. Most jurisdictions recognize that nonnative species can have adverse impacts and that there is a sound base of evidence for both self-regulation (e.g., taking care of personal equipment) and organization-based regulation (e.g., issuing guidelines and holding regular inspections) to reduce propagule transfer of nonnative organisms to the Antarctic (Huiskes et al. Citation2014). Several recent invasions in the maritime sub-Antarctic further strengthen the argument for careful regulation because the most likely cause is accidental human introduction, because the new populations were close to footpaths and areas used by station personnel, scientists, and others and far from other populations (e.g., Hughes et al. Citation2015; Fuentes-Lillo et al. Citation2017; Pertierra et al. Citation2017; Malfasi et al. Citation2019). Amenity plantings in ski resorts in Australia have been limited by government policy to reduce the risk of introducing climatically adapted nonnative species and, in Hawaii, control is implemented at low elevations for some nonnative species expected to move to higher elevations (McDougall et al. Citation2011).
In alpine areas, prevention is and will remain a pragmatic management solution because transport networks are few and the alternative—that is, responding to nonnative introductions where and when they occur—can be costly and difficult because of the rugged terrain. Although the Arctic and Antarctic are vast, prevention focusing on key transport networks seems a reasonable first line of defense. Because transport routes and invasion hubs are well known (e.g., tourist and research routes), these should be under stringent pathway control measures, including monitoring for early detection and rapid response to new introductions. Prevention is probably more difficult in the Arctic because of the closer proximity of propagule sources and greater connectivity with landmasses and easier in the terrestrial environment of the Antarctic, because despite its size there is little habitat suitable for plant growth and there are fewer entry points. Establishment of an early warning system in the Antarctic relies on scientists who work there, but in the Arctic and in many parts of the alpine it can be aided by the indigenous people who often have a good knowledge of the local flora and fauna that they utilize (Huntington et al. Citation2004). The capacity of indigenous people to detect and interpret changes in climate at a fine local scale has already been demonstrated in the Arctic (Huntington et al. Citation2004; Riseth et al. Citation2011).
Prevention is a desirable but complex management tool for two reasons, one applicable in all situations and one especially relevant in cold environments. Firstly, when prevention is successful, it is somewhat invisible and can be difficult to evaluate; that is, ecosystems that remain uninvaded because of chance and because of prevention look the same, meaning that support for the process can wane. Secondly, the borders of the Arctic and Antarctic areas are immense, so the likelihood of effective prevention might seem small. However, our synthesis and research experience show that most introductions in cold environments are along transportation networks and pathways where soil is more disturbed and have occurred mostly over the last few decades, meaning that we are often witnessing incipient impacts. Thus, because of the low level and size of nonnative populations in cold environments, prevention measures have a high chance of success and should be maintained as the main line of defense, focusing on new invasions and high-risk nonnative species (CAFF and PAME Citation2017; Committee for Environmental Protection Citation2019). However, rapid response to new invasions is problematic in all cold environments because of multiple land ownerships and jurisdictions, diverse stakeholder perceptions, and insufficient funding, all of which are compounded due to the isolation and vast areas involved.
Planning responses to range shifters is far more challenging than that required for nonnative movements. Our review shows that management concern can occur following range expansion of native species tracking their bioclimatic envelopes as the climate warms, and particularly when such movements are enhanced by human actions (e.g., the upward movement of montane species in China; Zong et al. Citation2016). However, the concern appears to be somewhat arbitrary. Responding to all native species movements would be impractical and, as Urban (Citation2020) argues, responding in general could be anathema to biodiversity conservation. Though we agree that allowing the free movement of native species is wise, there will be losers and negative impacts in some locations (Wallingford et al. Citation2020). Species currently growing in extreme climatic environments—such as alpine and Arctic ecosystems—will sometimes have nowhere to go and could well be among the losers. In addition, we have shown that some native species are taking advantage of human disturbances such as transport corridors and may potentially move more quickly than they would have because of climate warming alone. It has been suggested that range shifters whose movement is enhanced by humans, “neo-natives,” should be monitored more closely than those not enhanced by humans (Essl et al. Citation2019). There are no clear answers for how to relate to range shifts into cold environments. We suspect that responses will continue to be somewhat subjective and, as a consequence, arbitrary. Therefore, we do recommend that policymakers in cold environments declare what they will do with native species when such species arrive. For instance, will they distinguish neo-natives and evaluate for negative ecosystem impacts or treat all native plant species movements as desirable?
Conclusions
The climate is changing rapidly, and human influence is intensifying in cold environments. Plant species are responding by moving up in elevation and latitude, often moving over from adjacent, warmer environments. This is a problem for some alpine and arctic species that truly have nowhere else to go; consequently, species movements to these cold environments could have more impact than in most other ecosystems. High-latitude ecosystems are particularly vulnerable to the establishment of new species and assemblages, and neither the potential effects that these have on the ecosystems nor the resilience of the ecosystems have yet been well studied (Post et al. Citation2009).
Greater collaboration between resource managers, conservationists, and scientists in alpine, Arctic, and Antarctic regions could help identify nonnative and neo-native range-shifting species likely to be of greatest concern and of the best ways to manage them. In all cold environments, prevention is the best approach, and emphasis should be placed along transportation networks and areas with high levels of human-mediated disturbance where species movements will be greater. In these areas, species movements could be directly prevented, controlled, or minimized by changing land use so that it is less favorable to undesired movements. Because many of the nonnative introductions are at an early stage, eradication is possible in some cases if rapid response measures are taken. However, prioritization of nonnative species and assemblages to manage would be best achieved with an impact-based approach to identify species having negative effects on ecosystem functioning and or human health. It is worth noting that different stakeholders will have conflicting values and perceptions of nonnative species (Essl et al. Citation2017), which need to be incorporated into management plans (Crête et al. Citation2020), and different cultural values make creating stringent guidelines difficult or even foolish. Furthermore, there is growing sociocultural resistance to some conventional management tools (e.g., herbicides), so new management approaches will be necessary (Ricciardi et al. Citation2017). Management of range-shifting native species is complex. Climate-tracking native species movements are inevitable and will result in changing plant communities. Some range shifters may be considered locally undesirable and require local management, but this should be based on Arctic negative ecosystem impacts, not nativity. Alpine, Arctic, and Antarctic regions have entered a new era, and this requires new approaches that involve major shifts in policies and attitudes toward plant species movements.
Supplemental Material
Download Zip (39.9 KB)Acknowledgments
The workshop that led to this work was supported through funding by the Mountain Research Initiative (MRI) of the University of Bern (Switzerland), the Marcus Wallenberg Foundation for International Scientific Collaboration, the Oscar and Lili Lamms Remembrance Foundation, the Arctic Research Centre at Umeå University (ARCUM), and the Climate Impacts Research Centre (CIRC). LR is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture Hatch: MONB00363. JMA received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 678841. AP was funded by CONICYT PIA AFB170008 and Fondecyt 1180205. We thank Bridgett Naylor, USDA-FS, La Grande, Oregon, for .
Disclosure statement
The authors declare that they have no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- AKEPIC. 2020. Alaska exotic plant information clearinghouse database. Anchorage: Alaska Center for Conservation Science, University of Alaska. Accessed June 4, 2020. http://aknhp.uaa.alaska.edu/apps/akepic/
- Alexander, J. M., C. Kueffer, C. C. Daehler, P. J. Edwards, A. Pauchard, T. Seipel, J. Arevalo, L. Cavieres, H. Dietz, G. Jakobs, et al. 2011. Assembly of non-native floras along elevational gradients explained by directional ecological filtering. Proceedings of the National Academy of Sciences of the United States of America 108:656–61. doi:https://doi.org/10.1073/pnas.1013136108.
- Alexander, J. M., J. J. Lembrechts, L. A. Cavieres, C. Daehler, S. Haider, C. Kueffer, G. Liu, K. McDougall, A. Milbau, A. Pauchard, et al. 2016. Plant invasions into mountains and alpine ecosystems: Current status and future challenges. Alpine Botany 126 (2):89–103. doi:https://doi.org/10.1007/s00035-016-0172-8.
- Alexander, J. M., L. Chalmandrier, J. Lenoir, T. I. Burgess, F. Essl, S. Haider, C. Kueffer, K. McDougall, A. Milbau, M. A. Nunez, et al. 2018. Lags in the response of mountain plant communities to climate change. Global Change Biology 24 (2):563–79. doi:https://doi.org/10.1111/gcb.13976.
- Alsos, I. G., C. Ware, and R. Elven. 2015. Past Arctic aliens have passed away, current ones may stay. Biological Invasions 17 (11):3113–23.
- Ansong, M., and C. Pickering. 2014. Weed seeds on clothing: A global review. Journal of Environmental Management 144:203–11. doi:https://doi.org/10.1016/j.jenvman.2014.05.026.
- Antao, L. H., A. E. Bates, S. A. Blowes, C. Waldock, S. R. Supp, A. E. Magurran, M. Dornelas, and A. M. Schipper. 2020. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nature Ecology & Evolution 4 (7):927–33. doi:https://doi.org/10.1038/s41559-020-1185-7.
- Bennett, J. R., J. D. Shaw, A. Terauds, J. P. Smol, R. Aerts, D. M. Bergstrom, J. M. Blais, W. W. L. Cheung, S. L. Chown, M.-A. Lea, et al. 2015. Polar lessons learned: Long-term management based on shared threats in Arctic and Antarctic environments. Frontiers in Ecology and the Environment 13 (6):316–24. doi:https://doi.org/10.1890/140315.
- Bertrand, R., J. Lenoir, C. Piedallu, G. Riofrio-Dillon, P. De Ruffray, C. Vidal, J. C. Pierrat, and J. C. Gegout. 2011. Changes in plant community composition lag behind climate warming in lowland forests. Nature 479 (7374):517–20. doi:https://doi.org/10.1038/nature10548.
- Biuw, M., J. U. Jepsen, J. Cohen, S. H. Ahonen, M. Tejesvi, S. Aikio, P. R. Wali, O. P. L. Vindstad, A. Markkola, P. Niemela, et al. 2014. Long-term impacts of contrasting management of large ungulates in the Arctic tundra-forest ecotone: Ecosystem structure and climate feedback. Ecosystems 17:890–905. doi:https://doi.org/10.1007/s10021-014-9767-3.
- Bjerke, J. W., S. Rune Karlsen, K. Arild Høgda, E. Malnes, J. U. Jepsen, S. Lovibond, D. Vikhamar-Schuler, and H. Tømmervik. 2014. Record-low primary productivity and high plant damage in the Nordic Arctic Region in 2012 caused by multiple weather events and pest outbreaks. Environmental Research Letters 9:8. doi:https://doi.org/10.1088/1748-9326/9/8/084006.
- Blok, D., M. M. P. D. Heijmans, G. Schaepman-Strub, A. V. Kononov, T. C. Maximov, and F. Berendse. 2010. Shrub expansion may reduce summer permafrost thaw in Siberian Tundra. Global Change Biology 16 (4):1296–305. doi:https://doi.org/10.1111/j.1365-2486.2009.02110.x.
- CAFF and PAME. 2017. Arctic invasive alien species: Strategy and action plan. Akureyri, Iceland: Conservation of Arctic Flora and Fauna and Protection of the Arctic Marine Environment. http://www.abds.is/.
- Carlson, M. L., I. V. Lapina, M. Shephard, J. S. Conn, R. Densmore, P. Spencer, J. Heys, J. Riley, and J. Nielsen. 2008. Invasiveness ranking system for non-native plants of Alaska. 218. USDA Forest Service.
- Chapin, F. S., G. Peterson, F. Berkes, T. V. Callaghan, P. Angelstam, M. Apps, C. Beier, Y. Bergeron, A.-S. Crépin, K. Danell, et al. 2004. Resilience and vulnerability of northern regions to social and environmental change. AMBIO: A Journal of the Human Environment 33 (6):344–49. doi:https://doi.org/10.1579/0044-7447-33.6.344.
- Chen, I.-C., J. K. Hill, R. Ohlemuller, B. D. Roy, and C. D. Thomas. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–26. doi:https://doi.org/10.1126/science.1206432.
- Chown, S. L., A. H. Huiskes, N. J. Gremmen, J. E. Lee, A. Terauds, K. Crosbie, Y. Frenot, K. A. Hughes, S. Imura, K. Kiefer, et al. 2012. Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proceedings of the National Academy of Sciences of the United States of America 109 (13):4938–43. doi:https://doi.org/10.1073/pnas.1119787109.
- Chown, S. L., P. C. Le Roux, T. Ramaswiela, J. M. Kalwij, J. D. Shaw, and M. A. Mcgeoch. 2013. Climate change and elevational diversity capacity: Do weedy species take up the slack? Biological Letters 9 (1):20120806. doi:https://doi.org/10.1098/rsbl.2012.0806.
- Committee for Environmental Protection. 2019. Non-native species manual. Buenos Aires, Argentina: Secretariat of the Antarctic Treaty.
- Crête, G., T. M. Herrmann, C. Fortin, and E. Schüttler. 2020. Public perceptions of non-native plant species on a Chilean sub-Antarctic island. Polar Geography 43 (1):46–63. doi:https://doi.org/10.1080/1088937X.2019.1707321.
- Cuba-Díaz, M., J. M. Troncoso, C. Cordero, V. L. Finot, and M. Rondanelli-Reyes. 2012. Juncus bufonius, a new non-native vascular plant in King George Island, South Shetland Islands. Antarctic Science 25 (3):385–86. doi:https://doi.org/10.1017/S0954102012000958.
- Dainese, M., S. Aikio, P. E. Hulme, A. Bertolli, F. Prosser, and L. Marini. 2017. Human disturbance and upward expansion of plants in a warming climate. Nature Climate Change 7 (8):577–80. doi:https://doi.org/10.1038/nclimate3337.
- Damgaard, C., K. Raundrup, P. Aastrup, P. L. Langen, J. Feilberg, and J. Nabe-Nielsen. 2018. Arctic resilience: No evidence of vegetation change in response to grazing and climate changes in South Greenland. Arctic, Antarctic, and Alpine Research 48 (3):531–49. doi:https://doi.org/10.1657/AAAR0016-005.
- Davis, M. B. 1984. Climatic instability, time lags, and community disequilibrium. In Community ecology, ed. J. Diamond and T. J. Case, 269–84. New York: Harper and Row.
- Doherty, M. D., G. T. Wright, and K. L. McDougall. 2015. The flora of Kosciuszko National Park, New South Wales: Summary and overview. Cunninghamia 15:13–68.
- Dullinger, S., T. Dirnböck, and G. Grabherr. 2003. Patterns of shrub invasion into high mountain grasslands of the northern Calcareous Alps, Austria. Arctic, Antarctic, and Alpine Research 35 (4):434–41. doi:https://doi.org/10.1657/1523-0430(2003)035[0434:POSIIH]2.0.CO;2.
- Essl, F., P. Hulme, J. M. Jeschke, R. P. Keller, P. Pysek, D. M. Richardson, W. C. Saul, S. Bacher, S. Dullinger, R. A. Estevez, et al. 2017. Scientific and normative foundations for the valuation of alien-species impacts: Thirteen core principles. Bioscience 67:166–78.
- Essl, F., S. Dullinger, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Katsanevakis, I. Kühn, B. Lenzner, A. Pauchard, P. Pyšek, et al. 2019. A conceptual framework for range-expanding species that track human-induced environmental change. Bioscience 69 (11):908–19. doi:https://doi.org/10.1093/biosci/biz101.
- Essl, F., S. Dullinger, W. Rabitsch, P. E. Hulme, P. Pyšek, J. R. U. Wilson, and D. M. Richardson. 2015. Historical legacies accumulate to shape future biodiversity in an era of rapid global change. Diversity & Distributions 21:534–47. doi:https://doi.org/10.1111/ddi.12312.
- Forbes, B. C., M. Marcias Fauria, and P. Zetterberg. 2010. Russian Arctic warming and greening are closely tracked by tundra shrub willows. Global Change Biology 16:1542–54. https://doi.org/10.1111/j.1365/2486.2009.02047.x.
- Frenot, Y., S. L. Chown, J. Whinam, P. M. Selkirk, P. Convey, M. Skotnicki, and D. M. Bergstrom. 2005. Biological invasions in the Antarctic: Extent, impacts and implications. Biological Review 80:45–72. doi:https://doi.org/10.1017/S1464793104006542.
- Fuentes-Lillo, E., M. Cuba-Díaz, J. M. Troncoso-Castro, and M. Rondanelli-Reyes. 2017. Seeds of non-native species in King George Island soil. Antarctic Science 29 (4):324–30. doi:https://doi.org/10.1017/S0954102017000037.
- Galera, H., A. Rudak, E. A. Czyż, K. J. Chwedorzewska, A. Znój, and M. Wódkiewicz. 2019. The role of the soil seed store in the survival of an invasive population of Poa annua at point Thomas Oasis, King George Island, maritime Antarctica. Global Ecology and Conservation 19: e00679.
- Graziano, G., S. Seefeldt, and L. Clayton. 2014. Best management practices: Controlling the spread of invasive plants during road maintenance, ed. Service, UOaFCE, 39. University of Alaska, Fairbanks.
- Hallinger, M., M. Manthey, and M. Wilmking. 2010. Establishing a missing link: Warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist 186:890–99. doi:https://doi.org/10.1111/j.1469-8137.2010.03223.x.
- Harsch, M. A., P. E. Hulme, M. S. McGlone, and R. P. Duncan. 2009. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters 12:1040–49. doi:https://doi.org/10.1111/j.1461-0248.2009.01355.x.
- Hughes, K. A., L. R. Pertierra, M. A. Molina-Montenegro, and P. Convey. 2015. Biological invasions in terrestrial Antarctica: What is the current status and can we respond? Biodiversity and Conservation 24 (5):1031–55.
- Hughes, K. A., O. L. Pescott, J. Peyton, T. Adriaens, E. J. Cottier-Cook, G. Key, W. Rabitsch, E. Tricarico, D. K. A. Barnes, N. Baxter, et al. 2020. Invasive non-native species likely to threaten biodiversity and ecosystems in the Antarctic Peninsula region. Global Change Biology 13 (26):2702–16. doi:https://doi.org/10.1111/gcb.14938.
- Hughes, K. A., and P. Convey. 2012. Determining the native/non-native status of newly discovered terrestrial and freshwater species in Antarctica - Current knowledge, methodology and management action. Journal of Environmental Management 93 (1):52–66. doi:https://doi.org/10.1016/j.jenvman.2011.08.017.
- Hughes, K. A., P. Convey, L. R. Pertierra, G. C. Vega, P. Aragon, and M. A. Olalla-Tarraga. 2019. Human-mediated dispersal of terrestrial species between Antarctic biogeographic regions: A preliminary risk assessment. Journal of Environmental Management 232:73–89. doi:https://doi.org/10.1016/j.jenvman.2018.10.095.
- Huiskes, A. H. L., N. J. M. Gremmen, D. M. Bergstrom, Y. Frenot, K. A. Hughes, S. Imura, K. Kiefer, M. Lebouvier, J. E. Lee, M. Tsujimoto, et al. 2014. Aliens in Antarctica: Assessing transfer of plant propagules by human visitors to reduce invasion risk. Biological Conservation 171:278–84. doi:https://doi.org/10.1016/j.biocon.2014.01.038.
- Hulme, P. E. 2009. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. Journal of Applied Ecology 46 (1):10–18. doi:https://doi.org/10.1111/j.1365-2664.2008.01600.x.
- Huntington, H., T. Callaghan, S. Fox, and I. Krupnik. 2004. Matching traditional and scientific observations to detect environmental change: A discussion on Arctic terrestrial ecosystems. AMBIO: A Journal of the Human Environment 13:18–23.
- Intergovernmental Panel on Climate Change. 2013. Summary for policymakers. In Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change, ed. T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley, 222. Cambridge, UK and New York, NY, USA: Cambridge University Press.
- Kaltenborn, B. P. 2000. Arctic–alpine environments and tourism: Can sustainability be planned? Mountain Research and Development 20 (1):28–31. doi:https://doi.org/10.1659/0276-4741(2000)020[0028:AAEATC]2.0.CO;2.
- Kašák, J., M. Mazalová, J. Šipoš, and T. Kuras. 2015. Dwarf pine: Invasive plant threatens biodiversity of alpine beetles. Biodiversity and Conservation 24 (10):2399–415. doi:https://doi.org/10.1007/s10531-015-0929-1.
- Kasischke, E. S., and M. R. Turetsky. 2006. Recent changes in the fire regime across the North American boreal region—spatial and temporal patterns of burning across Canada and Alaska. Geophysical Research Letters 33 (9):1–5.
- Kent, A., T. D. Drezner, and R. Bello. 2018. Climate warming and the arrival of potentially invasive species into boreal forest and tundra in the Hudson Bay Lowlands, Canada. Polar Biology 41 (10):2007–22. doi:https://doi.org/10.1007/s00300-018-2341-2.
- Klein, J. A., J. Harte, and X.-Q. Zhao. 2004. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecology Letters 7 (12):1170–79. doi:https://doi.org/10.1111/j.1461-0248.2004.00677.x.
- Kopp, C. W., and E. E. Cleland. 2014. Shifts in plant species elevational range limits and abundances observed over nearly five decades in a western North America mountain range. Journal of Vegetation Science 25:135–46. doi:https://doi.org/10.1111/jvs.12072
- Kudo, G., Y. Amagai, B. Hoshino, and M. Kaneko. 2011. Invasion of dwarf bamboo into alpine snow-meadows in northern Japan: Pattern of expansion and impact on species diversity. Ecology and Evolution 1 (1):85–96. doi:https://doi.org/10.1002/ece3.9.
- Kueffer, C., C. Daehler, H. Dietz, K. McDougall, C. Parks, A. Pauchard, and L. Rew. 2014. The Mountain Invasion Research Network (MIREN). Linking local and global scales for addressing an ecological consequence of global change. GAIA - Ecological Perspectives for Science and Society 23 (3):263–65. doi:https://doi.org/10.14512/gaia.23.3.11.
- Kueffer, C., K. McDougall, J. Alexander, C. Daehler, P. Edwards, S. Haider, A. Milbau, C. Parks, A. Pauchard, Z. A. Reshi, et al. 2013. Plant invasions into mountain protected areas: Assessment, prevention and control at multiple spatial scales. In Plant invasions in protected areas. Invading nature - Springer series in invasion ecology, ed. L. Foxcroft, P. Pyšek, D. Richardson, and P. Genovesi, 89–113. Dordrecht: Springer.
- Le Roux, J. J., C. Hui, M. L. Castillo, J. M. Iriondo, J. H. Keet, A. A. Khapugin, F. Medail, M. Rejmanek, G. Theron, F. A. Yannelli, et al. 2019. Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Current Biology 29 (17):2912–18 e2. doi:https://doi.org/10.1016/j.cub.2019.07.063.
- Lembrechts, J. J., J. Lenoir, M. A. Nuñez, A. Pauchard, C. Geron, G. Bussé, A. Milbau, and I. Nijs. 2017. Microclimate variability in alpine ecosystems as stepping stones for non‐native plant establishment above their current elevational limit. Ecography 41 (6):900–09. doi:https://doi.org/10.1111/ecog.03263.
- Lenoir, J., R. Bertrand, L. Comte, L. Bourgeaud, T. Hattab, J. Murienne, and G. Grenouillet. 2020. Species better track climate warming in the oceans than on land. Nature Ecology and Evolution 4:1044–59. doi:https://doi.org/10.1038/s41559-020-1198-2
- Lenoir, J., and J. C. Svenning. 2013. Latitudinal and elevational range shifts under contemporary climate change, 599–611. Encyclopedia of Biodiversity. Cambridge: Academic Press.
- Lenoir, J., and J. C. Svenning. 2015. Climate-related range shifts - A global multidimensional synthesis and new research directions. Ecography 38 (1):15–28. doi:https://doi.org/10.1111/ecog.00967.
- MacDonald, D., J. R. Crabtree, G. Wiesinger, T. Dax, N. Stamou, P. Fleury, J. Gutierrez Lazpita, and A. Gibon. 2000. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. Journal of Environmental Management 59:47–69. doi:https://doi.org/10.1006/jema.1999.0335.
- Malfasi, F., P. Convey, S. Zaccara, and N. Cannone. 2019. Establishment and eradication of an alien plant species in Antarctica: Poa annua at Signy Island. Biodiversity and Conservation 29 (1):173–86. doi:https://doi.org/10.1007/s10531-019-01877-7.
- McDougall, K. L., G. Wright, and E. Peach. 2018. Coming to terms with ox-eye daisy (Leucanthemum vulgare) in Kosciuszko National Park, New South Wales. Ecological Management & Restoration 19 (1):4–13. doi:https://doi.org/10.1111/emr.12296.
- McDougall, K. L., J. M. Alexander, S. Haider, A. Pauchard, N. G. Walsh, and C. Kueffer. 2011. Alien flora of mountains: Global comparisons for the development of local preventive measures against plant invasions. Diversity & Distributions 17:103–11. doi:https://doi.org/10.1111/j.1472-4642.2010.00713.x.
- Meffin, R., A. L. Miller, P. E. Hulme, and R. P. Duncan. 2010. Biodiversity research: Experimental introduction of the alien plant Hieracium lepidulum reveals no significant impact on montane plant communities in New Zealand. Diversity & Distributions 16 (5):804–15. doi:https://doi.org/10.1111/j.1472-4642.2010.00684.x.
- Miller, C. M. 2015. The impact of introduced flowering species on alpine plant-pollinator networks in Southern New Zealand. PhD, University of Otago.
- Molina-Montenegro, M. A., D. M. Bergstrom, K. J. Chwedorzewska, P. Convey, and S. L. Chown. 2019. Increasing impacts by Antarctica’s most widespread invasive plant species as result of direct competition with native vascular plants. NeoBiota 51:19–40. doi:https://doi.org/10.3897/neobiota.51.37250.
- Molina-Montenegro, M. A., F. Carrasco-Urra, C. Rodrigo, P. Convey, F. Valladares, and E. Gianoli. 2012. Occurrence of the non-native annual bluegrass on the Antarctic mainland and its negative effects on native plants. Conservation Biology 26 (4):717–23. doi:https://doi.org/10.1111/j.1523-1739.2012.01865.x.
- Muñoz, A. A., and L. A. Cavieres. 2008. The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. Journal of Ecology 96 (3):459–67. doi:https://doi.org/10.1111/j.1365-2745.2008.01361.x.
- Niu, Y., S. Yang, J. Zhou, B. Chu, S. Ma, H. Zhu, and L. Hua. 2019. Vegetation distribution along mountain environmental gradient predicts shifts in plant community response to climate change in alpine meadow on the Tibetan Plateau. Science of the Total Environment 650 (Pt 1):505–14. doi:https://doi.org/10.1016/j.scitotenv.2018.08.390.
- Normand, S., C. Randin, R. Ohlemuller, C. Bay, T. T. Hoye, E. D. Kjaer, C. Korner, H. Lischke, L. Maiorano, J. Paulsen, et al. 2013. A greener Greenland? Climatic potential and long-term constraints on future expansions of trees and shrubs. Philosophical Transactions of the Royal Society of London B Biological Sciences 368 (1624):20120479. doi:https://doi.org/10.1098/rstb.2012.0479.
- Olofsson, J., L. Oksanen, T. Callaghan, P. E. Hulme, T. Oksanen, and O. Suominen. 2009. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biology 15 (11):2681–93. doi:https://doi.org/10.1111/j.1365-2486.2009.01935.x.
- Orsi, A., and U. Harris. 2019. Fronts of the Antarctic Circumpolar Current - GIS data, Ver. 1. Australian Antarctic Data Centre. Accessed November 21, 2020. https://data.aad.gov.au/metadata/records/antarctic_circumpolar_current_fronts
- Pajunen, A. M., J. Oksanen, and R. Virtanen. 2011. Impact of shrub canopies on understory vegetation in western Eurasian tundra. Journal of Vegetation Science 22:837–46. doi:https://doi.org/10.1111/j.1654-1103.2011.01285.x.
- Pauchard, A., A. Milbau, A. Albihn, J. M. Alexander, T. Burgess, C. C. Daehler, G. Englund, F. Essl, B. Evengård, G. B. Greenwood, et al. 2015. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: New challenges for ecology and conservation. Biological Invasions 18 (2):345–53. doi:https://doi.org/10.1007/s10530-015-1025-x.
- Pauli, H., M. Gottfried, K. Reiter, C. Klettner, and G. Grabherr. 2007. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994-2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global Change Biology 13 (1):147–56. doi:https://doi.org/10.1111/j.1365-2486.2006.01282.x.
- Pearson, R. G., S. J. Phillips, M. M. Loranty, P. S. A. Beck, T. Damoulas, S. J. Knight, and S. J. Goetz. 2013. Shifts in Arctic vegetation and associated feedbacks under climate change. Nature Climate Change 3 (7):673–77. doi:https://doi.org/10.1038/nclimate1858.
- Pecl, G. T., M. B. Araujo, J. D. Bell, J. Blanchard, T. C. Bonebrake, I. C. Chen, T. D. Clark, R. K. Colwell, F. Danielsen, B. Evengard, et al. 2017. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355:6332. doi:https://doi.org/10.1126/science.aai9214.
- Pepin, N., R. S. Bradley, H. F. Diaz, M. Baraer, E. B. Caceres, N. Forsythe, H. Fowler, G. Greenwood, M. Z. Hashmi, X. D. Liu, et al. 2015. Elevation-dependent warming in mountain regions of the world. Nature Climate Change 5 (5):424–30.
- Pertierra, L. R., K. A. Hughes, P. Tejedo, N. Enríquez, M. J. Luciañez, and J. Benayas. 2017. Eradication of the non-native Poa pratensis colony at Cierva Point, Antarctica: A case study of international cooperation and practical management in an area under multi-party governance. Environmental Science & Policy 69:50–56. doi:https://doi.org/10.1016/j.envsci.2016.12.009.
- Post, E., M. C. Forchhammer, M. S. Bret-Harte, T. V. Callaghan, T. R. Christensen, B. Elberling, A. D. Fox, O. Gilg, D. S. Hik, T. T. Høye, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325:1355–58. doi:https://doi.org/10.1126/science.1173113.
- Rew, L. J., T. J. Brummer, F. W. Pollnac, C. D. Larson, K. T. Taylor, M. L. Taper, J. D. Fleming, and H. E. Balbach. 2018. Hitching a ride: Seed accrual rates on different types of vehicles. Journal of Environmental Management 206:547–55. doi:https://doi.org/10.1016/j.jenvman.2017.10.060.
- Ricciardi, A., T. M. Blackburn, J. T. Carlton, J. T. A. Dick, P. E. Hulme, J. C. Iacarella, J. M. Jeschke, A. M. Liebhold, J. L. Lockwood, H. J. Macisaac, et al. 2017. Invasion science: A horizon scan of emerging challenges and opportunities. Trends in Ecology & Evolution 32 (6):464–74. doi:https://doi.org/10.1016/j.tree.2017.03.007.
- Riseth, J. Å., H. Tømmervik, E. Helander-Renvall, N. Labba, C. Johansson, E. Malnes, J. W. Bjerke, C. Jonsson, V. Pohjola, L.-E. Sarri, et al. 2011. Sámi traditional ecological knowledge as a guide to science: Snow, ice and reindeer pasture facing climate change. Polar Record 47 (3):202–17. doi:https://doi.org/10.1017/S0032247410000434.
- Sandvik, H., D. Dolmen, R. Elven, T. Falkenhaug, E. Forsgren, H. Hansen, K. Hassel, V. Husa, G. Kjærstad, F. Ødegaard, et al. 2019. Alien plants, animals, fungi and algae in Norway: An inventory of neobiota. Biological Invasions 21 (10):2997–3012. doi:https://doi.org/10.1007/s10530-019-02058-x.
- Sanz-Elorza, M., R. G. Mateo, and F. Bernardo. 2009. The historical role of agriculture and gardening in the introduction of alien plants in the western Mediterranean. Plant Ecology 202 (2):247–56.
- SAT. 2020. The Protocol on Environmental Protection to the Antarctic Treaty. Buenos Aires, Argentina: Secretariat of the Antarctic Treaty. Accessed November 21, 2020. Available http://ats.aq/e/protocol.html
- Sebastià, M.-T. 2006. Plant guilds drive biomass response to global warming and water availability in subalpine grassland. Journal of Applied Ecology 44 (1):158–67. doi:https://doi.org/10.1111/j.1365-2664.2006.01232.x.
- Seipel, T., C. Kueffer, L. J. Rew, C. C. Daehler, A. Pauchard, B. J. Naylor, J. M. Alexander, P. J. Edwards, C. G. Parks, J. R. Arevalo, et al. 2012. Processes at multiple scales affect richness and similarity of non-native plant species in mountains around the world. Global Ecology and Biogeography 21 (2):236–46. doi:https://doi.org/10.1111/j.1466-8238.2011.00664.x.
- Spellman, B., and T. L. Wurtz. 2011. Invasive sweetclover (Melilotus alba) impacts native seedling recruitment along floodplains of interior Alaska. Biological Invasions 13 (8):1779–90. doi:https://doi.org/10.1007/s10530-010-9931-4.
- Spellman, K. V., L. C. Schneller, C. P. Mulder, and M. L. Carlson. 2015. Effects of non-native Melilotus albus on pollination and reproduction in two boreal shrubs. Oecologia 179 (2):495–507. doi:https://doi.org/10.1007/s00442-015-3364-9.
- Steinbauer, M. J., J. A. Grytnes, G. Jurasinski, A. Kulonen, J. Lenoir, H. Pauli, C. Rixen, M. Winkler, M. Bardy-Durchhalter, E. Barni, et al. 2018. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556 (7700):231–34. doi:https://doi.org/10.1038/s41586-018-0005-6.
- Steyn, C., M. Greve, M. P. Robertson, J. M. Kalwij, and P. C. Le Roux. 2017. Alien plant species that invade high elevations are generalists: Support for the directional ecological filtering hypothesis. Journal of Vegetation Science 28:337–46.
- Sturm, M., C. Racine, and K. Tape. 2001. Increasing shrub abundance in the Arctic. Nature 441:546–47. doi:https://doi.org/10.1038/35079180.
- Taylor, K., T. Brummer, M. L. Taper, A. Wing, and L. J. Rew. 2012. Human mediated long distance dispersal: An empirical evaluation of seed dispersal by vehicles. Diversity & Distributions 18:942–51. doi:https://doi.org/10.1111/j.1472-4642.2012.00926.x.
- Te Beest, M., J. Sitters, C. B. Ménard, and J. Olofsson. 2016. Reindeer grazing increases summer albedo by reducing shrub abundance in Arctic tundra. Environmental Research Letters 11:125013. doi:https://doi.org/10.1088/1748-9326/aa5128.
- Tolvanen, A., P. Eilu, A. Juutinen, K. Kangas, M. Kivinen, M. Markovaara-Koivisto, A. Naskali, V. Salokannel, S. Tuulentie, and J. Simila. 2019. Mining in the Arctic environment - a review from ecological, socioeconomic and legal perspectives. Journal of Environmental Management 233:832–44. doi:https://doi.org/10.1016/j.jenvman.2018.11.124.
- Trump, B. D., M. Kadenic, and I. Linkov. 2018. A sustainable Arctic: Making hard decisions. Arctic, Antarctic, and Alpine Research 50:1. doi:https://doi.org/10.1080/15230430.2018.1438345.
- UN-WCMC. 2002. Cambridge, UK: United Nations Environment World Conservation Monitoring Centre. Accessed November 21, 2020. http://unep-wcmc.org
- Urban, M. C. 2020. Climate-tracking species are not invasive. Nature Climate Change 10:380–84. doi:https://doi.org/10.1038/s41558-020-0770-8.
- Vakhlamova, T., H.-P. Rusterholz, Y. Kanibolotskaya, and B. Baur. 2016. Effects of road type and urbanization on the diversity and abundance of alien species in roadside verges in western Siberia. Plant Ecology 217 (3):241–52. doi:https://doi.org/10.1007/s11258-016-0565-1.
- Vetter, V. M. S., N. B. Tjaden, A. Jaeschke, C. Buhk, V. Wahl, P. Wasowicz, and A. Jentsch. 2018. Invasion of a legume ecosystem engineer in a cold biome alters plant biodiversity. Frontiers of Plant Science 9:715. doi:https://doi.org/10.3389/fpls.2018.00715.
- Vilà, M., J. L. Espinar, M. Hejda, P. E. Hulme, V. Jarosik, J. L. Maron, J. Pergl, U. Schaffner, Y. Sun, and P. Pysek. 2011. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14 (7):702–08. doi:https://doi.org/10.1111/j.1461-0248.2011.01628.x.
- Wagner, W. L., D. R. Herbst, and S. H. Sohmer. 1999. Manual of the flowering plants of Hawaii. Revised ed. Hawaii: University of Hawaii Press.
- Wallingford, P. D., T. L. Morelli, J. M. Allen, E. M. Beaury, D. M. Blumenthal, B. A. Bradley, J. S. Dukes, R. Early, E. J. Fusco, D. E. Goldberg, et al. 2020. Adjusting the lens of invasion biology to focus on the impacts of climate-driven range shifts. Nature Climate Change 10 (5):398–405.
- Walther, G. R., A. Roques, P. E. Hulme, M. T. Sykes, P. Pysek, I. Kuhn, M. Zobel, S. Bacher, Z. Botta-Dukat, H. Bugmann, et al. 2009. Alien species in a warmer world: Risks and opportunities. Trends in Ecology & Evolution 24 (12):686–93. doi:https://doi.org/10.1016/j.tree.2009.06.008.
- Walther, G. R., E. Post, P. Convey, A. Menzel, C. Parmesan, T. J. C. Beebee, J. M. Fromentin, O. Hoegh-Guldberg, and F. Bairlein. 2002. Ecological responses to recent climate change. Nature 416 (6879):389–95.
- Ware, C., D. Berge, E. Muller, and I. G. Alsos. 2012. Humans introduce viable seeds to the Arctic on footwear. Biological Invasions 14:567–77. doi:https://doi.org/10.1007/s10530-011-0098-4.
- Wasowicz, P. 2016. Non-native species in the vascular flora of highlands and mountains of Iceland. PeerJ 4:e1559. doi:https://doi.org/10.7717/peerj.1559.
- Wasowicz, P., A. N. Sennikov, K. B. Westergaard, K. Spellman, M. Carlson, L. J. Gillespie, J. M. Saarela, S. S. Seefeldt, B. Bennett, C. Bay, et al. 2019. Non-native vascular flora of the Arctic: Taxonomic richness, distribution and pathways. Ambio 49 (3):693–703. doi:https://doi.org/10.1007/s13280-019-01296-6.
- Wasowicz, P., E. M. Przedpelska-Wasowicz, and H. Kristinsson. 2013. Alien vascular plants in Iceland: Diversity, spatial patterns, temporal trends, and the impact of climate change. Flora - Morphology, Distribution, Functional Ecology of Plants 208 (10–12):648–73. doi:https://doi.org/10.1016/j.flora.2013.09.009.
- Weiss, F., T. J. Brummer, and G. Pufal. 2016. Mountain bikes as seed dispersers and their potential socio-ecological consequences. Journal of Environmental Management 181:326–32. doi:https://doi.org/10.1016/j.jenvman.2016.06.037.
- Williams, M., J. Zalasiewicz, P. Haff, C. Schwa Gerl, A. D. Barnosky, and E. C. Ellis. 2015. The Anthropocene biosphere. The Anthropocene Review 2 (3):196–219. doi:https://doi.org/10.1177/2053019615591020.
- Yang, H., M. Ma, J. R. Thompson, and R. J. Flower. 2018. Transport expansion threatens the Arctic. Science 359 (6376):646–47.
- Zong, S., Y. Jin, J. Xu, Z. Wu, H. He, H. Du, and L. Wang. 2016. Nitrogen deposition but not climate warming promotes Deyeuxia angustifolia encroachment in alpine tundra of the Changbai Mountains, northeast China. Science of the Total Environment 544:85–93.
