?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We examined the effects of temperature and salt concentration on growth of the freshwater oomycete Saprolegnia parasitica that has recently (since 2013) been found to infect an important subsistence fish (in Iñupiaq, Aanaakłiq; broad whitefish, Coregonus nasus) on the Colville River in Nuiqsut, Alaska. Using two confirmed isolates (one from the Colville River and another from a southern British Columbia aquaculture facility), we tested the following hypotheses: (1) the isolate from Alaska will grow at a greater rate than the isolate from British Columbia at lower temperatures, (2) the isolate from British Columbia will grow at a greater rate at higher temperatures than the Alaska isolate, and (3) increasing salinity will reduce the growth rate of both isolates similarly at all temperatures. In addition, we used local observations—subsistence fishers and observations associated with scientific monitoring—to assist in interpreting the potential implications of our experimental results in the context of these environmental observations. In the habitat relevant to this study, water temperature ranges between <0°C and 18°C, and salinity ranges between 0 and 30 parts per thousand due to a seasonal (and occasional west wind-driven) saltwater intrusions. No statistically significant differences were detected in growth rate or salt tolerance between the two isolates at the temperatures and salinities tested; high temperature (24°C) and low salt concentration are associated with the highest growth rate for both isolates. From our lab study, one might conclude that the peak host colonization would occur during the seasonal period of warmest water temperature; however, the observations by local fishers and biologists show this not to be the case. We conclude that, at this time, we do not have evidence that peak warm water is the primary cause of an increased incidence of infection by this freshwater mold. Although indirect and lag analysis of temperature and timing of infection were not part of this study, we note that there is a greater role of complex interactions among biotic and abiotic factors (including temperature) that may predispose some individuals in the population to become infected during spawning season.
Introduction
The species Saprolegnia parasitica is an aquatic oomycete with a wide distribution in temperate freshwater ecosystems. In these regions, it commonly exists as a saprophyte, but this species has also been reported as an opportunistic pathogen of fish (van den Berg et al. Citation2013; de la Bastide, Leung, and Hintz Citation2015), and other members of this genus may infect amphibians (Kiesecker, Blaustein, and Belden Citation2001; Fernández-Benéitez et al. Citation2008; Petrisko et al. Citation2008) and invertebrates in aquatic ecosystems (Sarowar et al. Citation2013). Infections of freshwater fish are often observed in commercial aquaculture facilities and enhancement hatcheries when fish encounter stressors that weaken their immune response (Stueland, Hatai, and Skaar Citation2005; Fregeneda-Grandes, Rodrıguez-Cadenas, and Aller Citation2007; de la Bastide et al. Citation2016; de la Bastide, Naumann, and Hintz Citation2018). In contrast to temperate regions, the occurrence of this oomycete has rarely been recorded in freshwater ecosystems in the Arctic, and the prevalence of animal infections in natural arctic systems remained undescribed until recently. The first confirmed cases of saprolegniosis were observed in late September through November 2013 on Aanaakłiq, the Iñupiaq word for broad whitefish (Coregonus nasus), harvested by Iñupiaq fishers in the Colville River near Nuiqsut, Alaska, (; Sformo et al. Citation2017), although one case of saprolegniosis on the North Slope was documented in 1980 in a single whitefish (Coregonus sp., 81-0094, 1980) from the Inaru River, about 230 km west of the Colville River (Hauck Citation1980).
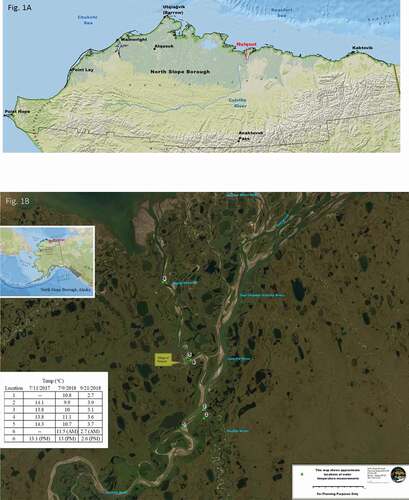
Figure 1. (A) Position of the Colville River in the North Slope of Alaska and the (B) sample sites in the Colville River Delta are shown. Locations of water sampling and temperature measurement on 21 September 2018 are indicated as green dots. Data loggers were placed at locations 1, 4, and 5. Inset table summarizes locations and dates of periodic water temperature measurement.
Nuiqsut is one of seven villages on the North Slope of Alaska (~89,000 mi2), located on the bank of the Niġliq (also Nechelik) Channel of the Colville River, approximately 80 km west of the Prudhoe Bay gas and oil development complex. The Colville River is the largest basin on the North Slope, a catchment draining over 50,000 km2 (Arnborg et al. Citation1966; McClelland et al. Citation2014) and annually discharging (modeled) ~19.7 km3 (McClelland et al., Citation2014). At the onset of freezing, discharge decreases and saltwater encroaches upstream as far as 60 km (Arnborg et al. Citation1966; Walker and Hudson Citation2003). There are few historical data that record the substantial seasonal variation in water temperature for the Colville River. Arnborg et al. (Citation1966) indicated a peak temperature of approximately 17°C in July 1962, and no data sources have been found for the period of 1970 to 2006. In 2007, the U.S. Geological Survey recorded a maximum water temperature of 18.3°C in July for other discrete projects in the upper Colville near Umiat (U.S. Geological Survey, Citation2016).
Aanaakłiq (broad whitefish, BWF) is found in the Colville River watershed and is a long-lived, high-fecundity species (Harper et al. Citation2012) that spawns in freshwater in autumn (September–October, concurrent with the observation of the infection) and is typically considered anadromous (Craig Citation1989; VanGerwen-Toyne, Tallman, and Gillis Citation2008). Though some tagged individuals remain in the same lake (sedentary), others travel tens of kilometers among connected lakes (nomadic); coastal migrants cover longer distances (one individual was estimated to have traveled up to 218 km) through both fresh and nearshore ocean waters (Morris Citation2003; pers. comm., William Morris, Owl Ridge Natural Resource Consultants, Inc, April 2020). The complex life history of BWF makes it difficult to define species boundaries, because some ecotypes are considered facultative anadromous and sensitive to saline conditions (Craig Citation1989; Gallaway et al. Citation1997; Morris Citation2003) and others are amphidromous (Morris Citation2003), indicating an ability to make nearshore migrations in the Beaufort Sea outside of life history parameters. Still others consider this species to be potadromous or lacustrine (VanGerwen-Toyne, Tallman, and Gillis Citation2008). Even a simplified diagram of their seasonal movements is complex (Morris Citation2003). All of these activities depend on watershed connectivity and the physiological plasticity of BWF that contributes to its variable life history. It is generally regarded, however, that high saline conditions for extended periods of time are not tolerated by BWF, which will migrate upstream to freshwater spawning and overwintering habitats. It should be noted that the opportunistic pathogen S. parasitica is generally described as being sensitive to salinity (van West Citation2006) and has not been known to infect marine fish species.
Aanaakłiq is an important subsistence resource for the community of Nuiqsut and is greatly valued for its size and abundance during its seasonal migrations. Fishing for BWF occurs from May to November in Nuiqsut, peaking in July, with a lower fishing effort occurring in May and late September to November (Braund Citation2010). The most recent survey conducted by the Alaska Department of Fish and Game survey involved a one-year recall for the calendar year of 2014 and estimated that 36,605 pounds of BWF were taken in Nuiqsut alone, with a 54 percent household response rate (Brown et al. Citation2016). Though based on past studies we have no reason to believe that there is a declining fishing effort or a decrease in fish taken (Craig Citation1989; Brower and Opie Citation1997; Braund Citation2010; Bacon et al. Citation2011), it should be noted with equal weight that some fishers have recently described a decline in catch. Annual catch of BWF in Nuiqsut and the North Slope during the past decade should be considered significant (in the tens of thousands of pounds).
Due to the importance of this local subsistence fishery and the finding of saprolegniosis (2013 to present; Sformo et al. Citation2017) as well as the worldwide increase in fungal and fungal-like infections of animals and plants (Fisher et al. Citation2012), the emergence of this newly observed animal pathogen on BWF in the Colville River has raised food security concerns for Iñupiaq residents of Alaska’s North Slope. A specific request from the Nuiqsut community in April 2017 asked that we examine growth rate of S. parasitica under arctic conditions, with the tacit assumption being that unusually warm waters on the Colville River may have contributed to the appearance of this infection in BWF. Elevated water temperature outside of the usual range can be a physiological stressor that contributes to saprolegniosis in some fish species (Teffer et al. Citation2017). However, because the infection has typically been observed and reported only in late September through November when water temperature is lower, we considered whether local isolates of S. parasitica may have a superior ability to grow at low water temperatures and are less tolerant of elevated summer temperatures.
To respond to the community request, the current study evaluated the growth of S. parasitica under a range of temperatures that BWF would encounter in the Colville River between June and September, as well as a higher temperature most likely not encountered. In addition, the sensitivity of S. parasitica to salinity was assessed to determine the effect of naturally saline river waters on the growth of this pathogen, in light of the seasonal salt wedge intrusion and migrations of BWF and other local fish species into saltwater. Using two confirmed isolates of S. parasitica, one from the Colville River (AK isolate) and the other from southern British Columbia (BC isolate), we tested the following hypotheses: (1) the AK isolate will grow at a greater rate than the BC isolate at lower temperatures, (2) the BC isolate will grow at a greater rate than the AK isolate at higher temperatures, and (3) increasing salinity will reduce the growth rate of both isolates similarly at all temperatures. In addition, we used local observations—subsistence fisher observations as well as observations associated with scientific monitoring related to gas and oil development—to assist in interpreting the potential implications of our results in the environmental context of these observations.
Materials and methods
Collection of pure culture isolates from field samples and river temperature data
Isolates were obtained from water samples collected on 21 September 2018 from the Colville River and tributaries (70.32716 N, −150.93837 W to 70.054966 N, −151.16307 W) in the vicinity of Nuiqsut, Alaska (). Water sampling locations were selected based on local knowledge of broad whitefish populations. Both water temperature and salinity were recorded at each location, and water samples were kept cool until processed. In addition, three temperature data loggers (BeadedStream Inc., Anchorage, AK) were placed in the main river channel and the Niġliq Channel and recorded water temperature hourly (°C) from 10 July to 21 September 2018. Each temperature probe was weighted down to approximately a 3-m depth.
To obtain pure cultures from environmental water samples, a sterile Petri dish (100 × 15 mm) containing a 15 to 20 mL volume was incubated at 25°C to 27°C with autoclaved hemp seeds as a nutritional substrate and colonization by spores or mycelia was visually scored within 24 to 72 hours. Single colonized hemp seeds were aseptically transferred to a glucose peptone agar (GPA; 3 g L−1 d-glucose, 1.25 g L−1 bacto peptone, and 15 g L−1 bacto agar) augmented with four antibiotics (Ab-GPA), which included Rifampicin (Calbiochem, La Jolla CA) at a final concentration of 50 mgL−1, Nystatin N1638 (Sigma-Aldrich, St. Louis, MO) at 10 mg L−1, Chloramphenicol (Sigma-Aldrich) at 25 mg L−1, and Streptomycin (Calbiochem) at 10 mg L−1. To obtain pure culture isolates, colonies were incubated for three to five days before transfer of a mycelial plug from the colony edge to a fresh Ab-GPA plate; this procedure was repeated at least three times.
Cultures of putative S. parasitica isolates were transported to the University of Victoria, British Columbia, Canada, and stored at 4°C prior to the confirmation of species identity and use in growth experiments. By a similar procedure, a second isolate of S. parasitica was obtained from a water sample collected at the Cermaq Canada aquaculture facility (50.337474 N, −125.521155 W) near Campbell River, British Columbia. A single isolate from each sample location was selected for growth experiments once species identity was confirmed by nucleotide sequence analysis.
DNA extractions, PCR amplification, and nucleotide sequence analysis
Extraction of total DNA from cultures of mycelia was completed by use of the Prepman Ultra Sample Preparation Reagent (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer’s instructions, with some modifications. Samples were combined in 2-mL screw cap microtubes (Starstedt, Nümbrecht, Germany) with 30 μg of zirconium/silica beads (0.5 mm diameter; Fisher Scientific, Ottawa, ON, Canada) and homogenized by the agitation of tubes with the Mini Beadbeater (Biospec Products Inc., Bartlesville, OK, USA) for 30 seconds, followed by centrifugation for 30 seconds at 13,000 ×g. The bead beating and centrifugation steps were carried out six times, three times prior to adding the Prepman solution and three times following the addition of 70 μL of Prepman solution. Samples were incubated in a heat block (100°C) for 10 to 15 minutes, followed by 2 minutes at room temperature to cool samples prior to centrifugation for 5 minutes at 13,000 ×g. A 40-μL volume of supernatant containing extracted DNA was subsequently transferred to a new 1.5-mL tube and subjected to further purification. A 320-μL volume of TE buffer (pH 8.0) and 40 μL of 3 M sodium acetate were added to each sample and mixed thoroughly. A 400-μL volume of isopropanol was subsequently added to each tube and mixed thoroughly. Samples were kept at room temperature for 15 minutes prior to centrifugation for 10 minutes at 13,000 ×g. The supernatant was decanted and dried DNA pellets were resuspended in 40 μL of sterile distilled water. Samples of DNA were analyzed to determine purity and concentration using the Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) prior to preparing DNA template as 1:10 dilutions in sterile distilled water for use in polymerase chain reaction (PCR).
To amplify oomycete DNA for nucleotide sequence analysis, the universal internal transcribed spacer (ITS) primers ITS4 and ITS5 (T. J. White et al. Citation1990) were used to amplify a 500 to 600 base pair (bp) region of the rDNA cistron containing the ITS1, 5.8S, and ITS2 regions. Each PCR reaction was completed in a 10-μL reaction volume using 1 U of DNA polymerase (Dream Taq; Thermo Scientific, Burlington, ON, Canada), a 1.0× concentration of Dream Taq Green buffer, 5 μg bovine serum albumin, 5 percent DMSO, 10 mM of deoxynucleoside triphosphate (dNTPs), a concentration of 10 μM for each primer, and 1 to 10 ng of template DNA. All reactions were performed using the Eppendorf Gradient MasterCycler model 5331 (Eppendorf Canada, Mississauga, ON, Ontario). Reaction conditions for the ITS primers included an initial denaturation step (3 minutes at 94°C), followed by thirty-five cycles of denaturation (45 seconds at 94°C), annealing (60 seconds at 55°C), and extension (40 seconds at 72°C), with a final extension (10 minutes at 72°C). A 5.0-μL volume of each post-PCR reaction was separated by gel electrophoresis on a 1.5 percent agarose gel (1.5 hours at 7 V cm−1) and visualized under ultraviolet light after 30 minutes in a 3× solution (v/v, from 10,000 stock) of GelRed nucleic acid gel stain (Biotium, Hayward, CA).
Amplified products (1–40 ng/μL) were sequenced by Eurofins MWG Operon (Operon, Huntsville, AL), following purification using the Column Pure PCR Clean-up Kit (ABM, Richmond, BC, Canada), according to the manufacturer’s instructions. All nucleotide sequence results were manually analyzed and manipulated using MEGA (v6.0) Alignment Explorer (Tamura et al. Citation2013). The opposing strands for each submitted sample were sequenced in each direction and aligned to verify nucleotide sequence quality and to obtain a consensus sequence for use in searches. Isolate ITS sequences were identified through queries against GenBank BLASTn (nucleotide query) search, using default parameters of the National Center for Biotechnology Information (NCBI) database (Citation2020).
Evaluation of Saprolegnia parasitica growth rate
We conducted quantitative and a series of qualitative (presence/absence) growth experiments. For the quantitative experiment, confirmed isolates were cultured on standard GPA plates using four incubation temperature treatments (4°C, 8°C, 12°C, and 24°C) and three salt concentrations (0, 1, and 2 percent of NaCl [w/v]) in solid agar medium. Growth determination used four replicates per factor combination per isolate for a total of ninety-six plates. After 17 hours, the growing colony perimeter was traced with a permanent marker on the back of each plate and photographed at a standard height of 13.8 cm and saved as TIFF files (72 × 72 dpi, 24-bit depth, file size ~1.4 MB). Scale calibration was completed for each plate with the accompanying scale bar in the original image (). The measurement of growth was terminated before the growth front reached the edge of the plate. All images were analyzed using the National Institutes of Health ImageJ v1.51 software measuring tool (Rasband, Citation1997-2018).
Figure 2. The plate illustrates an Alaska sample held at 4°C and 0 percent salinity; the colored concentric circles represent the sequential growth front for measuring perimeter, and the solid yellow lines are north–south, east–west radials (length) used to estimate perimeter.
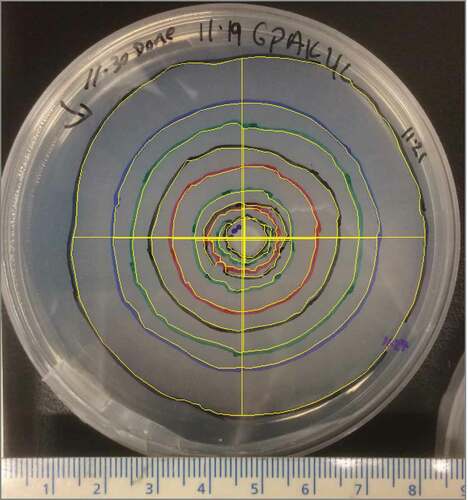
North–south and east–west radials were created on each image, ensuring that lines were perpendicular. These radials allowed us to compare estimated perimeter (via radials) to measured perimeter (via outlining growth front) by Spearman’s correlation to determine growth rate with different salt and temperature conditions, under the assumption that nutrients are equally dispersed and growth is concentric.
To more closely model natural growing conditions, four qualitative trials were conducted to evaluate mold growth and viability in water that contained a range of salt concentrations. Unless otherwise specified, all trials were incubated at 24°C and each included three replicates per treatment, with each replicate plate receiving an agar plug of viable S. parasitica inoculum. The first trial visually inspected growth from the agar plug in sterile distilled water containing a range of salt concentrations (0, 0.5, 1, 2, and 5 percent NaCl, w/v), with sterile bait seeds added after 96 hours to determine culture viability. In subsequent trials, seeds were added at time zero to salt concentrations of 1, 2, 5, 10, and 20 percent (trial 2); 4, 5, 6, 7, and 8 percent (trial 3); and 1, 2, 4, 5, and 6 percent (trial 4); all treatments were scored for culture viability after 96 hours. At the conclusion of the last trial, seeds at 6 percent were rinsed with distilled water and replated in pure water to determine whether the culture remained viable.
Statistical analysis
Because the growth curves for area appeared nonlinear by inspection, we chose to treat perimeter as the response variable in our statistical model because perimeter is proportional to the square root of area, which is an approximate linearizing transformation for our data.
These data constitute longitudinal repeated measures and must be modeled accordingly to account for correlation between measurements of the same plate; however, a preliminary assessment indicated visually that in many cases there is substantial between-plate variation in the growth curves, even for a fixed choice of salinity and temperature. Moreover, this variation appears to increase as salinity increases and temperature decreases.
Let Yi denote a perimeter measurement at time Hi, salinity Si, and temperature Ti. Let Bi = 1 if the isolate is BC and 0 otherwise.
This pattern of variation led us to fit the following mixed effects model (Galecki and Burzykowski Citation2013):
where the random effects (RE) components of this model are
and where αSi,i, δSi,i, γSi,i, ηTi,i, and εi are independent mean zero normal random variables with variances τ2α,Si, τ2δ,Si, τ2γ,Ti, τ2η,Ti, and 2, respectively. This model fits separate (linear) growth curves for each combination of salinity and temperature, with adjustments for isolate, while also assuming mean zero random effects (on the intercept and slope of the growth curve) having different variances for different salinity and temperature levels. In this manner, the model allows us to compare growth rates under different conditions while accounting for plate-specific variability.
We initially fit models of this sort using Maximum Likelihood/Restricted Maximum Likelihood (ML/REML) approaches and compared models using the Akaike information criterion, Bayesian information criterion, and likelihood ratio test with the lme4 package in R (Bates et al. Citation2015). During this exploratory phase, we learned what effects were likely important and we confirmed that treating salinity and temperature continuously (rather than as factors) was appropriate. Due to the complexity of our models, optimization was sometimes difficult, so we switched to a Bayesian approach for final model fitting, parameter estimation, and prediction. For this, we applied the rstanarm package in R (Stan Development Team Citation2016), with default priors, a conservative proposal step size (adapt_delta = 0.99), and 50,000 iterations in each of four chains, with half discarded as burn-in.
We assessed growth rates in two ways. First, we examined the parameters from the fixed effects portion of our model that involves time to evaluate how the slope (with respect to Hi) depends on covariates. This is a direct view of growth rate. It was evident, however, that in some cases growth did not begin until after some time delay. The slope does not describe this effect. Therefore, we also looked at model predictions of perimeter after 48 hours as a second measure of growth.
To compare rates with our statistical model while accounting for plate-specific variability, we calculated growth rate/hour and growth reduction/hour by temperature and salinity. In addition, using EquationEquation (1)(1)
(1) , we estimated colony growth rate under experimental conditions to try to understand whether growth rate experimentally determined in vitro can qualitatively account for the local observations that colonies grow quickly under arctic conditions (low water temperature and 0 percent salinity).
Table 2. The calculated rate of growth and inhibition of growth based on the model Equation (1)
Results
In situ temperature and salinity
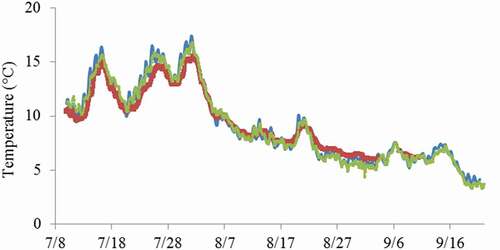
There was only slight variation in temperature recorded for a given time interval among the three logger locations in the Niġliq Channel of the Colville River between 10 July and 21 September 2018 (). The data are characterized by two peaks in July and the third peak on 1 August, corresponding to the warmest temperature recorded, 17.4°C. All three recordings appeared to decrease similarly after the maximum was reached, declining from a mean temperature of 15.9°C ± 0.08°C (mean ± standard error of mean) to 10.4°C ± 0.05°C between 4 and 5 August, after which there was an overall decline to the lowest levels recorded in late August and early September (3.4°C–5.9°C). Surface water temperature on the Colville River and the Niġliq Channel during point sampling (21 September) ranged from 2.6°C to 4.4°C, and salinity was 0.1 ppt, consistent with freshwater (). The inset temperature chart provides additional opportunistic surface temperature measurements taken in approximately the same locations as recordings made on 21 September. By contrast, freshwater temperature at the Cermaq Canada aquaculture facility in Campbell River, British Columbia, where the second isolate (BC) of S. parasitica was obtained, is maintained between 13°C and 16°C.
Culture isolation and species confirmation
Of the seven water samples from six locations on the Colville River, one showed growth within two days. This growth was subcultured on GPA plates and pure cultures were obtained for confirmation of species-level identity and experimental use. A total of two pure culture isolates were obtained from Colville River water samples, which were identified to species via nucleotide sequence analysis of the ITS-rDNA region. Sequence identity of confirmed isolates ranged from 99 to 100 percent in Blastn searches of the NCBI database. One confirmed isolate of S. parasitica (51-AK) was selected for use in growth studies, and the associated ITS-rDNA region nucleotide sequence data was submitted to the NCBI database (GenBank Accession No. MT820491). A similar process was followed to confirm the identity of the S. parasitica isolate (4A-1-BC) obtained from a freshwater aquaculture facility in Canada (GenBank Accession No. MT820490). These two confirmed isolates had greater than 99 percent sequence similarity for this region.
Growth rate and salinity tolerance
We examined growth cuves over time based on the estimates of the parameters associated with fixed effects in our model () We excluded plates run at 4°C and 2 percent salinity because no growth was observed ().
Table 1. Posterior medians and 95 percent posterior credible interval bounds for parameters associated with fixed effects in our model
Figure 4. Colony growth (perimeter) for AK (black) and BC (red) S. parasitica isolates over time (h), for a range of temperatures (°C) and salt concentrations (% w/v). Each curve represents the results from one plate for which there were four replicates per temperature–salt concentration per isolate.
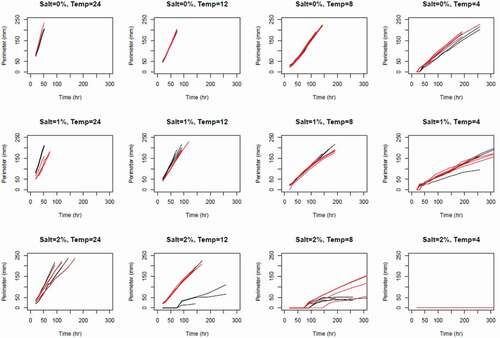
Notably, none of the effects associated with isolate (B in ) are statistically significant. Because β12 is approximately zero, the rate of growth (after growth has begun) does not significantly differ between the isolates. The other nonsignificant parameters indicate that the effect of salinity and temperature on growth does not significantly differ between the isolates. Indeed, omitting all model terms involving isolate yields a substantially improved model.
The other coefficient estimates in confirm that growth is significantly slowed as salinity increases and significantly increased when temperature increases. The significant interaction indicates that decreasing salinity and increasing temperature increases growth, but by less than would be expected if the effects of these factors were purely additive.
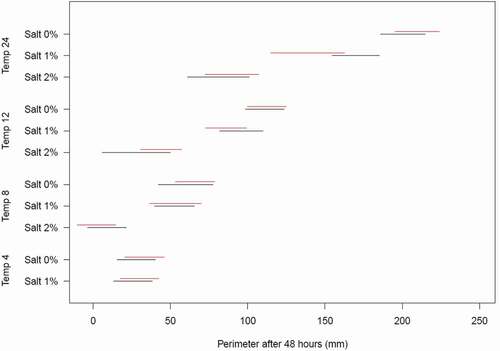
shows posterior predictive 95 percent intervals for perimeter at 48 hours, conditioned on the random effects. The predictions in reinforce our interpretation of the estimated model coefficients; specifically, that mold growth significantly increases with low salinity and high temperature. The results (and estimated random effects) for 1 percent salinity with temperature 24°C are somewhat anomalous; stronger growth of the BC isolate would be expected, due to its geographical origin.
Figure 5. Lines represent posterior predictive 95 percent intervals for AK (black) and BC (red) perimeter (mm) at 48 hours by temperature (°C), conditional on the random effects.
In terms of growth in millimeters per hour and/or inhibition of growth (), rates can be compared while accounting for plate-specific variability with this statistical model. Overall, the percentage of growth inhibition () depends on initial temperature, with the largest inhibitory effect (93 percent) occurring at low temperature (4°C) and high salinity (2 percent), whereas the inhibitory effect at 2 percent NaCl and at the highest temperature (24°C) is approximately 57 percent.
To assess the growth rate model in terms of field observations, we measured colonies on two host fish that were photographed (especially on dorsal and ventral sections that appeared on both sides of the fish) to estimate coverage. Colony size ranged from 76 to 300 mm in diameter, although the latter may have coalesced from two smaller colonies. Solving for Hi in our model, we determined that a mean colony size of 122 mm would require about 5.8 days to grow under experimental conditions (4°C and 0 percent salt); the smallest colony, about 76 mm, would take 3.5 days, and the largest at 300 mm would take 16 days.
The first qualitative trial for salt tolerance where no seeds were added showed no growth at any concentration. It was thought that the solid agar plug would provide enough nutrients to support growth. After sterile seeds were added 95 hours later, growth was observed within 24 hours at all salt concentrations. Growth in the highest salt treatment (5 percent) was unexpected and prompted the second qualitative trial in which growth was again observed in treatments containing 1, 2, and 5 percent NaCl within 24 hours, but not at 10 and 20 percent. To determine the percentage concentration of growth inhibition under these experimental conditions, a third trial was initiated, in which no growth was observed in treatments containing 4 to 8 percent NaCl. Finally, in the fourth trial growth was observed in treatments containing 1, 2, and 5 percent NaCl but for only the BC isolate in the latter treatment, and no growth was observed for either isolate at 4 and 6 percent NaCl; rinsing seeds from 6 percent NaCl plates and transferring them to freshwater confirmed that the mycelium was not viable, because no growth was observed.
Discussion
Our study determined and compared growth rates and temperature optima of two different isolates of S. parasitica under controlled laboratory conditions. Despite the disparate geographical origins of these isolates, our findings did not support our two hypotheses, namely (1) the AK isolate will have an optimal growth rate at a lower temperature and (2) the BC isolate will have an optimal growth rate at a higher temperature. Instead, we found no difference between the temperature (or salinity) responses of the AK and BC isolates. We speculated that the AK isolate possessed psychrothermic capability because saprolegniosis, which is only recently reported in Nuiqsut (Sformo et al. Citation2017), is observed on fish in late September and early October when water temperature is typically below 5°C. The waters of the Colville River and its tributaries remain at low temperatures and are ice-covered for long periods, although the river temperature can increase to 15°C to 17°C in the summer months (Arnborg et al. Citation1966; Fawcett, Moulton, and Carpenter Citation1985), sometimes exceeding 18°C. The seasonal variation in Colville River temperature, as well as the lower values observed in early autumn, contrast the narrow range of water temperatures experienced by S. parasitica in controlled aquaculture environments (13°C–16°C). Our results indicate that the Nuiqsut isolate is not psychrophilic but eurythermic. Based on controlled experiments at 12°C and 0 percent salinity, mycelial growth rate of S. parasitica is estimated to be 2.4 mm/h. Pathogen growth and host infection could potentially occur during the summer months; however, infected fish have not been observed and reported to the North Slope Borough-Department of Wildlife Management (NSB-DWM). In contrast to the warmer water temperature of the summer months, it should be noted that BWF spawning takes place between September and October at a temperature typically <5°C; however, this concurrence in timing of spawning and the appearance of the yearly infection cannot be eliminated as an important factor. It should be kept in mind that to our knowledge, and according to traditional ecological knowledge, the timing and conditions under which spawning takes place have not appeared to change in the collective memory of the subsistence fishermen. Their first observation of infected fish in 2013 and in subsequent years is closely correlated with the spawning season, suggesting a recent change in environmental conditions and/or susceptibility to infection for BWF that coincides with this season.
Temperature is an abiotic stressor in fish that is regularly cited as an important factor in the presence of this infection. Bly and Clem (Citation1991) and Bly et al. (Citation1992) implicated abrupt temperature declines (16°C/24 hours) as inducing immunosuppression in warm water adapted catfish leading to increased host susceptibility to Saprolegnia spp. infections, now typically described as “winter kill.” Other studies have noted that the combination of temperature change and spawning events, as well as the co-occurrence of preexisting injuries to the skin and mucus layer, are leading factors (van West Citation2006; Gozlan et al. Citation2014), especially if the fish can be shown to be already immune-compromised (van den Berg et al. Citation2013). Between 1968 and 1973, D. A. White (Citation1975) described an outbreak of saprolegniosis on wild brown trout (Salmo trutta) in the Provo River (Utah) associated with poor water quality and spawning-related wounds. The author suggested that warm waters and local water conditions probably increased the numbers of zoospores and spawning-related wounds, leading to an increased incidence of saprolegniosis, where 66 percent of spawning fish were positive for the infection. D. A. White (Citation1975) studied the distribution of saprolegniosis lesions and determined that they were related to gender and spawning activity; ventral, anterior lesions on males were presumed to be due to defending territories, and caudal peduncle lesions on females were associated with digging redds. Schaefer and Heckmann (Citation1981) attributed a die-off of wild rainbow smelt (Osmerus mordax) in Lake Superior to a combination of postspawning stress and high temperature, where surface water temperatures rose approximately 6°C above the ambient seasonal temperature.
Though the current study has focused to a large extent on the role of temperature in pathogen growth and host susceptibility, determining the importance of temperature in BWF susceptibility to infection is confounded by other environmental variables, including host physiology and life history. Different fish species are adapted to different temperature optima, and fish immune responses, such as macrophage-mediated activities, may differentially up- and downregulate particular genes in response to temperature changes and infection (Bly et al. Citation1992; Bly et al. Citation1993; Le Morvan, Troutaud, and Deschaux Citation1998; Nath et al. Citation2006; Kales et al. Citation2007). Host immune response to S. parasitica during early-stage infection can also be regulated by putative effector proteins released by the pathogen (van West et al. Citation2010; de Bruijn et al. Citation2012; Wawra et al. Citation2012). Seasonal movements and behaviors of BWF are complex (Craig Citation1989; Gallaway et al. Citation1997; Morris Citation2003) and add additional environmental variables. The pathogen itself can also display a differential response to temperature. Some S. parasitica isolates have the capacity to grow and produce motile zoospores over a wide range of temperatures (3°C–33°C; Willoughby and Copland Citation1984; Noga Citation1993; Willoughby Citation1994). In contrast, Koeypudsa et al. (Citation2005) did not observe S. parasitica growth in vitro at temperatures above 30°C. The complexity of host life history strategies, variable pathogen traits, and complex host–pathogen interactions makes it difficult to isolate the influence of temperature on host susceptibility to this pathogen under natural conditions.
This study showed a consistent pattern of increasing growth with increasing temperature. However, our experience in the field, as well as extensive observations from two independent expert sources (highly experienced Nuiqsut fishers and fisheries science researchers) have determined that S. parasitica infections are not observed in fish populations of the lower Colville River during the season of warmest water temperatures, corresponding to a period of medium to intense fishing effort by residents of Nuiqsut. From the observations of experienced Nuiqsut fishers, thousands of BWF are caught each year in subsistence nets (Craig Citation1989; Brower and Opie Citation1997; Bacon et al. Citation2011; Brown et al. Citation2016), and none of these fish have been found infected. In addition, there is an extensively monitored (thirty years) Qaaktaq (Arctic cisco, Coregonus autumnalis) fishery that coincides with the end of the BWF subsistence fishery. In the years since the first detection of infection on BWF (2013 to present), the Qaaktaq fishery has averaged over 35,700 fish/year (2013–2018), none of which have been reported with S. parasitica infections; the long-term average catch of Qaaktaq from 1985 to 2018, except for years 1999 and 2005–2006, was over 26,000 per year. Significant numbers of least cisco (Coregonus sardinella) are caught (on average 900 fish/year) and even some broad whitefish (on average approximately thirty-five fish per year) are also caught without infection (McCain, Raborn, and Fechhelm Citation2014; Seigle et al. Citation2008). However, it should be noted that the Qaaktaq fishery is conducted using small mesh nets (3 in.), whereas the Aanaakłiq fishery is conducted using larger mesh (5 in.), thereby largely excluding harvest of mature adult Aanaakłiq. One resource company (Conoco Philips of Alaska Inc.) has provided capture data on over 23,000 BWF from 1985 to 2014 (data not presented); none of the fish examined by fish biologists have been observed with saprolegniosis at any time.
Anecdotal reports suggest that average water temperatures on the North Slope are increasing, yet there are no reports of saprolegniosis from the Nuiqsut fishery during this peak in July or from other watersheds outside of the Nuiqsut fishery; furthermore, the infection seems to be largely targeting BWF, with only four specimens of humpback whitefish (Coregonus pidschian) collected since 2015 that visually appeared to have had the infection, although molecular identification of S. parasitica was not completed. Other locally common subsistence freshwater fish species such as Arctic grayling (Thymallus arcticus) and burbot (Lota lota) have not been reported with the infection, despite Saprolegnia spp. being considered a broad-spectrum pathogen (Sarowar et al. Citation2013). Although the growth of S. parasitica responds positively to elevated temperatures, field observations indicate that host infection is not observed during the summer months. At this time, elevated water temperature appears not to be a determining factor contributing to saprolegniosis outbreaks for BWF in this watershed.
Species of the genus Saprolegnia are opportunistic animal pathogens and generally do not infect healthy fish (de la Bastide, Leung, and Hintz Citation2015; de la Bastide et al. Citation2016). Increased host susceptibility to infection is therefore more likely to be the determining factor in the occurrence of saprolegniosis outbreaks, rather than simply the presence of viable zoospores in the environment, which can occur across a wide range of water temperatures. Host susceptibility can be a product of multiple factors, with temperature extremes, spawning stress, existing infections by other pathogens and parasites, and host injury being identified previously as factors that can weaken immune system function and increase the likelihood of Saprolegnia spp. infections (Miller et al. [Citation2014] and citations therein). Controlled laboratory trials would likely be required to dissect the role of known stressors in predisposing a host to a S. parasitica infection; host response to environmental variables may differ among fish species, and each may display different tolerances to stress factors that influence their immune response to an opportunistic pathogen (Miller et al. Citation2014).
We confirmed the hypothesis that increasing concentrations of NaCl will reduce growth rate on agar medium for both S. parasitica isolates, and we saw virtually no growth at the lowest temperature (4°C) and highest salt concentration (2 percent). Our study confirmed that salts and saline treatments have an inhibitory effect on S. parasitica growth. We considered this hypothesis for two reasons: salt is an accepted prophylactic treatment for saprolegniosis in freshwater finfish aquaculture (Dentler 1982; van West Citation2006); second, during the season associated with infections, two separate events are observed on the Colville River, including a saltwater intrusion that begins to move upriver (Arnborg et al., Citation1966; Walker Citation2001b) and the seasonal migrations of salmonids into the watershed from the brackish delta and the Beaufort Sea (George and Kovalsky Citation1986; Walker Citation2001b; Seigle et al. Citation2008-2021). We wanted to confirm that S. parasítica is sensitive to salt at a concentration similar to that of seawater (about 3.5 percent), because incoming salmonids such as Arctic cisco, least cisco, and chum and pink salmon (Oncorhynchus keta and O. gorbuscha, respectively) have been suggested as potential hosts to S. parasitica infections that may release viable zoospore inoculum into the freshwater system, thus exposing BWF. However, S. parasitica would not have tolerated the saltwater conditions experienced previously by these fish, which have not been reported to host this pathogen while in the Colville River. In addition, we noted a greater sensitivity of S. parasitica to salt at the lowest temperature tested (4°C) in growth experiments; this is similar to the temperature conditions experienced by these fish species in the river delta, where a salt wedge intrusion begins to move against the freshwater current during early fall at a time when infected BWF have been observed and reported.
Controlled experiments conducted in distilled water at 24°C generally supported the inhibitory effect of salt on S. parasitica growth within a concentration range similar to the average salinity of seawater (about 3.5 percent), which is due primarily to ions of chloride and sodium (National Oceanic and Atmospheric Administration, Citation2020). Salt tolerance of S. parasitica will vary with the isolate tested, their duration of exposure to saltwater, and the water source used (Marking, Rach, and Schreier Citation1994; Willoughby Citation1994; Koeypudsa et al. Citation2005). Based on our findings and previous studies, prolonged exposure to salinity of more than 3 percent is therefore likely to inhibit or prevent growth of Saprolegnia spp. Lastly, the presence of humic substances in natural waters may increase the tolerance of S. parasitica to salt, due to their buffering capacity (Ali Citation2005; Menzel et al. Citation2011; Meinelt et al. Citation2007; Steinburg Citation2016). Although arctic freshwater systems usually have a low organic carbon content, the extensive thawing of permafrost soils with global warming may increase the amount of permafrost-derived organic carbon in river waters (Zhang et al. [Citation2017] and references therein), thus influencing biological processes in these freshwater systems through changes in water chemistry and buffering capacity. Environmental samples of oomycota DNA with sequence homology to Saprolegnia spp. have been detected in sediment, seawater, and sea ice samples near Barrow, Alaska; however, this does not imply the geographical origin or the viability of such marine samples (Hassett et al. Citation2019).
Addendum
Recent communications with a local Nuiqsut fisher (26 July 2020), subsequent to the completion of this study, determined that at least one BWF appeared to be affected by saprolegniosis during the summer of 2020 (). Though we do not feel that a single case of fish infection negates our interpretation of factors contributing to outbreaks, this recent observation confirms that growth of S. parasitica and host colonization are possible during the summer months. However, infections are clearly more prevalent during late September in the Colville River delta.
Figure 6. Photo of fish taken in Nuiqsut in late July 2020. To the best of our knowledge, this is the first reported fish with putative saprolegniosis found outside of the time period (September–October) when saprolegniosis has typically been observed on this species (Sformo et al. Citation2017). Photo by Jerry Lee Pausanna. Used with permission.

Acknowledgments
In Nuiqsut, we thank Paul Kittick for his expertise on our river trips and Jerry Lee Pausanna for information and permission to use his photo of the first reported Aanaakłiq with S. parasitica prior to the month of September. We also acknowledge Daniel Heiner and Charmaine Hingada (NSB-GIS).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alaskan Inuit Food Security Conceptual Framework: How to Assess the Arctic from an Inuit Perspective, Technical Report. 2015. Inuit Circumpolar Council-Alaska. 114.
- Ali, E. H. 2005. Morphological and biochemical alterations of oomycete fish pathogen Saprolegnia parasitica as affected by salinity, ascorbic acid and their synergistic action. Mycopathologia 159 (2):231–43. doi:https://doi.org/10.1007/s11046-004-6670-z.
- Arnborg, L., H. J. Walker, and J. Peippo. 1966. Water Discharge in the Colville River, 1962. Geografiska Annaler: Series A, Physical Geography 48 (4):195–210. doi:https://doi.org/10.1080/04353676.1966.11879739.
- Bacon, J. J., T. R. Hepa, H. K. B. Brower Jr., M. Pederson, T. P. Olemaun, J. C. George, and B. G. Corrigan 2011. Estimates of subsistence harvest for village on the North Slope of Alaska, 1994-2003. Unpublished MS, The North Slope Borough Department of Wildlife Management, Barrow, AK.
- Bates, D., M. Mächler, B. Bolker, and S. Walker. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48. doi:https://doi.org/10.18637/jss.v067.i01.
- Bly, J. E., L. A. Lawson, A. J. Szalai, and L. W. Clem. 1993. Environmental factors affecting outbreaks of winter saprolegniosis in channel catfish, Ictalurus punctatus (Rafinesque). Journal of Fish Diseases 16:541–49. doi:https://doi.org/10.1111/j.1365-2761.1993.tb00890.x.
- Bly, J. E., L. A. Lawson, D. J. Dale, A. J. Szalai, R. M. Durborow, and L. W. Clem. 1992. Winter saprolegniosis in channel catfish. Diseases of Aquatic Organisms 13:155–64. doi:https://doi.org/10.3354/dao013155.
- Bly, J. E., and L. W. Clem. 1991. Temperature-mediated processes in teleost immunity: In vitro immunosuppression induced by in vivo low temperature in channel catfish. Veterinary Immunology and Immunopathology 28 (3–4):365–77. doi:https://doi.org/10.1016/0165-2427(91)90127-X.
- Braund, S. R. & Associates (SRB&A). 2010. Subsistence Mapping of Nuiqsut, Kaktovik, and Barrow. Prepared for the United States Department of the Interior, Minerals Management Service, Alaska OCS Region, Environmental Studies Program. MMS OCS Study Number 2009-003, Anchorage, Alaska.
- Brower, H. K., Jr., and R. Opie 1997. North Slope Borough subsistence harvest documentation project: Data for Nuiqsut, Alaska, for the period July 1, 1994, to June 30, 1995. Unpublished MS, The North Slope Borough Department of Wildlife Management, Barrow, AK.
- Brown, C. L., N. M. Braem, M. L. Kostick, A. Trainor, L. J. Slayton, D. M. Runfola, E. H. Mikow, H. Ikuta, C. R. McDevitt, J. Park, et al. 2016. Harvests and uses of wild resources in 4 Interior Alaska communities and 3 Arctic Alaska communities, 2014. ADF&G Division of Subsistence, Technical Paper No. 426.
- Craig, P. 1989. Subsistence fisheries at coastal villages in the Alaskan Arctic, 1970-1989. Biological Papers of the University of Alaska 24:131–52.
- de Bruijn, I., R. Belmonte, V. L. Anderson, M. Saraiva, T. Wang, P. van West, and C. J. Secombes. 2012. Immune gene expression in trout cell lines infected with the fish pathogenic oomycete Saprolegnia parasitica. Developmental & Comparative Immunology 38 (1):44–54. doi:https://doi.org/10.1016/j.dci.2012.03.018.
- de la Bastide, P. Y., C. Naumann, and W. E. Hintz. 2018. Assessment of the intraspecific variability of Saprolegnia parasitica populations in aquaculture facilities of British Columbia. Diseases of Aquatic Organisms 128:235–48. doi:https://doi.org/10.3354/dao03224.
- de la Bastide, P. Y., W. L. Leung, C. Naumann, and W. E. Hintz. 2016. Species composition and diversity of the genus Saprolegnia in fin fish aquaculture systems. Bulletin of the Aquaculture Association of Canada 2:6–12.
- de la Bastide, P. Y., W. L. Leung, and W. E. Hintz. 2015. Species composition of the genus Saprolegnia in fin fish aquaculture environments, as determined by nucleotide sequence analysis of the nuclear rDNA ITS regions. Fungal Biology 119:27–43. doi:https://doi.org/10.1016/j.funbio.2014.10.006.
- Fawcett, M. H., L. L. Moulton, and T. A. Carpenter. 1985. Chapter 2: Colville River Fishes: 1985 biological report, 171. Alaska: Entrix, Inc, ARCO.
- Fernández-Benéitez, M. J., M. E. Ortiz-Santaliestra, M. Lizana, and J. Diéguez-Uribeondo. 2008. Saprolegnia diclina: Another species responsible for the emergent disease ‘Saprolegnia infections’ in amphibians. FEMS Microbiology Letters 279:23–29.
- Fisher, M. C., D. A. Henk, C. J. Briggs, J. S. Brownstein, L. C. Madoff, S. L. McCraw, and S. J. Gurr. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484 (7393):186–94. doi:https://doi.org/10.1038/nature10947.
- Fregeneda-Grandes, J. M., F. Rodrıguez-Cadenas, and J. M. Aller. 2007. Fungi isolated from cultured eggs, alevins and broodfish of brown trout in a hatchery affected by saprolegniosis. Journal of Fish Biology 71 (2):510–18. doi:https://doi.org/10.1111/j.1095-8649.2007.01510.x.
- Galecki, A., and T. Burzykowski. 2013. Linear mixed-effects models using R: A step-by-step approach. New York: Springer-Verlag.
- Gallaway, B. J., R. G. Fechhelm, W. B. Griffiths, and J. G. Cole, 1997. Population dynamics of broad whitefish in the Prudhoe Bay region, Alaska. In ed. J. B. Reynolds, Fish Ecology in Arctic North America. American Fisheries Society Symposium 19, Proceedings of the fish Ecology in Arctic North America Symposium held at Fairbanks, 194–207. Alaska, USA. 19-21 May 1992.
- George, J. C., and R. Kovalsky 1986. Observations on the Kupigruak channel (Colville River) subsistence fishery October 1985. Unpublished MS, The North Slope Borough Department of Wildlife Management, Barrow, AK.
- Gozlan, R. E., W. L. Marshall, O. Lilje, C. N. Jessop, F. H. Gleason, and D. Andreou. 2014. Current ecological understanding of fungal-like pathogens of fish: What lies beneath? Frontiers in Microbiology 5:62. doi:https://doi.org/10.3389/fmicb.2014.00062Hauck1980.
- Harper, K. C., F. Harris, S. J. Miller, J. M. Thalhauser, and S. D. Ayers. 2012. Life history traits of adult broad whitefish and humpback whitefish. Journal of Fish and Wildlife Management 3 (1):56–75;e1944-687X. doi:https://doi.org/10.3996/022011-JFWM011Copyright:A.
- Hassett, B. T., M. Thines, A. Buaya, S. Ploch, R. Gradinger. 2019. A glimpse into the biogeography, seasonality, and ecological functions of arctic marine Oomycota. IMA Fungus 10:6. doi:https://doi.org/10.118/s43008-019-0006-6.
- Hauck, K. 1980. Alaska Department of Fish and Game, Fish Pathology Laboratory Report Accession Number 1981-0094. Unpublished MS, Alaska Department of Fish and Game, Fish Pathology Laboratory, Anchorage, Alaska.
- Kales, S. C., S. J. DeWitte-Orr, N. C. Bols, and B. Dixon. 2007. Response of rainbow trout monocyte/macrophage cell line. Molecular Immunology 44:2303–14. doi:https://doi.org/10.1016/j.molimm.2006.11.007.
- Kiesecker, J. M., A. R. Blaustein, and L. K. Belden. 2001. Complex causes of amphibian population declines. Nature 410:681–84. doi:https://doi.org/10.1038/35070552.
- Koeypudsa, W., P. Phadee, J. Tangtrongpiros, and K. Hatai. 2005. Influence of pH, temperature and sodium chloride concentration on growth rate of Saprolegnia sp. Journal of Scientific Research, Chulalongkorn University 30 (2):123–30.
- Le Morvan, C., D. Troutaud, and P. Deschaux. 1998. Differential effects of temperature on specific and nonspecific immune defenses in fish. The Journal of Experimental Biology 201 (Pt 2):165–68. doi:https://doi.org/10.1242/jeb.201.2.165.
- Marking, L. L., J. J. Rach, and T. M. Schreier. 1994. Search for Antifungal Agents in Fish Culture (chapter 7). In Salmon saprolegniasis, 131–148, ed. G. J. Mueller. Prepared for U.S. Dept. of Energy, Bonneville Power Administration, Division of Fish & Wildlife, April.
- McCain, K. M., S. W. Raborn, and R. G. Fechhelm. 2014. Year 31 of the long-term monitoring of nearshore Beaufort Sea fishes in the Prudhoe Bay region: 2013 annual report. Report for BP Exploration (Alaska) Inc. by LGL Alaska Research Associates, Inc., 76. Anchorage, Alaska.
- McClelland, J. W., A. Townsend‐Small, R. M. Holmes, Feifei Pan, M. Stieglitz, M. Khosh, and B. J. Peterson. 2014. River export of nutrients and organic matter from the North Slope of Alaska to the Beaufort Sea. Water Resources Research 50 (2): 1823–39.
- Meinelt, T., A. Paul, T. M. Phan, E. Zwirnmann, A. Krüger, A. Wienke, and C. E. Steinberg. 2007. Reduction in vegetative growth of the water mold Saprolegnia parasitica (Coker) by humic substance of different qualities. Aquatic Toxicology 83 (2):93–103. June 15. doi:https://doi.org/10.1016/j.aquatox.2007.03.013.
- Menzel, S., R. Bouchnak, R. Menzel, and C. E. Steinberg. 2011. Dissolved humic substances initiate DNA-methylation in cladocerans. Aquatic Toxicology 105 (3–4):640–42. doi:https://doi.org/10.1016/j.aquatox.2011.08.025.
- Miller, K. M., A. Teffer, S. Tucker, S. Li, A. D. Schulze, M. Trudel, F. Juanes, A. Tabata, K. H. Kaukinen, N. G. Ginther, et al. 2014. Infectious disease, shifting climates, and opportunistic predators: Cumulative factors potentially impacting wild salmon declines. Evolutionary Applications 7 (7):812–55. doi:https://doi.org/10.1111/eva.12164.
- Morris, W. 2003. Seasonal movements and habitat use of Arctic grayling (Thymallus arcticus), burbot (Lota lota), and broad whitefish (Coregonus nasus) within the Fish Creek drainage of the National Petroleum Reserve-Alaska, 2001-2002. Technical Report No. 03-02. Office of Habitat Management and Permitting, Alaska Department of Natural Resources, No. November 2003, 110.
- Nath, S., S. Kales, K. Fujiki, and B. Dixon. 2006. Major histocompatibility class II genes in rainbow trout (Oncorhynchus mykiss) exhibit temperature dependent downregulation. Immunogenetics 58 (5/6):443–53. doi:https://doi.org/10.1007/s00251-006-0094-5.
- National Center for Biotechnology Information (NCBI). 2020. September 15. https://blast.ncbi.nlm.nih.gov/
- National Oceanic and Atmospheric Administration. 2020. Why is the ocean salty? National Ocean Service. November 5. https://oceanservice.noaa.gov/facts/whysalty.html
- Noga, E. J. 1993. Water mold infections of freshwater fish: Recent advances. Annual Review of Fish Diseases 3:291–304. doi:https://doi.org/10.1016/0959-8030(93)90040-I.
- Petrisko, J. E., C. A. Pearl, D. S. Pilliod, P. P. Sheridan, C. F. Williams, C. R. Peterson, and R. B. Bury. 2008. Saprolegniaceae identified on amphibian eggs throughout the Pacific Northwest, USA, by internal transcribed spacer sequences and phylogenetic analysis. Mycologia 100:171–80. doi:https://doi.org/10.1080/15572536.2008.11832474.
- Rasband, W. S. 1997-2018. ImageJ, US National Institutes of Health, Bethesda, Maryland, USA.
- Sarowar, M. N., A. H. Van Den Berg, D. McLaggan, M. R. Young, and P. van West. 2013. Saprolegnia strains isolated from river insects and amphipods are broad spectrum pathogens. Fungal Biology 117:752–63. doi:https://doi.org/10.1016/j.funbio.2013.09.002.
- Schaefer, W. F., and R. A. Heckmann. 1981. Postspawning mortality of rainbow smelt in western Lake Superior. Journal of Great Lakes Research 7 (1):37–41. doi:https://doi.org/10.1016/S0380-1330(81)72021-5.
- Seigle J.C. 2008-2021. Annual report by ABR, Inc. for ConocoPhillips Alaska, Inc., Anchorage, AK.
- Sformo, T. L., B. Adams, J. C. Seigle, J. A. Ferguson, M. K. Purcell, R. Stimmelmayr, J. H. Welch, L. M. Ellis, J. C. Leppi, and J. C. George. 2017. Observations and first reports of saprolegniosis in Aanaakłiq, broad whitefish (Coregonus nasus), from the Colville River near Nuiqsut, Alaska. Polar Science 14:78–82. doi:https://doi.org/10.1016/j.polar.2017.07.002.
- Stan Development Team. 2016. rstanarm: Bayesian applied regression modeling via Stan. R package version 2.13.1. http://mc-stan.org.
- Steinberg, C. E. W. 2016. Umweltstress, die lange übersehene ökologische Stellgröße. (Environmental stress, the overlooked ecological driving force). Sber. Ges. Naturf. Freunde (NF) 52: 137-163. In Sitzungsberichte der Gesellschaft der Naturforschenden Freunde zu Berlin (Neue Folge). (Proceedings of the Society of Natural Scientific Friends at Berlin (New Series).
- Stueland, S., K. Hatai, and I. Skaar. 2005. Morphological and physiological characteristics of Saprolegnia spp. strains pathogenic to Atlantic salmon, Salmo salar L. Journal of Fish Diseases 28:445–53. doi:https://doi.org/10.1111/j.1365-2761.2005.00635.x.
- Tamura, K., G. Stecher, D. Peterson, A. Filipski, and S. Kumar. 2013. MEGA6: Molecular Evolutionary Genetics Analysis (ver. 6.0). Molecular Biology and Evolution 30:2725–29. doi:https://doi.org/10.1093/molbev/mst197.
- Teffer, A. K., S. G. Hinch, K. M. Miller, D. A. Patterson, A. P. Farrell, S. J. Cooke, A. L. Bass, P. Szekeres, and F. Juanes. 2017. Capture severity, infectious disease processes and sex influence post-release mortality of sockeye salmon bycatch. Conservation Physiology 5 (1):cox017. doi:https://doi.org/10.1093/conphys/cox017.
- U.S. Geological Survey. 2016. National Water Information System data available on the World Wide Web (USGS Water Data for the Nation). doi:https://doi.org/10.5066/F7P55KJN.
- van den Berg, A. H., D. McLaggan, J. Dieguez-Uribeondo, and P. Van West. 2013. The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biology Reviews 27:33–42. doi:https://doi.org/10.1016/j.fbr.2013.05.001.
- van West, P. 2006. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: New challenges for an old problem. Mycologist 20 (3):99–104. doi:https://doi.org/10.1016/j.mycol.2006.06.004.
- van West, P., I. de Bruijn, K. L. Minor, A. J. Phillips, E. J. Robertson, S. Wawra, J. Bain, V. L. Anderson, and C. J. Secombes. 2010. The putative RxLR effector protein SpHtp1 from the fish pathogenic oomycete Saprolegnia parasitica is translocated into fish cells. FEMS Microbiology Letters 310 (2):127–37. doi:https://doi.org/10.1111/j.1574-6968.2010.02055.x.
- VanGerwen-Toyne, M., R. F. Tallman, and D. Gillis. 2008. Comparison of life history traits between anadromous and lacustrine stocks of broad whitefish (Coregonus nasus): An intra-specific test of Roff’s hypothesis. Advances in Limnology 63:159–73. doi:https://doi.org/10.1127/advlim/63/2012/159.
- Walker, H. J. 2001a. Coastal processes and the influences on the people of Alaska’s North Slope. In Fifty more years below zero: tributes and meditations for the Naval Arctic Research Laboratory’s first half century at Barrow, Alaska, Norton, D. W. Arctic Institute of North America. (2001), 117–22. Calgary, Alta.: Arctic Institute of North America.
- Walker, H. J. 2001b. Research on the Colvilee River Delta: Lab and local support. In Fifty more years below zero: tributes and meditations for the Naval Arctic Research Laboratory’s first half century at Barrow, Alaska, Norton, D. W. Arctic Institute of North America. (2001), 15–21. Calgary, Alta.: Arctic Institute of North America.
- Walker, H. J., and P. F. Hudson. 2003. Hydrologic and geomorphic processes in the Colville River delta, Alaska. Geomorphology 56 (3–4): 291–303.
- Wawra, S., J. Bain, E. Durwarda, I. de Bruijn, K. L. Minor, A. Matena, L. Löbach, S. C. Whisson, P. Bayer, A. J. Porter, et al. 2012. Host-targeting protein 1 (SpHtp1) from the oomycete Saprolegnia parasitica translocates specifically into fish cells in a tyrosine-O-sulphate–dependent manner. Proceedings of the National Academy of Sciences 109 (6):2096–101. doi:https://doi.org/10.1073/pnas.1113775109.
- White, D. A. 1975. Ecology of an annual Saprolegnia sp. (Phycomycete) outbreak in wild brown trout. SIL Proceedings, 1922-2010 19 (3):2456–60. doi:https://doi.org/10.1080/03680770.1974.11896329.
- White, T. J., T. Bruns, T. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, ed. M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White, 315–22. New York: Academic Press, Inc.
- Willoughby, L. G. 1994. Rapid preliminary screening of Saprolegnia on fish (Short Communication). Journal of Fish Diseases 8:473–76. doi:https://doi.org/10.1111/j.1365-2761.1985.tb01282.x.
- Willoughby, L. G., and J. W. Copland. 1984. The temperature-growth relationships of Saprolegnia pathogenic to fish, especially eels cultivated in warm water. Nova Hedwigia 39:35–55.
- Zhang, X., T. S. Bianchi, X. Cui, B. E. Rosenheim, C. L. Ping, A. J. M. Hanna, M. Kanevskiy, K. M. Schreiner, and M. A. Allison. 2017. Permafrost organic carbon mobilization from the watershed to the Colville River delta: Evidence from 14 C ramped pyrolysis and lignin biomarkers. Geophysical Research Letters 44:11–491. doi:https://doi.org/10.1002/2017GL075543.