ABSTRACT
Vegetation removal during resource extraction in the Arctic causes long-lasting impacts requiring revegetation to accelerate plant reestablishment. This study focused on root development on shrub cuttings from seven common species at Diavik Diamond Mine, Northwest Territories. Two experiments were conducted; the first had six soaking times (zero, one, three, five, ten, twenty days), four indole-3-butyric acid (IBA) concentrations (0, 0.1, 0.4, 0.8 percent), and three seasons (summer, fall, spring). The second had a control, three IBA concentrations (0.1, 0.4, 0.8 percent) or alternative chemical compounds, either three Salix water or three smoke water extracts, in two seasons (summer, fall). After sixty days, all species developed at least primary and secondary roots in at least one season in one experiment, including one previously undocumented species, Kalmia procumbens. Rooting characteristics were highly variable, with maximum percentage of rooted cuttings from 3 to 55 percent and maximum number of roots per cutting from 1 to 117 across species, seasons, and experiments. Though rooting percentages were low, species-specific interactions between season and Salix water extract and smoke water extract were observed. Assessing multiple species highlights the potential of vegetative propagation to revegetate northern disturbed sites with common species that lack reliable seed sources.
Introduction
Exploration and extraction of mineral resources in the north has increased significantly over the past century, disturbing vast areas in Canada and around the world. Diamonds have been extracted from arctic mines since the mid-1990s, affecting the land through soil compaction and removal, road construction, infrastructure development, above- and belowground mining activities, and waste rock piling (Couch Citation2002; Drozdowski, Naeth, and Wilkinson Citation2012). Vegetation removal and changes in soil properties due to disturbances can have long-lasting impacts on northern landscapes, including on ecosystem services such as provision of food and habitat for fauna and indigenous communities (Johnson et al. Citation2005; Deshaies, Boudreau, and Harper Citation2009; Ficko, Smith, and Zeeb Citation2015). Natural recovery of these disturbances is predicted to take hundreds to thousands of years, as plant growth is inhibited by short growing seasons, low temperatures and rainfall, low species diversity, limited seed production and dispersal, low soil water, and low nutrient concentrations (Billings Citation1987; Harper and Kershaw Citation1996; Forbes, Ebersole, and Strandberg Citation2001; Miller and Naeth Citation2017).
Assisted revegetation is a common reclamation technique to accelerate plant establishment on disturbed sites. Successful revegetation of land in the north disturbed by mining and other anthropogenic activities will require development or amelioration of soil substrates and acquisition and propagation of plant material that can tolerate harsh conditions while allowing succession toward an appropriate plant community (Johnson Citation1987; Forbes and Jefferies Citation1999; Rausch and Kershaw Citation2007). Despite decades of research, few effective revegetation strategies have been developed for northern environments. Acquiring sufficient quantities of native species seed to reclaim large disturbances such as mine sites is challenging, as suppliers stock limited quantities of grass and forb seed and rarely seed for northern shrub species that dominate many tundra communities (Elliott, McKendrick, and Helm Citation1987; Vaartnou Citation1992; Matheus and Omtzigt Citation2011). A common revegetation technique has been to seed available early successional species such as cold tolerant grasses and legumes, expecting later successional species to invade as soil and nutrient properties improve, although this is unreliable (Densmore Citation1992; Forbes and Jefferies Citation1999; Jorgenson et al. Citation2003; Naeth and Wilkinson Citation2014).
Vegetative propagation of shrub species by cuttings is labor intensive but has good potential as a revegetation technique for reclamation of northern disturbances. Many northern shrub species have low, unknown, or cyclic seed production; seed handling, collection, and storage challenges; few if any commercial seed suppliers; slow growth that may require multiple years to become established from seed; and high costs of transporting seedlings to northern reclamation sites from more southern greenhouses (Holloway and Zasada Citation1979; Wright Citation2008; McTavish and Shopik Citation2010; Matheus and Omtzigt Citation2011). Planting stem cuttings will likely be a faster, more consistent, and effective method of establishing shrub species on disturbed sites if adventitious root development can be promoted directly in the field in a timely manner.
Adventitious root development on shrub cuttings has been documented for various horticulturally important species and some circumpolar species. Shrub species such as Populus balsamifera L. (balsam poplar), Salix alaxensis (Andersson) Coville (Alaska willow), Salix arctica Pallas (arctic willow), and Salix planifolia Pursh (diamond leaf willow) are known to easily develop adventitious roots from root primordia along the stem (Houle and Babeux Citation1993; Densmore, Vander Meer, and Dunkle Citation2000; Walter et al. Citation2005; Naeth and Wilkinson Citation2011; Ficko, Smith, and Zeeb Citation2015). However, other species rarely or never root from cuttings, and some require more intensive assistance such as application of growth hormones, soaking prior to planting, use of bottom heat or intermittent mist or fog systems, and control of environmental conditions such as light, shade, air temperature, and substrate pH (Davies et al. Citation2017).
A common method to stimulate root formation in cuttings is use of auxins such as indole-3-butyric acid (IBA), an endogenous growth hormone (Davies et al. Citation2017). Exogenous IBA application can improve rooting, root length, number of primary and secondary roots, root dry weight, time to root emergence, and survival in the field; timing and type of application and optimal IBA concentration vary significantly by species (Sharma and Aier Citation1989; Rehana et al. Citation2020; Abdel-Rahman Citation2020). Alternative methods such as soaking shoot cuttings in Salix water extract (chopped pieces of Salix ssp. shoot tissue in water; salicylic acid leaches into the water) have improved rooting percentage and number of roots for Olea europaea L. (European olive) and plant height and above- and belowground biomass for Coleus scutellarioides (L.) Benth. (common coleus) cuttings. Fire and fire by-products such as smoke water extract are known to induce germination in seeds from numerous plant species but have not been investigated to date as a stimulant for rooting of woody cuttings (Pierce, Esler, and Cowling Citation1995; Keely and Fotheringham Citation2000; Adkins and Peters Citation2001; Yao, Naeth, and Mollard Citation2017; Mackenzie and Naeth Citation2019). The only research with cuttings demonstrated that smoke water extract had a positive effect on rooting of Vigna radiata (L.) R. Wilczek (mung bean) hypocotyl cuttings (Taylor and Van Staden Citation1996), highlighting an important research gap.
Guidelines for using cuttings in revegetation are mostly from documents on stream bank restoration and slope bioengineering in northern boreal environments using easily rooting Salix and Populus species (Densmore, Vander Meer, and Dunkle Citation2000; Walter et al. Citation2005). Soaking one to ten days has promoted adventitious root formation, mostly on Salix species, indicating the need to research the effect of soaking on multiple other species (Schaff, Pezeshki, and Shields Citation2002; Pezeshki et al. Citation2005; Walter et al. Citation2005). Current guidelines recommend collecting dormant cuttings in fall or spring due to high carbohydrate reserves in tissues, but little information exists on species specific optimal times of year for collection and planting to induce root formation in northern species (Densmore and Zasada Citation1978; Houle and Babeux Citation1998; Gustavsson Citation1999; Holloway and Peterburs Citation2009). To date, limited research has been published on adventitiously derived root system architecture and morphology for various species from different ecosystems at different times of year and whether the root systems have similar growth patterns to seed grown plants from the same species. With an increasing need for revegetation in the north, the lack of knowledge about root development patterns and fine root systems of seed-grown and adventitiously derived root systems for arctic species is an important research gap.
In this study, effects of three common factors affecting rooting of stem cuttings of seven dominant shrub species at Diavik Diamond Mine Inc., Northwest Territories, were evaluated. The main objectives were to determine whether concentration of common growth hormones or alternative chemical compounds, soaking time, and time of year of collection could promote root initiation and development in growth chamber experiments to produce a more consistent source of plant material for reclamation of disturbed northern sites. We expected all of these factors to have a positive impact on the species assessed.
Materials and methods
Study site
Diavik Diamond Mine (Diavik) is located approximately 320 km northeast of Yellowknife, Northwest Territories (64°30′41″ N, 110°17′23″ W), on an island in the middle of Lac-de-Gras, approximately 100 km north of the treeline. Lac-de-Gras is in the Southern Arctic Ecozone and the Point Upland Arctic Ecoregion (Ecosystem Classification Group Citation2012), with mean annual precipitation of 299 mm (45 percent as rain) and mean annual temperature of −9°C. Mean monthly temperatures in the growing season were 8°C, 14°C, and 11°C, from June, July, and August, respectively. The landscape is dominated by large archean rock outcrops and the remnants of glaciers found as boulders, till, and eskers (Drozdowski, Anne Naeth, and Wilkinson Citation2012). Turbic and static cryosolic soils dominate upland areas, with dwarf-heath shrubs and lichen species. Organic cryosolic soils dominate lowland areas, with sedges and mosses (Drozdowski, Anne Naeth, and Wilkinson Citation2012).
Experimental design
Cuttings from the ends of the growing tip of seven common tundra species were collected as we meadered across an undisturbed upland community at Diavik. Betula glandulosa Michx. (bog birch), a large erect species in this community, was collected as less than 10-cm to 42-cm cuttings. For the other six smaller species, Arctous rubra (Rehder & Wilson) Fernald (red bearberry), Empetrum nigrum L. (crowberry), Kalmia procumbens (L.) Gift & Kron & P.F. Stevens ex Galasso, Banfi & F. Conti (alpine azalea), Rhododendron tomentosum Harmaja (marsh Labrador tea), Vaccinium uliginosum L. (bog bilberry) and Vaccinium vitis-idaea L. (bog cranberry), cuttings were less than 5 cm to 25 cm. Nomenclature for species from Diavik follows Northwest Territories Species Infobase (Government of the Northwest Territories Citation2021); nomenclature for all other species follows NatureServe (Citation2021). Cutting length varied based on available stem length from randomly selected plants for each species. Cutting stem diameter was 0.1 to 0.6 cm. Cuttings were transported in coolers and stored at 4°C until planting within one week of collection.
Two screening experiments were conducted to investigate effects of common horticultural and novel treatments on root initiation and development over sixty days in a growth chamber (). The first experiment was three factorial with seventy-two treatments. Cuttings were collected at common work times for reclamation practitioners; in summer (25 to 26 June) during active growth, fall (19 to 23 September) at the end of the growing season, and spring (20 to 22 and 24 May) prior to plants fully emerging from dormancy. Cuttings from each species in each season were randomly assigned to be treated with a soaking time (zero, one, three, five, ten, and twenty days) and a concentration of IBA (0, 0.1, 0.4, and 0.8 percent) (Stim Root #1, #2, #3, respectively).
Table 1. Species, treatments, and replication for experiments 1 and 2 in different seasons
The second experiment was two factorial with nine treatments and one control (). Cuttings were collected in summer (2 July) and/or fall (27 to 28 September) based on species differences observed in experiment 1. Cuttings from each time period were randomly assigned to untreated, treated with a common growth hormone (0.1, 0.4, and 0.8 percent IBA; Stim Root #1, #2, #3, respectively), or treated with an alternative chemical compound, either Salix water extract (Salix water) or smoke water extract (smoke water). Salix water extracts were prepared by cutting Salix shoots into 1- to 3-cm pieces and placing 300, 600, and 1,200 mL of cuttings in 2,400 mL boiling distilled water to soak for 12 hours to make three Salix water concentrations (0.5, 1, 2, respectively). Smoke water extracts were prepared by placing 4 L distilled water in a smoker with 1.2 kg wood chips for 4 hours until all wood chips had been burned and then diluting the extract with distilled water (1:20, 1:10, 1:1 volumes of extract to distilled water) to make three dilutions (0.05, 0.1, 0.5, respectively).
Planting and soaking
Growth chamber conditions for both experiments mimicked growing conditions at Diavik. Conditions were set at 17°C during the day for 16 hours and 10°C at night for 8 hours for experiment 1 and at 17°C during the day for 20 hours and 10°C at night for 4 hours for experiment 2. Cuttings were planted in a mix of 50:50 by volume peat moss and horticultural potting soil. Soaking treatments in tap water were topped up as needed to keep the bottom 1 to 5 cm of each stem wet. The bottom 1 to 5 cm of each IBA treatment cutting was dipped in IBA powder prior to planting. Cuttings from all species were dipped to approximately the same depth, with cuttings from larger species dipped in more powder than cuttings from smaller species due to surface areas. The bottom 1 to 5 cm of each cutting for Salix water and smoke water treatments was soaked for 12 hours prior to planting.
Planting containers were based on size of cuttings and growth patterns. For experiment 1, three cuttings per species per treatment (season, soaking time, IBA concentration) were nested in the same container due to growth chamber space restrictions (). For experiment 2, each cutting was planted in an individual container.
Measurements
Shoot health and vigor of each cutting were assessed at thirty and sixty days using a five-point scale with 1 = dead, 2 = poor (plant mostly dead or dying, <30 percent live green tissue), 3 = fair (average health and growth, 30 to 60 percent live green tissue), 4 = good (plant healthy and growing, 60 to 90 percent live green tissue), and 5 = excellent (plant robust and growing vigorously, 90 to 100 percent live green tissue). Adventitious root development was assessed at sixty days. Cuttings were gently washed under running water to remove all substrate material. Primary roots refers to adventitious roots that emerged from the stem cutting; secondary and tertiary roots refers to successive orders of lateral root branches off the primary root (modified from Jung and McCouch Citation2013). The number of primary roots were counted under a microscope, length of the longest primary and secondary roots was measured, and presence of tertiary roots was noted.
Statistical analyses
Interspecies comparisons were not conducted as different morphological and physiological characteristics could create confounding factors that affect interpretation of results. Cuttings in each season were assessed separately because modeling season as a fixed effect is problematic due to lack of season replication. Research has shown that many species likely have specific times of year that are more favorable for rooting than others (Teklehaimanot et al. Citation2004; Araya Citation2007; Holloway and Peterburs Citation2009); thus, season was not treated as a random effect. To analyze treatment effects, a threshold of two-thirds of cuttings with roots per species per season per experiment was selected for inclusion in a hurdle model, although due to low and/or inconsistent rooting, no species had sufficient rooting to meet this threshold. Given the exploratory nature of this screening study, results are presented graphically using ggplot2 (R, v4.0.2; Wickham Citation2016).
Results and discussion
Shoot health
Determining trends in shoot health at different times of year can indicate which cuttings have rooted without needing to physically check for roots, saving time and disruption to the cutting. Shoot health was generally higher at day 30 than day 60 for each species, in each season, in each experiment. Shoot health of rooted cuttings was variable for most species, between season within an experiment, and between experiments within a season. Shoot health was not a good indicator of rooting for evergreen species in any season in either experiment. Increased shoot health between days 30 and 60 was observed for rooted Betula glandulosa fall cuttings in experiment 1, indicating that this may be a useful technique for some deciduous species.
Adventitious root development
Reclamation of large-scale northern disturbances requires development of an appropriate self-sustaining and resilient plant community. All seven shrub species in our study produced adventitious roots in at least one season in one experiment, a significant milestone demonstrating future potential for plant propagation by arctic shrub cuttings. Maximum percentage of rooted cuttings was 3 to 55 percent across species, seasons, and experiments, with season having the most influence on rooting for each species (; Supplementary Table 1).
Figure 1. Percentage of rooted cuttings with 95 percent confidence intervals in experiment 1 for (a) each species (x-axis) grouped by time of year and (b) separated by all treatments; species (horizontal panels), time of year (x-axis), soaking time (vertical panels, zero, one, three, five, ten, twenty days), and growth hormones (0, 0.1, 0.4, 0.8 percent IBA). Summer cuttings of Arctous rubra, Rhododendron tomentosum, Vaccinium uliginosum, and Vaccinium vitis-idaea only received three IBA concentrations (0, 0.1, 0.8 percent IBA). Number of cuttings in (a) and (b) are summarized in .
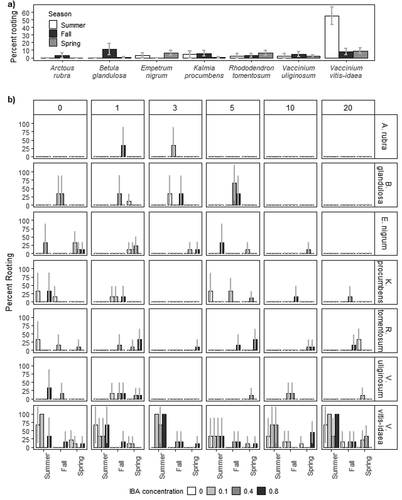
Figure 2. Percentage of rooted cuttings with 95 percent confidence intervals in experiment 2 for (a) each species (x-axis) grouped by time of year (n = 100) and (b) separated by species (horizontal panels), time of year (vertical panels), and treatment group (n = 10); untreated (control), growth hormone (0.1, 0.4, 0.8 percent IBA), Salix water extract concentrations (0.5, 1, 2), or smoke water extract dilutions (0.05, 0.1, 0.5). Arctous rubra and Betula glandulosa were only collected in fall.
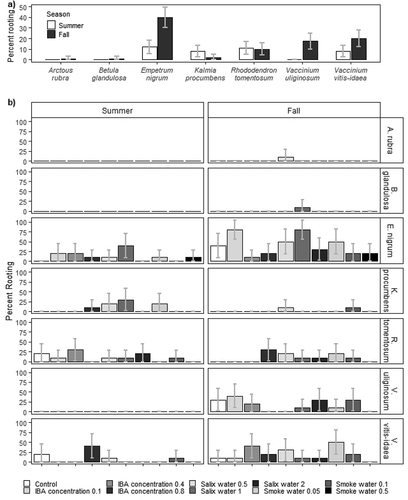
Although Kalmia procumbens cuttings had low rooting (<10 percent in all seasons and experiments), this was the first study to demonstrate development of adventitious roots (maximum twelve roots on one cutting in summer, experiment 1). Only one report was found describing rooting of Arctous rubra cuttings that had poor survival after four weeks (Naeth and Wilkinson Citation2011), similar to the low percentages in our study. Three other species, Betula glandulosa, Rhododendron tomentosum, and Vaccinium uliginosum, had less than 20 percent rooting regardless of treatments, similar to previous research (Holloway and Zasada Citation1979; Holloway Citation2006; Holloway and Peterburs Citation2009; Naeth and Wilkinson Citation2011), although Calmes and Zasada (Citation1982) observed up to 77 percent rooting for Vaccinium uliginosum summer cuttings. Despite low rooting for these five species, high variability in number of roots was common, with most cuttings having no roots and a few having a large number. Rooting is known to vary among species, among individuals within species, and between clones of individuals, due to interactions among genetic, physiological, and environmental factors (Leakey Citation1985; Bellini, Pacurar, and Perrone Citation2014).
Maximum number of roots on one cutting in our study was 1 to 117 across all species, seasons, and experiments. In experiment 1, 11 percent of fall Betula glandulosa cuttings rooted; one cutting had 12 roots, which is promising for a hard-to-root species (Holloway and Peterburs Citation2009; Naeth and Wilkinson Citation2011). Roots on Betula glandulosa cuttings generally only initiated from the base, whereas roots emerged from multiple locations up the stem for the other species (). Seasonal rooting trends for maximum and mean number of roots and maximum and mean length of the longest root were not always consistent between experiments for a given species (Supplementary Table 1). Due to the experimental design, differences between experiments, and low rooting, confirmatory statistical comparisons were not conducted within an experiment or between experiments.
Table 2. Number of cuttings with primary, secondary, and tertiary roots for each species and location of roots on cuttings
Rooting variability between years was more apparent for Empetrum nigrum and Vaccininum vitis-idaea and similar to some previous research. For example, 55 percent of Vaccininum vitis-idaea cuttings rooted in summer in experiment 1 but only 10 percent rooted in summer in experiment 2. Gustavsson (Citation1999) noted that variability in rooting for Vaccininum vitis-idaea cuttings was likely related to effects of weather on shoot health and development in the preceding year and interaction between year and seasonal rooting patterns for cuttings collected between April and August. Hagen (Citation2002) observed 60 to 85 percent rooting for Vaccininum vitis-idaea cuttings grown under saturated moist air and fog conditions, and Holloway (Citation2009) observed 44 to 91 percent rooting based on type of growth media and IBA treatment. Other studies have shown mixed results using softwood or hardwood cuttings and increased rooting for cuttings collected before bud break in spring or after shoot growth and berry production in fall (Lehmushovi Citation1975; Holloway Citation2009; Labokas and Budriuniene Citation1989).
No Empetrum nigrum cuttings rooted in fall in experiment 1; 40 percent rooted in fall in experiment 2. Hagen (Citation2002) observed 70 to 80 percent rooting for Empetrum nigrum ssp. hermaphroditum (Hagerup) Böcher cuttings in a peat, perlite, and sand mix under fog conditions or saturated moist air for two months. Other studies found good rooting capacity for Empetrum nigrum by stem cuttings but did not provide details of techniques or rooting percentages (Monni, Salemaa, and Millar Citation2000; Holloway Citation2006; Mallik and Karim Citation2008).
Rooting variability across and within species highlights the need for research to determine what other factors are affecting rooting behavior for these shrub species, because more consistency in rooting will make it a more effective reclamation technique. For example, conditions in our study were common for reclamation practitioners rather than typical horticultural procedures, so application of more specific techniques for hard-to-root species (e.g., mist chamber, bottom heat) may increase rooting and its consistency (Alder and Ostler Citation1989; Gustavsson Citation1999; Holloway and Peterburs Citation2009; Davies et al. Citation2017). Other factors known to affect rooting and potentially needing further investigation for these shrub species include cutting ontogenetic age, cutting location on a donor plant (terminal or lateral shoot), donor plant physiological status (e.g., carbohydrate concentration, carbon:nitrogen ratio, nutrient status, water status), photoperiod, seasonal influences, and weather conditions the preceding year (Hess Citation1963; Andersen Citation1986; Gustavsson Citation1999; Bellini, Pacurar, and Perrone Citation2014; Davies et al. Citation2017).
In a horticultural setting, less than 25 to 50 percent rooting is considered poor, depending on the species and grower, indicating that a species would not be grown commercially (Holloway and Peterburs Citation2009; Davies et al. Citation2017). However, for reclamation practitioners, other factors may take priority over low rooting, including reestablishment of keystone or rare species or development of a heterogenous plant community. In these cases, a higher cutting rate could be used to account for low rooting. When erosion control is a primary reclamation objective, selected species must provide sufficient live, litter, and ground cover to mitigate the impact of rain drops, with fibrous and deep taproots to stabilize surface and deeper soil layers (Hansen Citation1989). In northern environments, shrubs cuttings may be preferred for erosion control over seed grown shrubs, because their larger initial size provides greater ground cover. Cutting survival as low as 30 percent has led to successful streambank stabilization in riparian environments (Watson, Abt, and Derrick Citation1997), with similar benefits on large industrial disturbances in our experience.
Effect of treatment on adventitious root development
Determining treatment effects was challenging due to low rooting percentages, although responses to season, exogenous IBA concentration, and soaking length were species specific ( and 2). After wounding, exogenous auxins are taken up through the cut surface (Kenney, Sudi, and Blackman Citation1969), leading to an increase in endogenous auxin concentrations at the cutting base over time, which is needed for initiation of adventitious rooting (Gatineau et al. Citation1997; Benková et al. Citation2003; Yue et al. Citation2020). Because cuttings in this study were treated with IBA up to seven days after collection, rooting for some species may have improved by rewounding the base of each cutting prior to treatment (Howard Citation1971). Timing for peak auxin levels following wounding likely occurs on a species specific basis.
Exogenous IBA concentration and time of year of application had variable effects in both experiments in our study, indicating a potential seasonal interaction effect between endogenous and exogenous auxins for different species. Studies of different species have shown that levels of endogenous growth hormones such as Indole-3-acetic acid (IAA) vary naturally in roots of cuttings throughout the year, with some species needing application of different concentrations of exogenous auxin in different seasons for effective rooting (Nanda and Anand Citation1970; Blakesley, Weston, and Elliott Citation1991; Joshi et al. Citation1992; X. Guo et al. Citation2009). Studies with Arabidopsis thaliana L. Heynh. (thale cress) mutants indicated IAA and IBA may play different roles in adventitious rooting, with interactions between endogenous IAA and exogenous IBA promoting rooting (Ludwig-Müller, Vertocnik, and Town Citation2005). However, species-specific thresholds for auxin have been observed over which higher hormone concentrations can have a detrimental effect on rooting, number of roots, and root length (Houle and Babeux Citation1994; Lund, Smith, and Hackett Citation1996; Ricci et al. Citation2008). Further research is required to determine levels of endogenous auxins in different species in our study throughout the growing season and whether interactions between endogenous and exogenous growth hormones are occurring.
Once cut from a donor plant, cuttings are susceptible to desiccation prior to new root development, which can lead to low survival (Martin, Pezeshki, and Shields Citation2005). In our study, season influenced effect of soaking time in experiment 1. Only two other studies assessed effects of soaking cuttings in different seasons. Tilley and Hoag (Citation2009) found soaking for fourteeen days, and fall or spring planting did not affect rooting of either Salix amygdaloides Andersson (peach leaf willow) or Salix exigua Nutt (coyote willow) cuttings. However, fall Salix exigua cuttings soaked for fourteen days had higher root biomass than other treatments, and fall Salix amygdaloides cuttings soaked for fourteen days had higher shoot biomass. Pezeshki et al. (Citation2005) found that soaking nondormant Salix nigra Marsh. (black willow) cuttings for seven days was beneficial for cutting survival, root development, and bud flush, with no cuttings surviving after fifteen days of soaking. Results from these studies indicate a potential species-specific interaction between season and soaking, likely due to cutting physiological status. More research is required to decipher how species-specific differences in concentrations of various hormones, growth regulators, and carbohydrates between dormant and actively growing shrub cuttings influence adventitious rooting at different times of year. Though longer soaking times are not currently recommended for reclamation, results indicate that ensuring cuttings are turgid on a species-specific basis prior to planting will likely improve long-term survival in the field.
Rooting was generally low for the seven species in our study; however, species-specific interactions between Salix water concentration and season and smoke water concentration and season were observed in experiment 2 (). Similarly, Wise, Gill, and Selby-Pham (Citation2020) determined that concentration of a commercial willow bark extract that promoted root formation and root branching was species specific. Karrikins, the six active butenolide hormones isolated in plant-derived smoke and smoke water extract, have recently been shown to modulate root development, likely using a similar pathway as strigalactones (Swarbreck et al. Citation2019; Swarbreck, Mohammad-Sidik, and Davies Citation2020). Further research deciphering mechanisms of action for karrikins and strigolactones and biostimulants such as Salix water extract may enhance adventitious rooting in northern shrub cuttings and other species.
Lateral root development
All species in our study developed secondary and tertiary order roots on at least one primary root, except Arctous rubra cuttings, which did not develop tertiary roots in sixty days (; Supplementary and 2). Bell and Bliss (Citation1978) and Billings, Peterson, and Shaver (Citation1978) found that lateral root development may take several years to begin in some arctic species. Roots on Betula glandulosa cuttings were long and thick but easily broke into segments and had limited lateral root development. Empetrum nigrum, Kalmia procumbens, Rhododendron tomentosum, Vaccinium uliginosum, and Vaccinium vitis-idaea had small fine roots, and Empetrum nigrum, Rhododendron tomentosum, and Vaccinium vitis-idaea had considerable lateral root development. Vaccinium vitis-idaea roots had branching patterns similar to those of roots collected in the field in Alaska (Iversen et al. Citation2015) and was the only species to develop three or more orders of lateral roots in sixty days. Rhododendron tomentosum primary roots were easily detached from cuttings, similar to observations for Rhododendron groenlandicum (Oeder) Kron & Judd (bog Labrador tea), which developed tiny branched clumps of very thin roots, on a few roots (Holloway and Peterburs Citation2009). Maximum number of branching orders is likely controlled by species-specific genetic factors, although interactions with the environment can create significant variation in root system architecture within individuals of a species (Doussan, Pagès, and Pierret Citation2003). Due to the slow growth of arctic plants, it can take years for morphological characteristics and root branching patterns to fully develop (Bell and Bliss Citation1978; Billings, Peterson, and Shaver Citation1978), highlighting the need for further study of intact root systems of mature tundra plants to better understand growth, function, and phenology of arctic fine roots (Iversen et al. Citation2015) and how they compare to adventitiously developed root systems for different species.
Length of different root orders
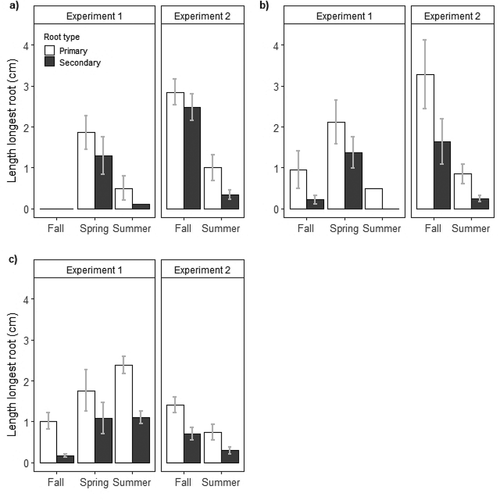
Primary roots were longer than secondary roots for Empetrum nigrum, Rhododendron tomentosum, and Vaccinium vitis-idaea in our study (other species not assessed due to limited root development; ). Root elongation followed by lateral root branching is an iterative developmental process, and root growth varies between species and for different root orders (H. Wilcox Citation1962; Pagès Citation1999; Malamy and Ryan Citation2001; Ito et al. Citation2006; Nibau, Gibbs, and Coates Citation2008). For example, Dittmer (Citation1937) observed a decrease in mean length of four successive root orders for Secale cereale L. (cultivated rye), and Fan and Guo (Citation2010) observed a similar decrease for six successive root orders for Fraxinus mandshurica Rupr. (Manchurian ash) and Larix gmelinii Rupr. (Dahurian larch). Basal diameter is correlated with potential root length, but many roots fail to reach their maximum potential (Wu, Pagès, and Wu Citation2016). In northern locations, plants can allocate 70 percent or more of their total biomass to roots (Chapin, Johnson, and McKendrick Citation1980; Poorter et al. Citation2012), and because lateral roots make up the majority of root biomass for most plant species, their growth and longevity play an important role in shaping root system architecture, particularly arctic species (Nibau, Gibbs, and Coates Citation2008; Jung and McCouch Citation2013).
Figure 3. Length of longest primary and secondary roots at day 60 with 95 percent confidence intervals for rooted (a) Empetrum nigrum, (b) Rhododendron tomentosum, and (c) Vaccinium vitis-idaea cuttings in experiments 1 and 2 at different times of year. Number of rooted cuttings is summarized in and Supplementary Table 1.
Within a specific root order in our study, experiment, season, and species influenced length of the longest root. Species-specific genetic factors in conjunction with hormonal interactions and environmental factors control cell division and elongation and determine growth and length of an emerged lateral root (Jung and McCouch Citation2013). Several studies demonstrated that elongation rates within a root order are related to root tip diameter because they reflect the size of the root meristem where new elongating cells are produced (Cahn, Zobel, and Bouldin Citation1989; Thaler and Pagès Citation1996; Lecompte and Pagès Citation2007).
Roots on cuttings in experiment 2 were generally longer than those in experiment 1 of a comparable root order for a specific season and species. Different results between experiments may have been influenced by experimental design, including cuttings growing individually versus together in one pot, photoperiod, and temperature. For example, Cakile edentula var. lacustris Fernald (Great Lakes sea rocket) plants can alter root growth if adjacent plants are related or not (Dudley and File Citation2007), and different species are known to have species-specific photoperiod and temperature requirements. Soil temperature has a strong influence on various parameters affecting root architecture including initiation, growth, branching, and orientation (G. E. Wilcox and Pfeiffer Citation1990; Kaspar and Bland Citation1992; Nagel et al. Citation2009; reviewed in Rich and Watt Citation2013). Though arctic species have adapted to growing at much lower optimal temperatures than related species in more temperate climates, tolerance for low temperatures still varies by species (Bell and Bliss Citation1978; Billings, Peterson, and Shaver Citation1978; Kummerow and Russell Citation1980).
Seasonal effects on root length varied by species in our study. Roots on summer cuttings in each experiment were generally shorter than roots of the same order on fall or spring cuttings, except for Vaccinium vitis-idaea cuttings in experiment 1 (). Resource partitioning between different tissue types varies by season and species. Though carbohydrate concentrations in cuttings are hypothetically considered an essential source of energy and material for adventitious root development, mixed results in various studies were based on numerous factors, including species, shrub type (deciduous, evergreen), donor plant maturity, cutting position (distal, basal), season (donor plant physiological status), and collection year, influencing carbohydrate type (soluble, insoluble) and quantity in different parts of a cutting (Fege and Brown Citation1984; reviewed in Haissig Citation1986, Citation1989; Davies et al. Citation2017; Tsafouros et al. Citation2019). Because plants have species-specific requirements for macro- and micronutrients at different physiological stages throughout the growing season, plant roots must adapt their root system architecture to optimize nutrient uptake (Chapin and Shaver Citation1989; Clark and Boldingh Citation1991; Drossopoulos, Kouchaji, and Bouranis Citation1996; Muhammad et al. Citation2015).
For reclamation practitioners, knowledge of species-specific root architecture can help inform revegetation practices by determining depth of required substrate, substrate properties and species selection to meet revegetation goals (e.g., stabilize disturbed soil, community restoration; indigenous needs for specific species) and indicate environmental stressors affecting a plant due to changes in root architecture. Future research directly comparing root system architecture of shrubs grown in the field versus those grown from cuttings in pots and in the field will provide further insight into revegetation practices for disturbed environments.
Conclusions
All seven shrub species in our study developed at least primary and secondary roots, including previously undocumented Kalmia procumbens. Season had the most influence on rooting for all species, although results were highly variable within and between species, indicating that other factors are likely influencing adventitious root development. Novel treatments of Salix water extract and smoke water extract were applied for the first time with cuttings from northern shrub species. Though rooting percentages were generally low, species-specific responses were apparent, highlighting the need for further research with these compounds.
To date, most propagation research has focused on individual and easy-to-root species such as Salix from northern plant communities. Our research addresses this critical gap by assessing multiple species and highlights the potential to use vegetative propagation to accelerate reestablishment of plant communities on disturbed northern sites.
Supplemental Material
Download Zip (425.9 KB)Acknowledgments
We gratefully acknowledge Dr. Peter Blenis and Dr. Sasha van der Klein for statistical analysis support, the two anonymous reviewers for their comments and suggestions, and the many people who assisted with all the various aspects of this research project.
Supplementary material
Supplemental material for this article can be accessed on the publisher’s website
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdel-Rahman, S. 2020. Influence of rooting media and indole-3-butyric acid (IBA) concentration on rooting and growth of different types of Conocarpus erectus L. stem cuttings. Scientific Journal of Flowers and Ornamental Plants 7 (3):199–219. doi:https://doi.org/10.21608/sjfop.2020.109363.
- Adkins, S. W., and N. C. B. Peters. 2001. Smoke derived from burnt vegetation stimulates germination of arable weeds. Seed Science Research 11:213–22.
- Alder, G. M., and W. K. Ostler. 1989. Native shrub propagation and nursery stock production. In The biology and utilization of shrubs, ed. C. M. McKell, 535–52. 1st ed. San Diego, USA: Academic Press Inc.
- Andersen, A. S. 1986. Environmental influences on adventitious rooting in cuttings of non-woody species. In New root formation in plants and cuttings, ed. M. B. Jackson, 223–53. Dordrecht: Springer Netherlands.
- Araya, H. T. 2007. Influence of cutting position, medium, hormone and season on rooting of bush tea (Athrixia phylicoides DC.) stem cuttings. Medicinal and Aromatic Plant Science and Biotechnology 1:243–52.
- Bell, K. L., and L. C. Bliss. 1978. Root growth in a polar semidesert environment. Canadian Journal of Botany 56 (20):2470–90. doi:https://doi.org/10.1139/b78-299.
- Bellini, C., D. I. Pacurar, and I. Perrone. 2014. Adventitious roots and lateral roots: Similarities and differences. Annual Review of Plant Biology 65 (1):639–66. doi:https://doi.org/10.1146/annurev-arplant-050213-035645.
- Benková, E., M. Michniewicz, M. Sauer, T. Teichmann, D. Seifertová, G. Jürgens, and J. Friml. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 (5):591–602. doi:https://doi.org/10.1016/S0092-8674(03)00924-3.
- Billings, W. D. 1987. Constraints to plant growth, reproduction, and establishment in Arctic environments. Arctic and Alpine Research 19 (4):357. doi:https://doi.org/10.2307/1551400.
- Billings, W. D., K. M. Peterson, and G. R. Shaver. 1978. Growth, turnover, and respiration rates of roots and tillers in tundra graminoids. In Vegetation and production ecology of an Alaskan Arctic Tundra, ed. L. L. Tieszen, 415–34. New York: Springer New York.
- Blakesley, D., G. D. Weston, and M. C. Elliott. 1991. Endogeneous levels of indole-3-acetic acid and abscisic acid during the rooting of Cotinus coggygria cuttings taken at different times of the year. Plant Growth Regulation 10 (1):1–12. doi:https://doi.org/10.1007/BF00035126.
- Cahn, M. D., R. W. Zobel, and D. R. Bouldin. 1989. Relationship between root elongation rate and diameter and duration of growth of lateral roots of maize. Plant and Soil 119 (2):271–79. doi:https://doi.org/10.1007/BF02370419.
- Calmes, M. A., and J. C. Zasada. 1982. Some reproductive traits of four shrub species in the black spruce forest type of Alaska. Canadian Field-Naturalist 96:35–40.
- Chapin, F. S., D. A. Johnson, and J. D. McKendrick. 1980. Seasonal movement of nutrients in plants of differing growth form in an Alaskan tundra ecosystem: Implications for herbivory. Journal of Ecology 68 (1):189–209. doi:https://doi.org/10.2307/2259251.
- Chapin, F. S., and G. R. Shaver. 1989. Differences in growth and nutrient use among Arctic plant growth forms. Functional Ecology 3 (1):73. doi:https://doi.org/10.2307/2389677.
- Clark, C. J., and H. L. Boldingh. 1991. Biomass and mineral nutrient partitioning in relation to seasonal growth of Zantedeschia. Scientia Horticulturae 47 (1–2):125–35. doi:https://doi.org/10.1016/0304-4238(91)90034-V.
- Couch, W. J. 2002. Strategic resolution of policy, environmental and socio-economic impacts in Canadian Arctic diamond mining: BHP’s NWT diamond project. Impact Assessment and Project Appraisal 20 (4):265–78. doi:https://doi.org/10.3152/147154602781766564.
- Davies Jr, F., R. Geneve, S. Wilson, H. Hartmann, and D. Kester. 2017. Hartmann & Kester’s plant propagation: Principles and practices. 9th ed. NY: Pearson.
- Densmore, R. V. 1992. Succession on an Alaskan tundra disturbance with and without assisted revegetation with grass. Arctic and Alpine Research 24 (3):238–43. doi:https://doi.org/10.2307/1551663.
- Densmore, R. V., and J. C. Zasada. 1978. Rooting potential of Alaskan willow cuttings. Canadian Journal of Forest Research 8 (4):477–79. doi:https://doi.org/10.1139/x78-070.
- Densmore, R. V., M. E. Vander Meer, and N. G. Dunkle. 2000. Native plant revegetation manual for Denali National Park and preserve. Anchorage, Alaska: US Geological Survey, Biological Resources Division.
- Deshaies, A., S. Boudreau, and K. A. Harper. 2009. Assisted revegetation in a subarctic environment: Effects of fertilization on the performance of three indigenous plant species. Arctic, Antarctic, and Alpine Research 41 (4):434–41. doi:https://doi.org/10.1657/1938-4246-41.4.434.
- Dittmer, H. J. 1937. A quantitative study of the roots and root hairs of a winter rye plant (Secale cereale). American Journal of Botany 24 (7):417–20. doi:https://doi.org/10.1002/j.1537-2197.1937.tb09121.x.
- Doussan, C., L. Pagès, and A. Pierret. 2003. Soil exploration and resource acquisition by plant roots: An architectural and modelling point of view. Agronomie 23 (5–6):419–31. doi:https://doi.org/10.1051/agro:2003027.
- Drossopoulos, B., G. G. Kouchaji, and D. L. Bouranis. 1996. Seasonal dynamics of mineral nutrients and carbohydrates by walnut tree leaves. Journal of Plant Nutrition 19 (3–4):493–516. doi:https://doi.org/10.1080/01904169609365138.
- Drozdowski, B. L., M. Anne Naeth, and S. R. Wilkinson. 2012. Evaluation of substrate and amendment materials for soil reclamation at a diamond mine in the Northwest Territories, Canada. Canadian Journal of Soil Science 92 (1):77–88. doi:https://doi.org/10.4141/cjss2011-029.
- Dudley, S. A., and A. L. File. 2007. Kin recognition in an annual plant. Biology Letters 3 (4):435–38. doi:https://doi.org/10.1098/rsbl.2007.0232.
- Ecosystem Classification Group. 2012. Ecological regions of the Northwest Territories – southern arctic. Yellowknife NT: Department of Environment and Natural Resources, Government of the Northwest Territories.
- Elliott, C. L., J. D. McKendrick, and D. Helm. 1987. Plant biomass, cover, and survival of species used for stripmine reclamation in South-Central Alaska, USA. Arctic and Alpine Research 19 (4):572–77. doi:https://doi.org/10.2307/1551427.
- Fan, P., and D. Guo. 2010. Slow decomposition of lower order roots: A key mechanism of root carbon and nutrient retention in the soil. Oecologia 163 (2):509–15. doi:https://doi.org/10.1007/s00442-009-1541-4.
- Fege, A. S., and G. N. Brown. 1984. Carbohydrate distribution in dormant Populus shoots and hardwood cuttings. Forest Science 30:999–1010.
- Ficko, S., B. Smith, and B. Zeeb. 2015. Assisted revegetation following contaminated site remediation in the arctic: Four-year case study of a former radar site. American Journal of Plant Sciences 6 (8):1301–12. doi:https://doi.org/10.4236/ajps.2015.68130.
- Forbes, B. C., J. J. Ebersole, and B. Strandberg. 2001. Anthropogenic disturbance and patch dynamics in circumpolar Arctic ecosystems. Conservation Biology 15 (4):954–69. doi:https://doi.org/10.1046/j.1523-1739.2001.015004954.x.
- Forbes, B. C., and R. L. Jefferies. 1999. Revegetation of disturbed Arctic sites: Constraints and applications. Biological Conservation 88 (1):15–24. doi:https://doi.org/10.1016/S0006-3207(98)00095-0.
- Gatineau, F., J. G. Fouché, C. Kevers, J. F. Hausman, and T. Gaspar. 1997. Quantitative variations of indolyl compounds including IAA, IAA-aspartate and serotonin in walnut microcuttings during root induction. Biologia Plantarum 39 (1):131–37. doi:https://doi.org/10.1023/A:1000377511120.
- Government of the Northwest Territories. 2021. NWT Species Infobase. Accessed May 31, 2021. https://www.enr.gov.nt.ca/en/services/biodiversity/nwt-species-infobase.
- Guo, D., M. Xia, X. Wei, W. Chang, Y. Liu, and Z. Wang. 2008. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist 180 (3):673–83. doi:https://doi.org/10.1111/j.1469-8137.2008.02573.x.
- Guo, X., X. Fu, D. Zang, and Y. Ma. 2009. Effect of auxin treatments, cuttings’ collection date and initial characteristics on Paeonia ‘Yang Fei Chu Yu’ cutting propagation. Scientia Horticulturae 119 (2):177–81. doi:https://doi.org/10.1016/j.scienta.2008.07.022.
- Gustavsson, B. A. 1999. Effects of collection time and environment on the rooting of Lingonberry (Vaccinium vitis-idaea L.) stem cuttings. Acta Agriculturae Scandinavica, Section B - Soil & Plant Science 49 (4):242–47. doi:https://doi.org/10.1080/713782030.
- Hagen, D. 2002. Propagation of native Arctic and alpine species with a restoration potential. Polar Research 21 (1):37–47. doi:https://doi.org/10.1111/j.1751-8369.2002.tb00065.x.
- Haissig, B. E. 1986. Metabolic processes in adventitious rooting in cuttings. In New root formation in plants and cuttings, ed. M. B. Jackson, 141–89. Dordrecht: Martinus Nijhoff Publishers.
- Haissig, B. E. 1989. Carbohydrate relations during propagation of cuttings from sexually mature Pinus banksiana trees. Tree Physiology 5 (3):319–28. doi:https://doi.org/10.1093/treephys/5.3.319.
- Hansen, D. J. 1989. Reclamation and erosion control using shrubs. In The biology and utilization of shrubs, ed. C. M. McKell, 459–78. 1st ed. San Diego, USA: Academic Press Inc.
- Harper, K. A., and G. P. Kershaw. 1996. Natural revegetation on borrow pits and vehicle tracks in shrub tundra, 48 years following construction of the CANOL No. 1 Pipeline, N.W.T., Canada. Arctic and Alpine Research 28 (2):163. doi:https://doi.org/10.2307/1551756.
- Hess, C. 1963. Why certain cuttings are hard to root. Plant Propagator 13:63–70.
- Holloway, P. S. 2006. Managing wild bog blueberry, lingonberry, cloudberry and crowberry stands in Alaska. Fairbanks, Alaska: Natural Resources Conservation Service.
- Holloway, P. S. 1985. Rooting of lingonberry, Vaccinium vitis-idaea stem cuttings. Plant Propagator 31:7–9.
- Holloway, P. S., and J. C. Zasada. 1979. Vegetative propagation of 11 common Alaska woody plants. Portland, Oregon: Dept. of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station.
- Holloway, P. S., and M. R. Peterburs. 2009. Propagation of twelve Alaska native plants by summer stem cuttings. Journal of Environmental Horticulture 27 (4):207–10. doi:https://doi.org/10.24266/0738-2898-27.4.207.
- Houle, G., and P. Babeux. 1993. Temporal variations in the rooting ability of cuttings of Populus balsamifera and Salix planifolia from natural clones–populations of subarctic Quebec. Canadian Journal of Forest Research 23 (12):2603–08. doi:https://doi.org/10.1139/x93-323.
- Houle, G., and P. Babeux. 1994. Variations in rooting ability of cuttings and in seed characteristics of five populations of Juniperus communis var. depressa from subarctic Quebec. Canadian Journal of Botany 72 (4):493–98. doi:https://doi.org/10.1139/b94-066.
- Houle, G., and P. Babeux. 1998. The effects of collection date, IBA, plant gender, nutrient availability, and rooting volume on adventitious root and lateral shoot formation by Salix planifolia stem cuttings from the Ungava Bay area (Quebec, Canada). Canadian Journal of Botany 76 (10):1687–92. doi:https://doi.org/10.1139/b98-177.
- Howard, B. H. 1971. Nursery experiment report: The response of cuttings to basal wounding in relation to time of auxin treatment. Plant Propagator 21:267–74.
- Ito, K., K. Tanakamaru, S. Morita, J. Abe, and S. Inanaga. 2006. Lateral root development, including responses to soil drying, of maize (Zea mays) and wheat (Triticum aestivum) seminal roots. Physiologia Plantarum 127 (2):260–67. doi:https://doi.org/10.1111/j.1399-3054.2006.00657.x.
- Iversen, C. M., V. L. Sloan, P. F. Sullivan, E. S. Euskirchen, A. D. McGuire, R. J. Norby, A. P. Walker, J. M. Warren, and S. D. Wullschleger. 2015. The unseen iceberg: Plant roots in Arctic tundra. New Phytologist 205 (1):34–58. doi:https://doi.org/10.1111/nph.13003.
- Johnson, C. J., M. S. Boyce, R. L. Case, H. D. Cluff, R. J. Gau, A. Gunn, and R. Mulders. 2005. Cumulative effects of human developments on Arctic wildlife. Wildlife Monographs 160 (1):1–36.
- Johnson, L. A. 1987. Management of northern gravel sites for successful reclamation: A review. Arctic and Alpine Research 19 (4):530–36. doi:https://doi.org/10.2307/1551421.
- Jorgenson, M. T., J. G. Kidd, T. C. Carter, S. Bishop, and C. H. Racine. 2003. Long-term evaluation of methods for rehabilitation of lands disturbed by industrial development in the Arctic. In Social and environmental impacts in the north: Methods in evaluation of socio-economic and environmental consequences of mining and energy production in the Arctic and sub-Arctic, ed. R. O. Rasmussen and N. E. Koroleva, 173–90. Dordrecht: Springer Science & Business Media.
- Joshi, N. K., S. Sharma, G. S. Shamet, and R. C. Dhiman. 1992. Studies on the effect of auxin and season on rooting stem cuttings of some important shrubs in nursery beds. Indian Forester 118:893–900.
- Jung, J. K. H., and S. R. M. McCouch. 2013. Getting to the roots of it: Genetic and hormonal control of root architecture. Frontiers in Plant Science 4. doi:https://doi.org/10.3389/fpls.2013.00186.
- Kaspar, T. C., and W. L. Bland. 1992. Soil temperature and root growth. Soil Science 154 (4):290–99. doi:https://doi.org/10.1097/00010694-199210000-00005.
- Keely, J. E., and C. J. Fotheringham. 2000. Role of fire in regeneration from seed. In Seeds: The ecology of regeneration in plant communities, ed. M. Fenner, 311–30. 2nd ed. New York: CABI.
- Kenney, G., J. Sudi, and G. E. Blackman. 1969. The uptake of growth substances: XIII. Differential uptake of indol-3yl-acetic acid through the epidermal and cut surfaces of etiolated stem segments. Journal of Experimental Botany 20 (4):820–40. doi:https://doi.org/10.1093/jxb/20.4.820.
- Kummerow, J., and M. Russell. 1980. Seasonal root growth in the Arctic tussock tundra. Oecologia 47 (2):196–99. doi:https://doi.org/10.1007/BF00346820.
- Labokas, J., and D. Budriuniene. 1989. Vegetative propagation of lingonberry. Acta Horticulturae (241):270–72. doi:https://doi.org/10.17660/ActaHortic.1989.241.45.
- Leakey, R. R. B. 1985. The capacity for vegetative propagation in trees. In Attributes of trees as crop plants, ed. M. G. R. Cannell and J. E. Jackson, 110–33. Abbotts Ripton, Great Britain: Institute of Terrestrial Ecology.
- Lecompte, F., and L. Pagès. 2007. Apical diameter and branching density affect lateral root elongation rates in banana. Environmental and Experimental Botany 59 (3):243–51. doi:https://doi.org/10.1016/j.envexpbot.2006.01.002.
- Lehmushovi, A. 1975. Methods of propagating the cowberry. Annales Agriculturae Fenniae 14:325–33.
- Ludwig-Müller, J., A. Vertocnik, and C. D. Town. 2005. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. Journal of Experimental Botany 56 (418):2095–105. doi:https://doi.org/10.1093/jxb/eri208.
- Lund, S. T., A. G. Smith, and W. P. Hackett. 1996. Cuttings of a tobacco mutant, rae, undergo cell divisions but do not initiate adventitious roots in response to exogenous auxin. Physiologia Plantarum 97 (2):372–80. doi:https://doi.org/10.1034/j.1399-3054.1996.970223.x.
- Mackenzie, D. D., and M. A. Naeth. 2019. Effect of plant-derived smoke water and potassium nitrate on germination of understory boreal forest plants. Canadian Journal of Forest Research 49 (12):1540–47. doi:https://doi.org/10.1139/cjfr-2019-0016.
- Malamy, J. E., and K. S. Ryan. 2001. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiology 127 (3):899–909. doi:https://doi.org/10.1104/pp.010406.
- Mallik, A. U., and M. N. Karim. 2008. Roadside revegetation with native plants: Experimental seeding and transplanting of stem cuttings. Applied Vegetation Science 11 (4):547–54. doi:https://doi.org/10.3170/2008-7-18570.
- Martin, L. T., S. R. Pezeshki, and F. D. Shields. 2005. Soaking treatment increases survival of black willow posts in a large-scale field study. Ecological Restoration 23 (2):95–98. doi:https://doi.org/10.3368/er.23.2.95.
- Matheus, P., and T. Omtzigt. 2011. Yukon revegetation manual: Practical approaches and methods. Whitehorse, YT: Mining and Petroleum Environmental Research Group.
- McTavish, B., and T. Shopik. 2010. Propagation and use of native woody plants in northern latitudes, 158–81. Victoria: British Columbia Technical and Research Committee on Reclamation.
- Miller, V. S. and M. A. Naeth. 2017. Amendments and substrates to develop anthroposols for northern mine reclamation. Canadian Journal of Soil Science 97 (2):266–77. doi:https://doi.org/10.1139/cjss-2016-0145.
- Monni, S., M. Salemaa, and N. Millar. 2000. The tolerance of Empetrum nigrum to copper and nickel. Environmental Pollution 109 (2):221–29. doi:https://doi.org/10.1016/S0269-7491(99)00264-X.
- Muhammad, S., B. L. Sanden, B. D. Lampinen, S. Saa, M. I. Siddiqui, D. R. Smart, A. Olivos, K. A. Shackel, T. DeJong, and P. H. Brown. 2015. Seasonal changes in nutrient content and concentrations in a mature deciduous tree species: Studies in almond (Prunus dulcis (Mill.) D. A. Webb). European Journal of Agronomy 65:52–68. doi:https://doi.org/10.1016/j.eja.2015.01.004.
- Naeth, M. A., and S. R. Wilkinson. 2011. Reclamation at the Diavik Diamond Mine in the Northwest Territories: Substrates, soil amendments and native plant community development. Phase II final report, 1–31. Edmonton: University of Alberta.
- Naeth, M. A., and S. R. Wilkinson. 2014. Establishment of restoration trajectories for upland tundra communities on diamond mine wastes in the Canadian Arctic. Restoration Ecology 22 (4):534–43. doi:https://doi.org/10.1111/rec.12106.
- Nagel, K. A., B. Kastenholz, S. Jahnke, D. van Dusschoten, T. Aach, M. Mühlich, D. Truhn, H. Scharr, S. Terjung, A. Walter, et al. 2009. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Functional Plant Biology 36 (11):947–59. doi:https://doi.org/10.1071/FP09184.
- Nanda, K. K., and V. K. Anand. 1970. Seasonal changes in auxin effects on rooting of stem cuttings of Populus nigra and its relationship with mobilization of starch. Physiologia Plantarum 23 (1):99–107. doi:https://doi.org/10.1111/j.1399-3054.1970.tb06396.x.
- NatureServe. 2021. NatureServe Explorer [web application]. Arlington, VA: NatureServe. Accessed May 31, 2021. https://explorer.natureserve.org/.
- Nibau, C., D. J. Gibbs, and J. C. Coates. 2008. Branching out in new directions: The control of root architecture by lateral root formation. New Phytologist 179 (3):595–614. doi:https://doi.org/10.1111/j.1469-8137.2008.02472.x.
- Pagès, L. 1999. Root system architecture: From its representation to the study of its elaboration. Agronomie 19 (3–4):295–304. doi:https://doi.org/10.1051/agro:19990309.
- Pendall, E., S. Bridgham, P. J. Hanson, B. Hungate, D. W. Kicklighter, D. W. Johnson, B. E. Law, Y. Luo, J. P. Megonigal, M. Olsrud, et al. 2004. Below-ground process responses to elevated CO2 and temperature: A discussion of observations, measurement methods, and models. New Phytologist 162 (2):311–22. doi:https://doi.org/10.1111/j.1469-8137.2004.01053.x.
- Pezeshki, S. R., C. E. Brown, J. M. Elcan, and F. Douglas Shields. 2005. Responses of nondormant black willow (Salix nigra) cuttings to preplanting soaking and soil moisture. Restoration Ecology 13 (1):1–7. doi:https://doi.org/10.1111/j.1526-100X.2005.00001.x.
- Pierce, S. M., K. Esler, and R. M. Cowling. 1995. Smoke-induced germination of succulents (Mesembryanthemaceae) from fire-prone and fire-free habitats in South Africa. Oecologia 102 (4):520–22. doi:https://doi.org/10.1007/BF00341366.
- Poorter, H., K. J. Niklas, P. B. Reich, J. Oleksyn, P. Poot, and L. Mommer. 2012. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytologist 193 (1):30–50. doi:https://doi.org/10.1111/j.1469-8137.2011.03952.x.
- Rausch, J., and G. P. Kershaw. 2007. Short-term revegetation performance on gravel-dominated, human-induced disturbances, Churchill, Manitoba, Canada. Arctic, Antarctic, and Alpine Research 39 (1):16–24. doi:https://doi.org/10.1657/1523-0430(2007)39[16:SRPOGH]2.0.CO;2.
- Rehana, S., M. Madhavi, A. D. Rao, and P. Subbaramamma. 2020. Effect of cutting types and IBA treatments on success of vegetative propagation in crossandra (Crossandra infundibuliformis L.) var. Arka shravya. Journal of Pharmacognosy and Phytochemistry 9:1469–75.
- Ricci, A., E. Rolli, L. Dramis, and C. Diaz-Sala. 2008. N,N′-bis-(2,3-methylenedioxyphenyl)urea and N,N′-bis-(3,4-methylenedioxyphenyl)urea enhance adventitious rooting in Pinus radiata and affect expression of genes induced during adventitious rooting in the presence of exogenous auxin. Plant Science 175 (3):356–63. doi:https://doi.org/10.1016/j.plantsci.2008.05.009.
- Rich, S. M., and M. Watt. 2013. Soil conditions and cereal root system architecture: Review and considerations for linking Darwin and Weaver. Journal of Experimental Botany 64 (5):1193–208. doi:https://doi.org/10.1093/jxb/ert043.
- Schaff, S. D., S. R. Pezeshki, and F. D. Shields. 2002. Effects of pre-planting soaking on growth and survival of black willow cuttings. Restoration Ecology 10 (2):267–74. doi:https://doi.org/10.1046/j.1526-100X.2002.02035.x.
- Sharma, S. D., and N. B. Aier. 1989. Seasonal rooting behaviour of cuttings of plum cultivars as influenced by IBA treatments. Scientia Horticulturae 40 (4):297–303. doi:https://doi.org/10.1016/0304-4238(89)90103-9.
- Swarbreck, S. M., A. Mohammad-Sidik, and J. M. Davies. 2020. Common components of the strigolactone and karrikin signaling pathways suppress root branching in Arabidopsis. Plant Physiology 184 (1):18–22. doi:https://doi.org/10.1104/pp.19.00687.
- Swarbreck, S. M., Y. Guerringue, E. Matthus, F. J. C. Jamieson, and J. M. Davies. 2019. Impairment in karrikin but not strigolactone sensing enhances root skewing in Arabidopsis thaliana. The Plant Journal 98 (4):607–21. doi:https://doi.org/10.1111/tpj.14233.
- Taylor, J. L. S., and J. Van Staden. 1996. Root initiation in Vigna radiata (L.) Wilczek hypocotyl cuttings is stimulated by smoke-derived extracts. Plant Growth Regulation 18 (3):165–68. doi:https://doi.org/10.1007/BF00024377.
- Teklehaimanot, Z., P. L. Mwang’ingo, A. G. Mugasha, and C. K. Ruffo. 2004. Influence of the origin of stem cutting, season of collection and auxin application on the vegetative propagation af African Sandalwood (Osyris lanceolata) in Tanzania. The Southern African Forestry Journal 201 (1):13–24. doi:https://doi.org/10.1080/20702620.2004.10431770.
- Thaler, P., and L. Pagès. 1996. Root apical diameter and root elongation rate of rubber seedlings (Hevea brasiliensis) show parallel responses to photoassimilate availability. Physiologia Plantarum 97 (2):365–71. doi:https://doi.org/10.1034/j.1399-3054.1996.970222.x.
- Tilley, D. J., and J. C. Hoag. 2009. Evaluation of fall versus spring dormant planting of hardwood willow cuttings with and without soaking treatment. Native Plants Journal 10 (3):288–94. doi:https://doi.org/10.2979/NPJ.2009.10.3.288.
- Tsafouros, A., A. Frantzeskaki, A. Assimakopoulou, and P. A. Roussos. 2019. Spatial and temporal changes of mineral nutrients and carbohydrates in cuttings of four stone fruit rootstocks and their contribution to rooting potential. Scientia Horticulturae 253:227–40. doi:https://doi.org/10.1016/j.scienta.2019.04.049.
- Vaartnou, M. 1992. The northern native grass seed industry - spring 1992. Proceedings of the 16th Annual British Columbia Mine Reclamation Symposium 75–109. British Columbia, Canada: Smithers.
- Walter, J., D. Hughes, N. J. Moore, and G. Muhlberg. 2005. Streambank revegetation and protection: A guide for Alaska. Juneau: Alaska Department of Fish and Game.
- Watson, C. C., S. R. Abt, and D. Derrick. 1997. Willow posts bank stabilization. Journal of the American Water Resources Association 33 (2):293–300. doi:https://doi.org/10.1111/j.1752-1688.1997.tb03510.x.
- Wickham, H. 2016. ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag.
- Wilcox, G. E., and C. L. Pfeiffer. 1990. Temperature effect on seed germination, seedling root development and growth of several vegetables. Journal of Plant Nutrition 13 (11):1393–403. doi:https://doi.org/10.1080/01904169009364161.
- Wilcox, H. 1962. Growth studies of the root of Incense Cedar, Libocedrus decurrens. II. Morphological features of the root system and growth behavior. American Journal of Botany 49 (3):237–45. doi:https://doi.org/10.1002/j.1537-2197.1962.tb14933.x.
- Wise, K., H. Gill, and J. Selby-Pham. 2020. Willow bark extract and the biostimulant complex Root Nectar® increase propagation efficiency in chrysanthemum and lavender cuttings. Scientia Horticulturae 263:109108. doi:https://doi.org/10.1016/j.scienta.2019.109108.
- Wright, S. J. 2008. A revegetation manual for Alaska. Palmer: Alaska Plant Materials Center, Division of Agriculture, Alaska Department of Natural Resources.
- Wu, Q., L. Pagès, and J. Wu. 2016. Relationships between root diameter, root length and root branching along lateral roots in adult, field-grown maize. Annals of Botany 117 (3):379–90. doi:https://doi.org/10.1093/aob/mcv185.
- Yao, L., M. A. Naeth, and F. P. O. Mollard. 2017. Ecological role of pyrolysis by-products in seed germination of grass species. Ecological Engineering 108:78–82. doi:https://doi.org/10.1016/j.ecoleng.2017.08.018.
- Yue, J., H. Yang, S. Yang, and J. Wang. 2020. TDIF regulates auxin accumulation and modulates auxin sensitivity to enhance both adventitious root and lateral root formation in poplar trees. Tree Physiology 40 (11):1534–47. doi:https://doi.org/10.1093/treephys/tpaa077.
