ABSTRACT
The McMurdo Dry Valleys are a cold and arid environment with low biomass relative to most ice-free environments. The ice-covered lakes in the valleys, however, provide a refuge for diverse microbial communities where liquid water persists year-round. Within these lakes, benthic microbial assemblages form ornate structures in the absence of burrowing and grazing organisms. In Lake Vanda, the microbial communities create pinnacles with features including tip, web, and ridge ornaments and brown, green, purple, and beige pigmented zones. Bacterial 16S rRNA gene composition differed between lake depths for all sampled features. Within each depth, community composition correlated with the relative distance into the pinnacle and there were also some significant differences between assemblages in certain zones. The bacterial community composition in the zones may reflect how they respond to environmental changes as the mat is buried, altering the internal light environment and affecting 16S rRNA gene assemblages across niches from the surface to the interior.
Introduction
The McMurdo Dry Valleys (MDVs) are among the coldest and driest environments on Earth (Fountain et al. Citation1999). As a result, they contain little biomass with few macroscopic organisms (Friedmann Citation1982; Matsumoto et al. Citation1992; Vincent and James Citation1996). However, the valleys contain seasonal streams and ice-covered lakes teeming with life—oases in an otherwise inhospitable environment (Spaulding et al. Citation1997; Priscu et al. Citation1998; Zhang et al. Citation2015; Horn et al. Citation2016; Jungblut et al. Citation2016; Sumner et al. Citation2016; Kwon et al. Citation2017; Mackey et al. Citation2018; Dillon et al. Citation2020a, Citation2020b; Salvatore et al. Citation2021). In particular, unusually elaborate phototropic microbial mats, dominated by filamentous cyanobacteria coat the floor of the ice-covered lakes in the MDVs to depths where sufficient light penetrates. These microbial mats therefore contribute much of the biomass in Lake Vanda as well as many of the terrestrial aquatic ecosystems in Antarctica. In some cases, the productivity of these mats exceeds that of planktonic phototrophs (Hawes et al. Citation2001; Moorhead, Schmeling, and Hawes Citation2005). The mats accumulate biomass in the form of annual growth layers. In the virtual absence of disturbance, elaborate macroscopic features emerge during multidecadal growth, including pinnacles and flat and honeycomb-shaped structures (Sumner et al. Citation2016; Mackey et al. Citation2017; Dillon et al. Citation2020a, Citation2020b). These structural features appear to host unique microbial communities (Zhang et al. Citation2015; Sumner et al. Citation2016; Dillon et al. Citation2020b). Annual laminations allow growth to be hindcast (Sutherland and Hawes Citation2009), and their similarity to ancient stromatolites (Wharton, Parker, and Simmons Citation1983) can help inform the interpretation of fossil stromatolites in the rock record (Sumner et al. Citation2016; Mackey et al. Citation2017) and as analogs of potential life on other planets.
Both microbial community composition and emergent microbial structures vary along environmental gradients within and among the MDV lakes, though many cyanobacterial taxa are shared, including genera of Oscillatoriales, such as Phormidium, Microcoleus, Tychonema, Leptolyngbya, and Pseudanabaena (Zhang et al. Citation2015; Greco et al. Citation2020). Several factors are potentially influential, though these can be difficult to isolate, because lakes and depth zones within lakes differ in multiple physicochemical parameters that often co-vary, including the availability of photosynthetically active radiation, temperature, pH, ionic composition, and the concentration of nitrogen and phosphorus (Zhang et al. Citation2015). Within a single lake, the availability of photosynthetically active radiation decreases with depth, and geochemical parameters, including dissolved oxygen, the concentration of sulfide, and conductivity, also vary (Dillon et al. Citation2020a, Citation2020b). These gradients can influence microbial communities (Zhang et al. Citation2015; Ramoneda et al. Citation2020), including at the millimeter scale (Dillon et al. Citation2020b). Similarly, photosynthetically active radiation decreases rapidly (on millimeter scales) with distance into a single mat (Vopel and Hawes Citation2006; Sumner et al. Citation2016). The microenvironments within a mat may be associated with differences in microbial community structure, including in populations of cyanobacteria (Mackey et al. Citation2015; Jungblut et al. Citation2016; Dillon et al. Citation2020a, Citation2020b).
In this study, we focus on Lake Vanda a large, endorheic (closed basin) perennially ice-covered lake in Wright Valley of the MDVs, with an approximately 3.5- to 4-m-thick perennial ice cover that transmits 15 to 20 percent of incoming solar irradiance (Hawes and Schwarz Citation2001; Castendyk et al. Citation2016). Lake Vanda contains two thermohaline convection cells separated by a distinct pycnocline (Spigel and Priscu Citation1998). In 2015, the upper convection cell (4.5°C, 900 μS/cm specific conductivity) occupied the depth range 4 to 24 m, and the lower convection cell (6.5°C, 1,555 μS/cm specific conductivity) occupied the depth range 26 to 50 m (Castendyk et al. Citation2016). Based on sediment accumulation and historical records, the upper convection cell likely formed within the last 100 years as lake level rose following a climate shift that altered the balance between the influx of meltwater and efflux via ablation and evaporation (Castendyk et al. Citation2016).
The water column is exceptionally clear (Howard-Williams et al. Citation1998; Hawes and Schwarz Citation2001), and microbial pinnacles coat the lake floor to depths of at least 50 m (Mackey et al. Citation2017). A back-of-the-envelope calculation of lake bottom area shallower than 50 m suggests that Lake Vanda contains more than 109 microbial pinnacles >1 mm tall, with some reaching tens of centimeters. The physiology of these photosynthetic mats and their temporal evolution were studied previously (Hawes et al. Citation2013; Sumner et al. Citation2016; Mackey et al. Citation2017), which revealed within-mat vertical organization evident as zones of different pigmentation (Hawes and Schwarz Citation2001).
Compositional and functional differences among the mat communities that develop in the distinct zones of the Lake Vanda water column have been demonstrated and suggest that variation in mat composition between depths can provide insights into the ecological drivers controlling community composition (Ramoneda et al. Citation2020). This initial study, however, did not resolve microbial attributes within the various layers within mats. Given that these zones are temporally related (the surface of the mat at time zero will be subsurface in several years’ time), more spatially resolved analysis of these pinnacles may provide the opportunity to examine the relationship between community composition and (1) between the depth in the lake and (2) zone in the mat. Lastly, we can test the competing hypotheses that unique microbial communities form unique pinnacle structures or that the structures are independent of community composition.
Methods
Site description
Lake Vanda occupies a closed basin in Wright Valley of the MDVs, Antarctica (77°31.60 S, 161°36.30 E). The ice cover is 3.5 to 4.0 m thick, although open water leads can form, and the ice around the lakeshore melts in summer to produce an open-water moat. The ice cover transmits 15 to 20 percent of incident photosynthetically active radiation, and the lake water is clear with a vertical extinction coefficient of 0.06 m−1 (Howard-Williams et al. Citation1998).
In 2013, scientific divers collected samples at two depths, 19 and 31 m, which correspond to absolute elevations of 76 and 64 m, respectively (Castendyk et al. Citation2016). In 2013, mats at 19 m received 32 percent of irradiance penetrating the ice cover. Mats at 31-m depth received approximately 16 percent of irradiance (Howard-Williams et al. Citation1998). The mats at 19-m depth grew in a slowly convecting cell within the lake with a temperature of ~4°C and conductivity of ~0.6 mS/cm. Mats at 31-m depth grew within a lower convecting cell at ~6°C and conductivity of 1.55 mS/cm (Castendyk et al. Citation2016). At both depths, pH was 8 to 9, and the water was oxic year-round (Castendyk et al. Citation2016).
The water column contained a planktonic community that had a low biomass, likely due to nutrient limitations (Vincent and Vincent Citation1982), whereas benthic microbial mats had high biomass (Love et al. Citation1983; Hawes and Schwarz Citation2001; Hawes et al. Citation2013; Sumner et al. Citation2016). Photosynthetic organisms, including filamentous cyanobacteria and pennate diatoms, were abundant in the mats, and moss was present, but not abundant, in mats below ~30-m depth (Kaspar et al. Citation1982; Love et al. Citation1983; Rankin et al. Citation2017).
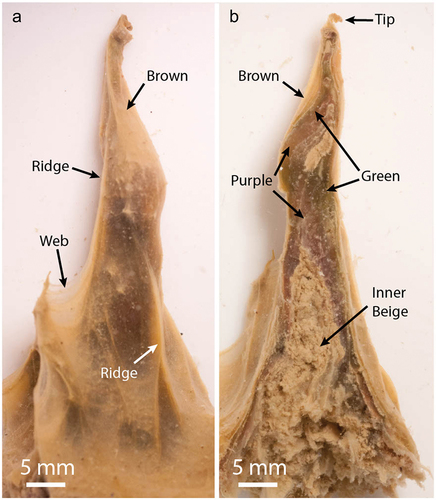
Benthic mats down to ~50-m depth formed pinnacles <1 to 300 mm tall (Sumner et al. Citation2016). The maximum height and the abundance of tall pinnacles increased with depth through the first convecting layer, and lake depth correlated with the duration of mat growth due to lake level rise (Hawes et al. Citation2013). The pinnacles had multiple features including ornaments on their surfaces, including banners of entwined filaments extending from their tips, ridges running up their sides, and webs of vertically oriented biofilm between pinnacles or between a pinnacle and surrounding flat mat (; Sumner et al. Citation2016).
The upper parts of the mats were cohesive and laminated distinctly on millimeter scales, with alternating sediment-rich and sediment-poor bands that have previously been shown to represent annual growth (Sutherland and Hawes Citation2009; Hawes et al. Citation2013; Sumner et al. Citation2016). These laminae change color with depth into the mat, with the upper two to four laminae forming an orange-brown zone, rich in carotenoids, below which laminae form zones with patchy green and purple pigmentation due to phycobilin pigmentation (Hawes and Schwarz Citation2001). Below approximately a dozen annual laminations, the mat became flocculent and lamination became difficult to distinguish, with a beige color due to a paucity of photosynthetic pigments (Hawes et al. Citation2013). Throughout we use the terms “lamina” to describe an annual growth layer and “zone” to describe a collection of laminae forming an optically coherent group. The mat’s ornaments and brown, green, and purple zones contained Cyanobacteria with active photosynthetic systems (Hawes et al. Citation2013; Sumner et al. Citation2016). Photosynthetically active wavelengths of light penetrated these mat regions, and biomass accumulated within the subsurface of the pinnacles, suggesting active photosynthesis in green- and purple-pigmented subsurface areas (Hawes et al. Citation2013; Sumner et al. Citation2016).
Pinnacle sample collection and processing
In December 2013, scientific divers collected microbial mat into alcohol-sterilized containers with a knife and spatula. The containers were sealed and transported to the surface. Sampling equipment was sterilized with alcohol or antimicrobial wipes before use.
Twenty-two pinnacles were collected for this project, sixteen from 19-m depth and six from 31-m depth. Pinnacles were dissected into subsamples in the field using aseptic sampling techniques using ethanol and flame-sterilized forceps and scalpels. Zones were selected based on previous descriptions of the macroscopic structure and pigmentations of pinnacles in Lake Vanda (Sumner et al. Citation2016). Subsamples were obtained of the outer brown-pigmented, inner green- and purple-pigmented, and nonpigmented beige microbial biomass at the center of the pinnacles. The pinnacle web, ridge, and tip features were also subsampled for sequencing analyses (, ).
Table 1. The number of samples, average number of ASVs, and Shannon diversity for each subsample type.
The dissected mat subsamples were placed in Zymo Xpedition buffer in Zymo ZR Bashing Bead tubes. Cells were disrupted via bead beating in the field. The stabilized samples were frozen on dry ice and transported frozen to University of California Davis and stored at −80°C until further analysis.
DNA extraction and 16S rRNA gene sequencing
DNA was extracted from the dissected microbial mat sections using the Zymo Soil/Fecal miniprep kit following the manufacturer’s instructions. The extracted DNA was quantified using Qubit. 16S rRNA genes were amplified via polymerase chain reaction (PCR) using the Kapa HotStart PCR kit. Concentrations of PCR mix components were as follows: 1× Kapa Hot Start Kit Buffer A, 10 μM DNTPs, 100 nM of each primer, and 1 U Kapa HotStart Taq per reaction. Cycling conditions were as follows: initial melting step 95°C for 2 minutes; then twenty-eight cycles of 95°C for 15 seconds, 52°C for 30 seconds, 72°C for 1.5 minutes; and a final extension step of 3 minutes at 72°C. The primers used were barcoded universal 16S rRNA gene primers 515 F (TGCCAGCMGCCGCGGTAA) and 806 R (GGACTACHVGGGTWTCTAAT; Caporaso et al. Citation2011). Positive PCR reactions were normalized, pooled, and sequenced at the University of California Davis Genome Center Core Facility using paired-end 250 base pair read length and paired-end Illumina MiSeq sequencing.
Data Analysis
Reads were trimmed to removed regions less than Q20 and then screened for adapter and primer contamination using Trimmomatic 0.36 (Bolger, Lohse, and Usadel Citation2014).
16S rRNA data were processed in DADA2 1.26.0 (Callahan et al. Citation2016). Based on their quality profiles, forward reads were trimmed to 160 bp and reverse reads to 225 bp and reads with Ns or more than two expected errors were removed. Error rates for forward and reverse reads were modeled using the learnErrors command and applied using the dada command to infer true sequence variants. Forward and reverse reads were merged using the mergePairs command to obtain the amplicon sequence variants (ASVs). ASVs that were longer than 254 or shorter than 252 bp were removed. The target product was 253 bp. Chimeras were identified and removed using the removeBimeraDenovo command. Taxonomy was assigned to the remaining sequences using the assignTaxonomy command and the Silva nonredundant training set (v132; Quast et al. Citation2013). Bacterial-specific primers were used; therefore, mitochondrial, chloroplast, and sequences not classified as Bacteria were removed. Samples containing fewer than 1,000 sequences were removed. The number of ASVs present in and Simpson diversity for each sample was calculated using the plot_richness command in the R package phyloseq v1.42.0 (McMurdie and Holmes Citation2013). Sequence counts were transformed to relative abundance. Individual permutational multivariate analysis of variance tests using a Bray-Curtis distance matrix were performed independently for features within each depth and the same zones between depths using the adonis function in the R vegan package v2.6.4 and implemented by the adonis.pair function (Oksanen et al. Citation2020). p Values were adjusted for multiple comparisons using the p.adjust function and Benjamini-Hochberg method (Benjamini and Hochberg Citation1995). For features that differed significantly, the simper function in the R Vegan package v2.6.4 (Oksanen et al. Citation2020) was used to identify taxa that differed between the features. Here, we report on taxa that have significantly different abundances between zones (p < .05) and contribute to 1 percent or more of the dissimilarity between the communities. The Basic Local Alignment Search Tool was used to search for its nearest relatives of taxa that are different between zones and contribute to 1 percent of the dissimilarity between communities (Altschul et al. Citation1990).
Nonmetric multidimensional scaling (NMDS) was performed using Bray-Curtis distance using the ordinate function of the phyloseq package and standard parameters (McMurdie and Holmes Citation2013). Separate NMDS analyses were performed for each of the 19- and 31-m depths using the same methodology. For each of the depth-specific NMDS ordinations, the envfit function in the R Vegan package was used to fit the variable “relative depth into pinnacle” to the NMDS ordination using standard parameters including 999 permutations. The “relative depth into pinnacle” variable identified the relative position of each feature. It was a dimensionless unit with surface samples (web, tip, rod, and brown) equal to zero, green and purple zones equal to one, and beige zone equal to two. The relative abundance of photosynthetic cyanobacteria, members of the class Cyanobacteria, in surface communities (web, tip, ridge, and brown) was compared to that in interior communities (green, pink, and beige) using a two-tailed heteroscedastic t test in Microsoft Excel.
16S rRNA sequences were deposited to the NCBI Sequence Read Archive under BioProject accession PRJNA891318.
Results
After quality control and removal of samples with less than 1,000 sequences, our data set contained eighty-nine samples (). The samples included web communities from 19 m and tip, ridge, brown, green, purple, and beige communities from 19- and 31-m depth in Lake Vanda. The median number of reads per sample was 20,968. After the removal of nonbacterial sequences, there were 3,725 unique ASVs from thirty-six phyla. The most abundant phyla included the Cyanobacteria, Proteobacteria, Chloroflexi, Bacteroidota, and Planctomycetota (). The average number of ASVs in a feature was between 113 and 260 and the average Simpson diversity was 3.1 to 4.8 (). The most abundant Cyanobacterial orders were the RD011, Pseudanabaenales, Cyanobacteriales, RD017, and Obscuribacterales ().
Figure 2. Relative abundance of the eight most abundant phyla in Lake Vanda. Samples are separated by depth, with 19-m samples on the left and 31-m samples on the right, and by feature (indicated by gray bars).
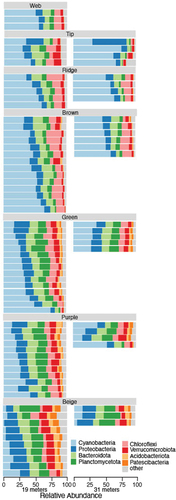
Figure 3. Relative abundance of Cyanobacteria. The six most abundant orders are indicated. Less abundant orders are grouped into the “other” category. Samples are separated by depth, with 19-m samples on the left and 31-m samples on the right, and by feature (indicated by gray bars).
The communities at 19- and 31-m depths differed for all subsample types that could be compared (). Fifteen taxa had different abundances and contributed to 1 percent or more of the dissimilarity between two or more of these communities (Supplemental Table S1). These taxa included members of the Cyanobacteria (ASVs 2, 4, 7, 11, 51, and 188), Chloroflexi (ASVs 5 and 8), Bacteroidota (ASV 10), Proteobacteria (ASVs 14, 331, and 449), Planctomycetota (ASV 38 and 44), and Zixibacteria (ASV 47; Supplemental Table S1).
Table 2. Adonis comparisons between the same subsample types at 19- and 31-m depth.
Within 19-m depth samples, the bacterial communities obtained from the purple, green, and beige pigmented zones differed from the communities in all other features. The communities from the web and ridge features and brown-pigmented biomass did not differ from one another (). Twenty-one taxa had different abundances and contributed to 1 percent or more of the dissimilarity between two or more of these communities. These taxa included members of the Cyanobacteria (ASVs 1, 2, 3, 4, 7, 11, 12, 17, 20, 22, and 51), Chloroflexi (ASVs 5 and 8), Bacteroidota (ASV 6, 10, and 27), Planctomycetota (ASVs 13 and 21), and Proteobacteria (ASVs 14, 15 and 40; Supplemental Table S2).
Table 3. Adonis comparisons between different mat subsample types within the same depth.
Within 31-m depth, the bacteria assemblages in the purple-pigmented zone did not differ from the tip or ridge feature communities, and the ridge community did not differ from the community in the brown-pigmented zone of the pinnacles (). All other comparisons were significantly different. Twenty taxa had different abundances and contributed to 1 percent or more of the dissimilarity between two or more communities, including members of the Cyanobacteria (ASVs 1, 2, 3, 4, 7, 12, 17, 20, and 188), Chloroflexi (ASVs 5 and 8), Bacteroidota (ASV 6), Planctomycetota (ASVs 19, 38, and 44), Acidobacteriota (ASV 43), Proteobacteria (ASVs 14, 331, and 449), and Zixibacteria (ASV47; Supplemental Table S3).
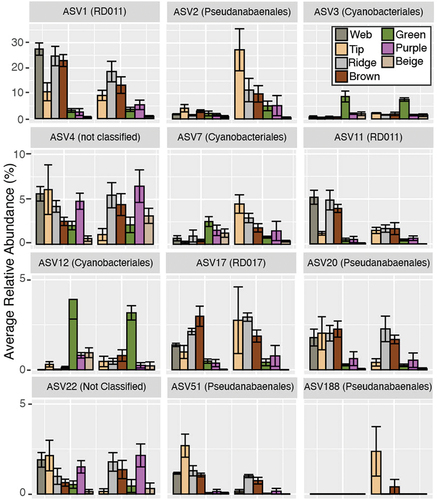
Twelve cyanobacterial taxa differed in one or more community comparisons (, Supplemental Tables S1–S3). The taxonomy and nearest relatives of each of these taxa can be found in Supplemental Table S4.
Figure 4. Average relative abundance of cyanobacterial ASVs in Lake Vanda. Only taxa that differ between two or more communities and contribute 1 percent or more toward their dissimilarity are shown. Error bars denote standard error. Note that axes differ between rows.
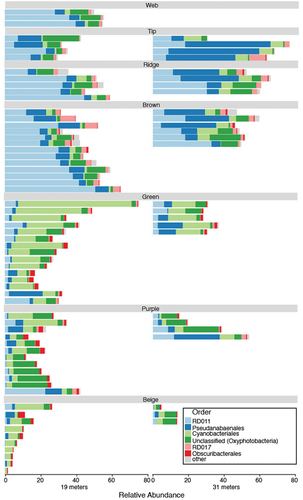
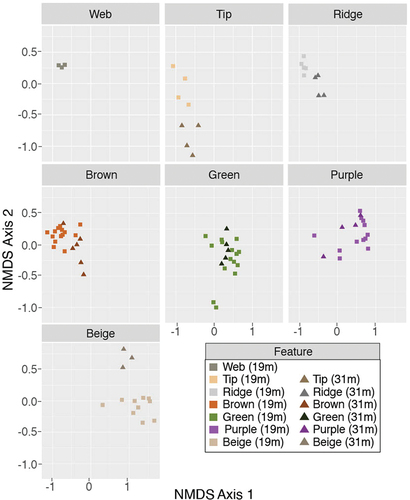
The zone within the pinnacle was significantly correlated with the NMDS ordinations of the full microbial community at 19 m (p < .01, R2 = 0.81) and 31-m depth (p < .01, R2 = 0.73) and with the photosynthetic cyanobacterial community at 19- and 31-m depths (p < .01, R2 = 0.84 and 0.86, respectively). Photosynthetic cyanobacteria (members of the Cyanobacteria based on SILVA database) were more abundant in surface communities than in the interior communities in both the 19- and 31-m depth pinnacles (p < .01; and Supplemental Figure S1).
Discussion
Pinnacle-shaped microbial mats cover the Lake Vanda in the McMurdo Wright Valley from under the lake ice to at least 50 m. Community composition varies inside the differently pigmented zones within the microbial mats and by lake depth. Irradiance, water chemistry, and community history may influence microbial community composition, but the mechanisms are not well understood yet.
Microbial communities vary with lake depth
The microbial communities that form pinnacles at 19 m are distinct from those growing at 31 m (). The pinnacles growing at 31 m receive less photosynthetically active radiation than those at 19 m. Additionally, these pinnacles have had a longer growth history because the lake depth has increased since the first observations in 1947 (Castendyk et al. Citation2016). The level of photosynthetically active radiation may influence the composition of the photosynthetic communities, which may, in turn, have cascading effects on the heterotrophic communities. Eight cyanobacterial taxa differed between the shallow and deep communities. ASV 4 and ASV 22 (Leptolyngbya ANT.L67.1) and ASV 51 (Pseudanabaenaceae) were more common in deep subsamples (; Supplemental Table S1). These three cyanobacterial groups are common in Antarctic lakes (Komárek and Elster Citation2008; Mackey et al. Citation2015; Koo et al. Citation2017), soils (Davey and Clarke Citation1991), meltwater ponds (Kleinteich et al. Citation2014), and cryoconite holes (Buda et al. Citation2020). Members of these groups inhabit many light environments. However, the distribution of these taxa suggests a potential that the taxa or genotypes inhabiting Lake Vanda are also capable of inhabiting low-light environments. Differences in bacterial community composition by layer and lake depth have also been observed in three-dimensional microbial mats in the Antarctic ice-covered Dry Valley lakes Fryxell and Joyce (Zhang et al. Citation2015; Jungblut et al. Citation2016). However, in Lake Fryxell, changes in the community composition were associated with different macroscopic morphologies of the microbial mats (Jungblut et al. Citation2016; Dillon et al. Citation2020a).
Microbial mats in the Dry Valley lakes contain microbial eukaryotes including diatoms, protist, and fungi as shown by microscopy analysis and limited number of DNA-based papers (Sutherland and Hawes Citation2009; Rojas-Jimenez et al. Citation2017; Dillon et al. Citation2020a, Citation2020b). To our knowledge, there are no 18S rRNA gene studies for the pinnacle-shaped microbial mats in Lake Vanda. However, in the ice-covered Lake Untersee, 18S rRNA gene eukaryote communities comprised microalgae, fungi, rhizaria, and other protist groups. These communities differed between pinnacle-, cone-, and prostrate-shaped microbial mats. However, cyanobacteria and other bacterial groups were more heterogeneous than eukaryotes (Greco et al. Citation2020). Similarly, study of 16S rRNA gene and 18S rRNA gene communities in microbial mats in meltwater ponds on the McMurdo Ice Shelf described a stronger habitat filtering in particular salinity for Bacteria and Archaea than microbial eukaryotes (Jackson, Hawes, and Jungblut Citation2021).
Microbial communities vary with the zone in the mat
The microbial pinnacles contain multiple distinctly pigmented zones. The upper zones of the mat are brown and overlie zones with patchy green and purple pigmentation. Deeper into the interior of the mats, it becomes flocculent, lacks lamination, and has a beige color due to a paucity of photosynthetic pigments (Sumner et al. Citation2016). Similar pigmentation has also been reported from microbial mats in the perennially ice-covered Dry Valley Lake Hoare, Antarctica (Hawes et al. Citation2016). These zones host different bacterial communities.
In Lake Vanda, the availability of photosynthetically active radiation is a key environmental factor that can influence microbial community composition. As the mats grow and as sediment is deposited, less light is available in the interior of the pinnacle (Sumner et al. Citation2016). In NMDS space, microbial community composition correlates with the relative distance into the pinnacle. The cyanobacterial communities also correlate with the relative distance into the pinnacles. The surface ornaments and brown surface zones group together and grade into the green and purple communities, which grade into the inner beige communities (). This pattern supports the hypothesis that the different pigmented mat zones represent community progression as the local light environment changes with the distance into the pinnacle. The findings agree with microscopic evaluation of cyanobacteria in Lake Vanda, where differences in morphotypes were observed in differently pigmented zones in microbial mats (Hawes et al. Citation2013). Stratification of distinct cyanobacteria and bacteria communities in three-dimensional microbial mats has also been documented from Lake Fryxell, McMurdo Taylor Valley, Antarctica, where photosynthetically active radiation and O2 were suggested to be important drivers of community richness and composition (Jungblut et al. Citation2016; Dillon et al. Citation2020a).
Higher light–adapted cyanobacteria would likely have a growth advantage in in newly developing regions of the pinnacle—in all newly forming pinnacles and on the exterior surfaces of existing pinnacles. Through time, lower light–adapted phototrophs may predominate in the zones that had been buried. Based on these data, we expect that ASV 1 (RD011) cyanobacterium may be capable of living in higher light than other cyanobacteria in the pinnacle communities because it is more abundant in the surface features of the pinnacles at 19- and 31-m depths (, ). This species may be a taxon that is one of the first to colonize newly available lake bed or the space above the pinnacle surface. However, these data are only a snapshot of the community at a single point in time. Therefore, it is not clear whether the different pigmented zones represent the temporal sequences of succession or whether differences in their community composition are merely due to the recruitment of species well suited for environmental conditions at each location within the mat, which has been shown to be an important process in structuring microbial communities in Lake Vanda (Ramoneda et al. Citation2020). Careful experimental analyses could detangle these patterns (Cole et al. Citation2014) and would lead to a greater understanding of ecology in this and other microbially dominated ecosystems and their potential response to climatic-driven environmental change.
Pinnacle surface ornaments are not associated with distinct microbial communities
Surface ornaments in pinnacles, like tips, webs, and ridges, may form due to unique microbial communities. Alternately, these ornaments may result from microbial behaviors rather than specific microbial communities. If unique communities are responsible for forming the ornaments, we would expect (1) that the ornaments contain distinct microbial communities and (2) that these communities are consistent regardless of depth. The tip communities were distinct from all other communities except the web communities at 19 m and from the brown, green, and beige communities at 31 m. ASV 2, a member of the family Pseudanabaenaceae, was more abundant in the tip communities than all other communities except the brown community at 19-m depth and the purple community at 31-m depth. Similarly, ASV 7, a member of the family Phormidiaceae, often distinguishes the tip community from other communities, especially at 31-m depth (, Supplemental Table S3). Therefore, these taxa may play an essential role in forming the tip structure. However, although tip ornaments form at both 19- and 31-m depths, the tip community differs between these depths. Additionally, no single taxon distinguished tip communities from other communities. Therefore, though ASVs 2 and 7 appear to be important components of the tip-building communities, they are not solely responsible for forming these ornaments; other community members are likely important. It is possible that a microbial behavior, such as upward motility, shared across these taxa leads to this ornament type. For example, some filamentous cyanobacteria move using gliding and twitching motility as a strategy to response to physical and chemical conditions, and it has been shown that cyanobacteria in Antarctic microbial mats can respond to changes in irradiation (Vincent et al. Citation1993; Quesada and Vincent Citation1997).
Other surface ornaments were not distinguishable by their component microbial communities. The web community was not distinguishable from any other surface community at 19-m depth. The ridges were not distinct from the brown zones at 19- or 31-m depths (). Therefore, the web and ridge features are likely not formed due to unique microbial consortia. Furthermore, the communities from the same structures but found at different depths were distinct. This may indicate that these mat ornaments are common emergent structures that form independently or quasi-independently from microbial community composition. Multiple species can act as “builders,” “tenants,” and “squatters” (Petryshyn et al. Citation2021) and create structures that are morphologically similar despite originating from and hosting different microbial taxa. Thus, the ornaments result from microbial community behavior in response to environmental or ecological conditions.
Conclusions
The community composition of phototrophic mats inhabiting Lake Vanda differed between different pigmented zones and by lake depth. We hypothesize that the availability of photosynthetically active radiation is likely a key environmental factor influencing the community composition. Ornaments on the mat surface do not appear to be formed by unique microbial communities and may be the result of microbial behavior rather than a specific microbial community.
Supplemental Material
Download ()Acknowledgments
We thank Dr. Blythe Durbin-Johnson at the University of California Davis Bioinformatics Core Facility for helpful advice regarding the statistical analyses. We thank the reviewers for their insightful comments and suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15230430.2023.2276578.
Additional information
Funding
References
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–13. doi:10.1016/s0022-2836(05)80360-2.
- Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 57: 289–300. doi:10.1111/j.2517-6161.1995.tb02031.x.
- Bolger, A. M., M. Lohse, and B. Usadel. 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–20. doi:10.1093/bioinformatics/btu170.
- Buda, J., E. Łokas, M. Pietryka, D. Richter, W. Magowski, N. S. Iakovenko, D. L., Porazinska, et al. 2020. Biotope and biocenosis of cryoconite hole ecosystems on Ecology Glacier in the maritime Antarctic. Science of the Total Environment 724: 138112. doi:10.1016/j.scitotenv.2020.138112.
- Callahan, B. J., P. J. McMurdie, M. J. Rosen, A. W. Han, A. J. A. Johnson, and S. P. Holmes. 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods 13: nmeth.3869. doi:10.1038/nmeth.3869.
- Caporaso, J. G., C. L. Lauber, W. A. Walters, D. Berg-Lyons, C. A. Lozupone, P. J. Turnbaugh, and R. Knight. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences 108: 4516–22. doi:10.1073/pnas.1000080107.
- Castendyk, D. N., M. K. Obryk, S. Z. Leidman, M. Gooseff, and I. Hawes. 2016. Lake Vanda: A sentinel for climate change in the McMurdo Sound region of Antarctica. Global and Planetary Change 144: 213–27. doi:10.1016/j.gloplacha.2016.06.007.
- Cole, J. K., J. R. Hutchison, R. S. Renslow, Y.-M. Kim, W. B. Chrisler, H. E. Engelmann, and S. R. Lindemann. 2014. Phototrophic biofilm assembly in microbial-mat-derived unicyanobacterial consortia: Model systems for the study of autotroph-heterotroph interactions. Frontiers in Microbiology 5: 109. doi:10.3389/fmicb.2014.00109.
- Davey, M. C., and K. J. Clarke. 1991. The spatial distribution of microalgae on Antarctic fellfield soils. Antarctic Science 3: 257–63. doi:10.1017/s0954102091000317.
- Dillon, M. L., I. Hawes, A. D. Jungblut, T. J. Mackey, J. A. Eisen, P. T. Doran, and D. Y. Sumner. 2020a. Energetic and environmental constraints on the community structure of benthic microbial mats in Lake Fryxell, Antarctica. FEMS Microbiology Ecology 96: fiz207. doi:10.1093/femsec/fiz207.
- Dillon, M. L., I. Hawes, A. D. Jungblut, T. J. Mackey, J. A. Eisen, P. T. Doran, and D. Y. Sumner. 2020b. Environmental control on the distribution of metabolic strategies of benthic microbial mats in Lake Fryxell, Antarctica. PLoS ONE 15: e0231053. doi:10.1371/journal.pone.0231053.
- Fountain, A. G., W. B. Lyons, M. B. Burkins, G. L. Dana, P. T. Doran, K. J. Lewis, D. M. McKnight, et al. 1999. Physical controls on the Taylor Valley ecosystem, Antarctica. BioScience 49: 961–71. doi:10.1525/bisi.1999.49.12.961.
- Friedmann, E. I. 1982. Endolithic microorganisms in the Antarctic cold desert. Science 215: 1045–53. doi:10.1126/science.215.4536.1045.
- Greco, C., D. T. Andersen, I. Hawes, A. M. C. Bowles, M. L. Yallop, G. Barker, and A. D. Jungblut. 2020. Microbial diversity of pinnacle and conical microbial mats in the perennially ice-covered Lake Untersee, East Antarctica. Frontiers in Microbiology 11: 607251. doi:10.3389/fmicb.2020.607251.
- Hawes, I., A. D. Jungblut, M. K. Obryk, and P. T. Doran. 2016. Growth dynamics of a laminated microbial mat in response to variable irradiance in an Antarctic lake. Freshwater Biology 61: 396–410. doi:10.1111/fwb.12715.
- Hawes, I., D. Moorhead, D. Sutherland, J. Schmeling, and A.-M. Schwarz. 2001. Benthic primary production in two perennially ice-covered Antarctic lakes: Patterns of biomass accumulation with a model of community metabolism. Antarctic Science 13: 18–27. doi:10.1017/s0954102001000049.
- Hawes, I., and A. J. Schwarz. 2001. Absorption and utilization of irradiance by cyanobacteria mats in two ice-covered Antarctic lakes with contrasting light climates. Journal of Phycology 37: 5–15. doi:10.1046/j.1529-8817.1999.014012005.x.
- Hawes, I., D. Y. Sumner, D. T. Andersen, A. D. Jungblut, and T. J. Mackey. 2013. Timescales of growth response of microbial mats to environmental change in an ice-covered Antarctic lake. Biology 2: 151–76. doi:10.3390/biology2010151.
- Horn, D. J. V., C. R. Wolf, D. R. Colman, X. Jiang, T. J. Kohler, D. M. McKnight, L. F. Stanish, et al. 2016. Patterns of bacterial biodiversity in the glacial meltwater streams of the McMurdo Dry Valleys, Antarctica. FEMS Microbiology Ecology 92: fiw148. doi:10.1093/femsec/fiw148.
- Howard-Williams, C., A.-M. Schwarz, I. Hawes, and J. C. Priscu. 1998. Optical properties of the McMurdo Dry Valley lakes, Antarctica. In Antarctic research series, ed. J. C. Priscu, pp. 189–203. Washington, DC: American Geophysical Union. doi:10.1029/ar072p0189.
- Jackson, E. E., I. Hawes, and A. D. Jungblut. 2021. 16S rRNA gene and 18S rRNA gene diversity in microbial mat communities in meltwater ponds on the McMurdo Ice Shelf, Antarctica. Polar Biology 44: 823–36. doi:10.1007/s00300-021-02843-2.
- Jungblut, A. D., I. Hawes, T. J. Mackey, M. Krusor, P. T. Doran, D. Y. Sumner, J. A. Eisen, et al. 2016. Microbial mat communities along an oxygen gradient in a perennially ice-covered Antarctic lake. Applied and Environmental Microbiology 82: 620–30. doi:10.1128/aem.02699-15.
- Kaspar, M., G. M. Simmons, B. C. Parker, K. G. Seaburg, R. A. Wharton, and R. I. L. Smith. 1982. Bryum Hedw. Collected from Lake Vanda, Antarctica. The Bryologist 85: 424. doi:10.2307/3242912.
- Kleinteich, J., F. Hildebrand, S. A. Wood, S. Ciŕs, R. Agha, A. Quesada, and D. R. Dietrich. 2014. Diversity of toxin and non-toxin containing cyanobacterial mats of meltwater ponds on the Antarctic Peninsula: A pyrosequencing approach. Antarctic Science 26: 521–32. doi:10.1017/s0954102014000145.
- Komárek, J., and J. Elster. 2008. Ecological background of cyanobacterial assemblages of the northern part of James Ross Island, Antarctica. Polish Polar Research 29: 17–32.
- Koo, H., N. Mojib, J. A. Hakim, I. Hawes, Y. Tanabe, D. T. Andersen, and A. K. Bej. 2017. Microbial communities and their predicted metabolic functions in growth laminae of a unique large conical mat from Lake Untersee, East Antarctica. Frontiers in Microbiology 8: 1347. doi:10.3389/fmicb.2017.01347.
- Kwon, M., M. Kim, C. Takacs‐Vesbach, J. Lee, S. G. Hong, S. J. Kim, O. Kim, and O.-S. Kim. 2017. Niche specialization of bacteria in permanently ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Environmental Microbiology 19: 2258–71. doi:10.1111/1462-2920.13721.
- Love, F. G., Jr., G. M. Simmons, B. C. Parker Jr., R. A. Wharton, and K. G. Seaburg. 1983. Modern conophyton‐like microbial mats discovered in Lake Vanda, Antarctica. Geomicrobiology Journal 3: 33–48. doi:10.1080/01490458309377782.
- Mackey, T. J., D. Y. Sumner, I. Hawes, and A. D. Jungblut. 2017. Morphological signatures of microbial activity across sediment and light microenvironments of Lake Vanda, Antarctica. Sedimentary Geology 361: 82–92. doi:10.1016/j.sedgeo.2017.09.013.
- Mackey, T. J., D. Y. Sumner, I. Hawes, A. D. Jungblut, and D. T. Andersen. 2015. Growth of modern branched columnar stromatolites in Lake Joyce, Antarctica. Geobiology 13: 373–90. doi:10.1111/gbi.12138.
- Mackey, T. J., D. Y. Sumner, I. Hawes, S. Z. Leidman, D. T. Andersen, and A. D. Jungblut. 2018. Stromatolite records of environmental change in perennially ice-covered Lake Joyce, McMurdo Dry Valleys, Antarctica. Biogeochemistry 137: 73–92. doi:10.1007/s10533-017-0402-1.
- Matsumoto, G. I., E. I. Friedmann, K. Watanuki, and R. Ocampo-Friedmann. 1992. Novel long-chain anteiso-alkanes and anteiso-alkanoic acids in Antarctic rocks colonized by living and fossil cryptoendolithic microorganisms. Journal of Chromatography. A 598: 267–76. doi:10.1016/0021-9673(92)85056-y.
- McMurdie, P. J., and S. Holmes. 2013. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8: e61217. doi:10.1371/journal.pone.0061217.
- Moorhead, D., J. Schmeling, and I. Hawes. 2005. Modelling the contribution of benthic microbial mats to net primary production in Lake Hoare, McMurdo Dry Valleys. Antarctic Science 17: 33–45. doi:10.1017/s0954102005002403.
- Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, and H. Wagner 2020: vegan: Community ecology package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan
- Petryshyn, V. A., E. N. Junkins, B. W. Stamps, J. V. Bailey, B. S. Stevenson, J. R. Spear, and F. A. Corsetti. 2021. Builders, tenants, and squatters: The origins of genetic material in modern stromatolites. Geobiology 19: 261–77. doi:10.1111/gbi.12429.
- Priscu, J. C., C. H. Fritsen, E. E. Adams, S. J. Giovannoni, H. W. Paerl, C. P. McKay, J. L. Pinckney, D. A. Gordon, B. D. Lanoil, and J. L. Pinckney. 1998. Perennial Antarctic lake ice: An oasis for life in a polar desert. Science 280: 2095–8. doi:10.1126/science.280.5372.2095.
- Quast, C., E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, F. O. Glöckner, and F. O. Glöckner. 2013. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Research 41: D590–D596. doi:10.1093/nar/gks1219.
- Quesada, A., and W. F. Vincent. 1997. Strategies of adaptation by Antarctic cyanobacteria to ultraviolet radiation. European Journal of Phycology 32: 335–42. doi:10.1080/09670269710001737269.
- Ramoneda, J., I. Hawes, A. Pascual-García, T. J. Mackey, D. Y. Sumner, and A. D. Jungblut. 2020. Importance of environmental factors over habitat connectivity in shaping bacterial communities in microbial mats and bacterioplankton in an Antarctic freshwater system. FEMS Microbiology Ecology 97. doi:10.1093/femsec/fiab044.
- Rankin, A. H., S. Pressel, J. Duckett, W. R. Rimington, I. Hawes, D. Y. Sumner, A. D. Jungblut, D. Castendyke, H. Schneider, and A. D. Jungblut. 2017. Characterisation of a deep-water moss from the perennially ice-covered Lake Vanda, Antarctica. Polar Biology 40: 2063–76. doi:10.1007/s00300-017-2127-y.
- Rojas-Jimenez, K., C. Wurzbacher, E. C. Bourne, A. Chiuchiolo, J. C. Priscu, and H.-P. Grossart. 2017. Early diverging lineages within Cryptomycota and Chytridiomycota dominate the fungal communities in ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Scientific Reports 7: 15348. doi:10.1038/s41598-017-15598-w.
- Salvatore, M. R., J. E. Barrett, S. R. Borges, S. N. Power, L. F. Stanish, E. R. Sokol, and M. N. Gooseff. 2021. Counting carbon: Quantifying biomass in the McMurdo Dry Valleys through orbital & field observations. International Journal of Remote Sensing 42: 8597–623. doi:10.1080/01431161.2021.1981559.
- Spaulding, S. A., D. M. McKnight, E. F. Stoermer, and P. T. Doran. 1997. Diatoms in sediments of perennially ice-covered Lake Hoare, and implications for interpreting lake history in the McMurdo Dry Valleys of Antarctica. Journal of Paleolimnology 17: 403–20. doi:10.1023/a:1007931329881.
- Spigel, R. H., and J. C. Priscu. 1998. Physical limnology of the McMurdo Dry Valleys lakes. In Ecosystem dynamics in a polar desert: The McMurdo Dry Valleys, Antarctica, ed. J. C. Priscu, 153–87. Washington DC: American Geophysical Union. doi:10.1029/ar072p0153.
- Sumner, D. Y., A. D. Jungblut, I. Hawes, D. T. Andersen, T. J. Mackey, and K. Wall. 2016. Growth of elaborate microbial pinnacles in Lake Vanda, Antarctica. Geobiology 14: 556–74. doi:10.1111/gbi.12188.
- Sutherland, D. L., and I. Hawes. 2009. Annual growth layers as proxies of past growth conditions for benthic microbial mats in a perennially ice‐covered Antarctic lake. FEMS Microbiology Ecology 67: 279–92. doi:10.1111/j.1574-6941.2008.00621.x.
- Vincent, W. F., M. T. Downes, R. W. Castenholz, and C. Howard-Williams. 1993. Community structure and pigment organisation of cyanobacteria-dominated microbial mats in Antarctica. European Journal of Phycology 28: 213–21. doi:10.1080/09670269300650321.
- Vincent, W. F., and M. R. James. 1996. Biodiversity in extreme aquatic environments: Lakes, ponds and streams of the Ross Sea sector, Antarctica. Biodiversity and Conservation 5: 1451–71. doi:10.1007/bf00051987.
- Vincent, W. F., and C. L. Vincent. 1982. Factors controlling phytoplankton production in Lake Vanda (77S). Canadian Journal of Fisheries and Aquatic Sciences 39: 1602–9. doi:10.1139/f82-216.
- Vopel, K., and I. Hawes. 2006. Photosynthetic performance of benthic microbial mats in Lake Hoare, Antarctica. Limnology and Oceanography 51: 1801–12. doi:10.4319/lo.2006.51.4.1801.
- Wharton, R. A., B. C. Parker, and G. M. Simmons. 1983. Distribution, species composition and morphology of algal mats in Antarctic dry valley lakes. Phycologia 22: 355–65. doi:10.2216/i0031-8884-22-4-355.1.
- Zhang, L., A. D. Jungblut, I. Hawes, D. T. Andersen, D. Y. Sumner, and T. J. Mackey. 2015. Cyanobacterial diversity in benthic mats of the McMurdo Dry Valley lakes, Antarctica. Polar Biology 38: 1097–110. doi:10.1007/s00300-015-1669-0.