 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Red snow algae seasonally color glacier and alpine snow surfaces with characteristic red blooms. These blooms significantly reduce the albedo of the snow surface resulting in increased snow ablation. The global cryosphere is sensitive to the melt effect of expansive reoccurring blooms; however, the primary dispersal path by which snow algae seasonally recolonize snow surfaces is currently unresolved. Using an experimental field approach that inhibited resurfacing populations with a physical barrier, then sampled algal surface abundance the following growing season, we assessed two pathways of algal surface colonization on the Harding Icefield, Alaska. Our results provide the first experimental depiction of active resurfacing as the primary pathway of seasonal snow surface colonization by red snow algae above the equilibrium line elevation on an Alaskan glacier. Results suggest that, at the peak of the growing season, 65 percent of surface abundance was derived from actively resurfacing cells and 35 percent from passively dispersing cells.
KEYWORDS:
Introduction
Red snow algae (snow algae)—a taxon of unicellular photosynthetic green algae (Chlorophyceae) containing the red pigment astaxanthin—seasonally reappear on alpine snowpacks and glacier surfaces around the world. Snow algae are known to significantly reduce surface albedo (Lutz et al. Citation2016; Cook et al. Citation2017), thereby increasing the rate of snowpack melt (Lutz et al. Citation2014; Ganey et al. Citation2017). Increased rates of seasonal snowpack ablation may have cascading effects on ecological and human systems (Barnett, Adam, and Lettenmaier Citation2005), ultimately contributing to ice mass loss and atmospheric warming (Hotaling et al. Citation2021; Intergovernmental Panel on Climate Change, Citation2022). Despite a growing body of research focused on snow algae genomics and morphology, the primary pathway of snow alga seasonal surface reappearance is currently unresolved. Resolving the relative contributions of active resurfacing from local cyst banks vs. reappearance due to passive dispersal is necessary for understanding environmental limitations of snow algae and so their impact on snowmelt.
Sanguina (formerly often identified as Chlamydomonas nivalis), Rosetta, Chlainomonas, and Chloromonas are the primary genera of snow algae associated with red blooms (Remias et al. Citation2016; Procházková et al. Citation2019; Hoham and Remias Citation2020; Engstrom et al. Citation2024). Currently, Sanguina, Rosetta, and Chlainomonas have not been successfully cultured. Thus, hypothesized snow alga life cycles () are largely informed by morphological observations of field samples and phylogenetic comparisons to cultured Chloromonas species. Here we distinguish two pathways of snow alga surface reappearance: active resurfacing and passive dispersal.
Figure 1. Red snow alga dispersal in a glacial environment and Exit Glacier experimental design. A1: Red snow algae overwinter on the previous summer surface as dormant cysts. A2: Cysts germinate into green flagellated cells in response to spring meltwater. Motile cells follow gradients in liquid water and visible light through the interannual snowpack to the snow surface. A3: At the snow surface cells transform into non-motile cysts and develop their signature red pigment. Red cysts clonally divide throughout the summer forming dense snow algal blooms. A4: Passive dispersal by wind, water, or birds. C1: Physical barriers to resurfacing were installed on the previous summer surface directly atop patches of visible snow algae treated with a 10 percent bleach solution. C2: The barrier and bleach prevent the upward migration of motile cells. C3: Samples at each experimental block were collected on the summer 2023 snow surface as n = 9 treatment samples located above barrier (open circles) and n = 8 proximal control samples (closed circles) located at the perimeter of a 5 m diameter circle centered at the barrier center.
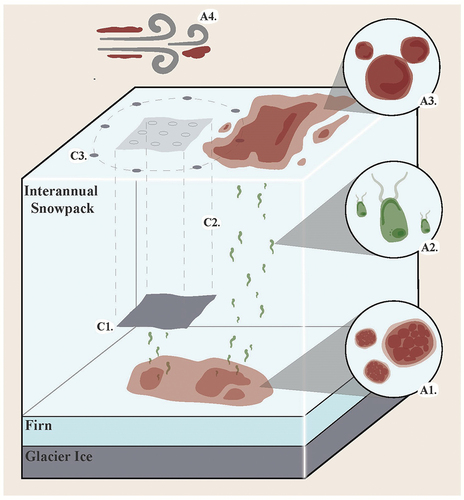
Active resurfacing from cyst banks of the previous year (Procházková et al. Citation2019) is analogous to annual plants which repopulate from local seed banks. Three life stages defined by cell morphology, mobility, and snowpack location are associated with active resurfacing. The first life stage is characterized by encysted snow algal cells that lay dormant at the previous summer surface in dense cyst banks, buried beneath the interannual snowpack (A1 in ). During spring, encysted cells germinate in response to temperature, meltwater, and penetrating light cues (Cur et al. Citation1972; Dove et al. Citation2012; Hoham and Remias Citation2020). This germination releases the second stage, motile green flagellates, that, under phototaxis, actively swim upward through melt water that is interstitial among snow grains (Remias Citation2012). Flagellates at this stage may reproduce sexually or asexually (Hoham and Duval Citation2001). As the snowpack continues to ablate, flagellates experience stress at the snow surface, such as intense solar radiation, nutrient deprivation, and surface freezing (Dial, Ganey, and Skiles Citation2018). These environmental stressors signal cells to transform into the red-colored, immobile spheroid cells (Leya et al. Citation2009) that are diagnostic of most snow algae blooms (A3 in ). The functional roles of the red-colored secondary carotenoid, astaxanthin, are thought to include protection of the photosynthetic apparatus of the chloroplasts and dissipation of absorbed radiation that increases access to meltwater and nutrients proximal to the cell (Dial, Ganey, and Skiles Citation2018). Influenced by local environmental conditions, cells in this stage may continue to proliferate clonally or cease reproductive activity as resting hypnozygotes (Hoham and Duval Citation2001; Procházková et al. Citation2019). Proliferation of snow algae likely ceases near the end of the growing season when substantial newly fallen snow covers the cells, metabolism slows, and the next year’s cyst bank establishes beneath the autumn and winter snowpack.
Hypotheses of passive dispersal maintain that the primary source of snow algae surface abundance is derived from dispersal of encysted cells that is facilitated by wind, water, and/or avian vectors (Müller, Leya, and Fuhr Citation2001; Procházková et al. Citation2019; A4 in ). Because snowpacks deeper than two meters are considered prohibitive for actively resurfacing cells, both because of light attenuation initiating phototaxis and because of the physical barriers to active swimming, snow algal blooms observed at the surface of interannual snowpacks deeper than two meters (Bischoff Citation2007) are considered evidence of passive dispersal. Atmospheric dispersal of microalgae has been well documented (Tesson et al. Citation2016) and the resting cyst cells of snow algae are known to be resistant to drought and freezing stress (Hoham and Remias Citation2020). Thus, given their small size and surface location exposed to high winds and blowing precipitation at the end of the melt season, cysts are prime candidates for passive dispersal (Procházková et al. Citation2019). Moreover, long-distance dispersal by large-scale atmospheric currents would explain the genetic similarity found between Arctic and Antarctic populations of Sanguina (Segawa et al. Citation2018).
Ultimately, passive dispersal is a necessary pathway for algal colonization of new habitats. However, the relative contributions of the passive and active dispersal pathways to algal seasonal population dynamics has not yet been estimated. These two dispersal processes are not mutually exclusive, and both may operate over the melt season, whereby snow alga populations underlying thin snowpacks actively resurface and are then passively dispersed to thicker or unseeded snowpacks where new populations then colonize. Further, cysts may be dispersed during winter storms scouring bear earth snow or ice surfaces, lifting and then depositing cysts between layers of snowfall resulting in shorter resurfacing distances. Given the range of possibilities for both pathways, it is likely that the balance of the two at any given snow algal bloom changes over a single melt season.
Here we present results of a novel, replicated field experiment that quantitatively evaluates the relative contributions by active vs. passive colonization of snow algae to the summer snow surface. Physical barriers to potential cell resurfacing were installed over snow algae cyst banks at the end of one summer season. The following year, snow alga surface abundance above the barriers (experimental plots) and at adjacent paired untreated plots (controls) were sampled and compared. These comparisons provide, for the first time, estimates of the relative contributions of active resurfacing and passive dispersal of snow algae to abundance on a glacial snowpack surface.
Methods
Study site
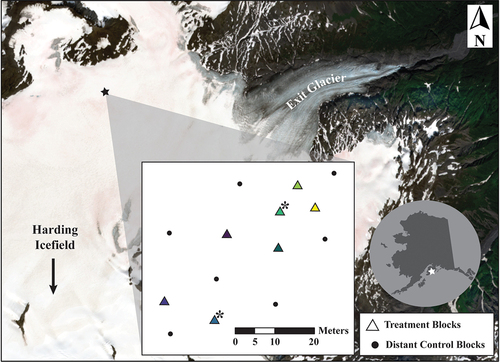
We selected a study site (60.171° N, 149.756° W) at the northeast corner of the Harding Icefield near Seward, Alaska (). The Harding Icefield, located at the southern end of the Kenai Peninsula, covers nearly 1,900 km2 of the Kenai Mountains and is contained almost entirely within the Kenai National Wildlife Refuge and Kenai Fjords National Park. We applied the normalized difference index of Ganey et al. (Citation2017) to eight years of Landsat-8 imagery between 2013–2021 to identify potential experimental sites with more than five years of perennial snow algae. The site selected was relatively flat, free of large crevasses, and located within one hectare immediately above the equilibrium line of Exit Glacier at approximately 1,100 m above sea level (asl). The locations of individual experimental blocks were determined by the appearance of visible snow alga patches (), such that each block was chosen with an obvious bloom.
Figure 2. Experimental location (black star at 60.171° N, 149.756° W) on Exit Glacier of Harding Icefield (white star on inset map of Alaska) and map of sample locations (square inset). Base map is true color Sentinel-2 scene captured on July 28, 2022. Note red hue of the snowpack at the experimental site. Triangles on inset map locate experimental blocks with colors matching block colors in . Experimental barriers to vertical algal movement were installed on September 9, 2022 at centers of experimental blocks. Asterisks on inset map indicate blocks without RECCO reflectors. Circles on inset map locate distant control blocks.
Experimental design
To prevent the resurfacing of snow algae from the previous summer surface to the current summer surface, we installed a membrane barrier of Tyvek home wrap (https://www.dupont.com/products/tyvek-homewrap.html), a strong, non-woven, compressed polyethylene material. Tyvek home wrap has a hydrostatic head potential of 250 cm, providing significant water resistance such that snow algae cells likely cannot pass through the membrane. Further inhibiting active resurfacing, Tyvek home wrap blocks ~90 percent of visible light transmittance, the critical stimulus for phototaxis. Barriers were installed on September 9, 2022. Only patchy and frequently water-saturated firn covered the study site (A in Supplemental Figure 5). Fourteen individual blooms of visible red snow algae on non-saturated snowpacks were selected for the experiment and apportioned randomly as seven experimental blocks (n = 7 blocks), each consisting of eight barrier-treatment plots (treatments) matched to eight control (proximal controls) plots for a total of n = 56 plot pairs (112 total plots). The seven additional sample sites (distant controls) served as measures of variability at a larger spatial scale.
Experimental treatments consisted of physical barriers of Tyvek installed directly on the firn surface (C1 in ; Supplemental Figure 5). These barriers likely reduced both light and meltwater from reaching cysts below as well as preventing their upward passage. To further limit cells from active resurfacing, an inhibitory 10 percent bleach solution (Ganey et al. Citation2017) was also sprayed over each treatment plot before barriers were installed. Treatment blocks included 2.4 × 2.4 m (n = 1) and 1.2 × 1.2 m (n = 6) Tyvek sheets secured to the icefield by nylon cord (3 mm) affixed to wooden dowels (5 cm diameter) inserted into holes drilled 30 cm into the ice. Each barrier was oriented so that one edge was in line with a NW direction (315°). The remaining seven blooms were designated as distant control blocks. Each distant control block contained nine plots (0.4 m x 0.4 m). On average distant controls blocks were 10 m from the nearest experimental block.
Within each experimental block, we matched the eight treatment plots (0.4 x 0.4 m) with the eight proximal control plots, the latter approximately 2 m from the barrier edge (C3 in ). In addition, a ninth, unmatched treatment plot, established above the center of the barrier, was used to test for barrier edge effects.
Block detection the following season
The equilibrium line area of Exit Glacier regularly receives 4–5 m of snow each winter (D. Kurtz, personal communication, March 23, 2023). During installation, Global Positioning System (GPS) locations of all blocks were recorded using a Garmin inReach Mini device (https://www.garmin.com/en-US/p/592606); additionally, the relative distance and direction among blocks were surveyed using a fiberglass tape measure and magnetic compass. To ensure location detection for sampling of algal abundance, we also installed passive RECCO system reflectors (https://recco.com/technology/) on the northernmost wooden dowels of five of the experimental blocks. The RECCO system is an avalanche rescue tool that uses a two-part harmonic radar system to locate objects beneath snowpacks. A certified RECCO detector operator assisted with relocation on the day of sampling. Bamboo wands were placed at the point of strongest RECCO reflector return signal, as determined by the RECCO detector operator. All surface sampling occurred relative to the bamboo wands (C in Supplemental Figure 5). After sampling was complete on July 18, 2023, we uncovered the summer 2022 surface within two of the experimental blocks, finding the snowpack depth averaged 1.2 m at the time of algal sampling. Uncovering the previous summer surface confirmed that the RECCO located sampling locations were above the installed barriers. In both cases, the RECCO reflector was within about 15 cm of the bamboo wands.
Relocation of all distant control blocks and the two experimental blocks (B3 & B5) without RECCO reflectors used the relative distance and direction survey measurements made during installation. We assumed that glacier motion moved all blocks within the study area the same distance and same direction, as reported by a previous study (Ganey et al. Citation2017) that occurred nearby. This assumption was partially confirmed as there was little variation in the relative distances of blocks relocated with RECCO reflectors.
Sampling and cell counts
We sampled snow algae abundance in the seven experimental blocks and seven distant control blocks on July 18, 2023, the general timing of historical periods of peak bloom. Within each plot, we collected a single snow surface sample by inserting a section of 10 cm diameter polyvinyl chloride (PVC) tube 2 cm into the snow surface and extracting the 157 cm3 sample with a snow knife (Engstrom et al. Citation2022). This method sampled ~5 percent of the area of each plot of the distant control blocks and the tarp-treatment plots of experimental blocks. Future studies may benefit from denser sampling methods to better capture the patchiness of the natural bloom. Snow samples were sealed in plastic bags and allowed to melt in the field. Snow density varied between samples (0.35–0.9 g/cm3). We added a 3–4 percent formalin solution to preserve the cells (Takeuchi Citation2001) in the field and then stored samples at −20°C until laboratory processing began in September.
Snow algal cells in each sample were counted under a Lecia ATC 2000 compound microscope at 40x using a hemocytometer counting chamber. Samples were inverted three times to resuspend cells and detritus; then, about 1 mL was placed onto the hemocytometer and covered with a 0.15 mm coverslip. All algal cells within the four 1 mm x 1 mm corner grids of the hemocytometer were enumerated. This process was replicated four times for each sample. Total algal cell abundance within each sample was extrapolated from the mean of the four hemocytometer counts () as
where
is the mean number of algal cells per hemocytometer volume and
is the total liquid sample volume of melted snow. Total algal cell counts were normalized to volume of snow using the PVC sampler volume (157 cm3), giving cell count per cm3 of surface snow. No effort was made to classify cells beyond identifying red pigmented spherical cells as members of the Chlamydomonadaceae family (Supplemental Figure 6).
Statistical tests
All statistical analyses were performed in R (version 4.2.3). Because both blooms and alga abundance within blooms are patchy, we compared treatment abundances to abundances in both proximal and distal controls and compared proximal and distal controls to each other, for a total of three comparisons. The first comparison between treatments and proximal controls was accomplished using two similar approaches: a linear mixed-effects model and a paired samples t-test. In the linear mixed-effects model, alga abundance was the dependent variable, treatment was the fixed factor and experimental block was the random factor accounting for variance within experimental blocks and estimating variance among blocks. However, because the among-block variance was near zero, the mixed-effects model failed to converge. Thus, we treated treatment-control pairs (n = 56) as independent pairs in a paired samples t-test. In contrast to the experimental investigation, proximal (n = 7 block means) and distal (n = 7 block means) control means were compared using an independent samples t-test. For completeness, the mean of treatment blocks (n = 7) was compared to the mean of distal control blocks (n = 7) using an independent samples t-test.
Snow algae flagellates are thought to follow melt water and light intensity cues through snowpack to the snow surface. It is unlikely that the path motile snow algae follow to the snow surface is directly vertical, given meltwater flow within snowpacks (Hoham and Duval, Citation2001). Thus, we tested the idea that snow algae blocked by the barrier might move around it, or that nearby, unrestricted populations of algae may migrate horizontally to emerge within the treatment plots. To test for evidence of horizontal movement across treatment plots, we compared abundance between treatment margins (n = 56) and centers (n = 7) using both a linear-mixed model (with block as random factor) and an independent samples t-test.
Results
Overall, the abundance of snow algae on the summer surface (, Supplemental Dataset 1) of paired control plots (± se cells per cm3 = 4,459 ± 251, n = 56) was nearly three times the abundance of treatment (barriers present) plots (1,570 ± 145, n = 63), suggesting that most red snow algae actively resurface. While the distal control plots (4,025 ± 324, n = 72) and paired experimental controls had similar means, the variability of the distal controls was greater. This variability in the distal controls captured the larger-scale patchiness of snow alga distribution ().
Figure 3. Cell count probability density by sample type. Distant Control = samples from naturally occurring populations >10 m from experimental blocks. Proximal Control = samples from naturally occurring populations in plots matched to treatment plots approximately 2 m distant. Treatment = samples from experimentally manipulated populations underlain by barriers located on previous summer surface of glacier. Distribution of algal counts of treatment samples were much lower than both sets of control samples.
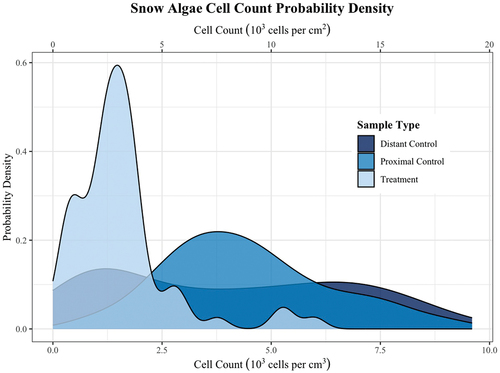
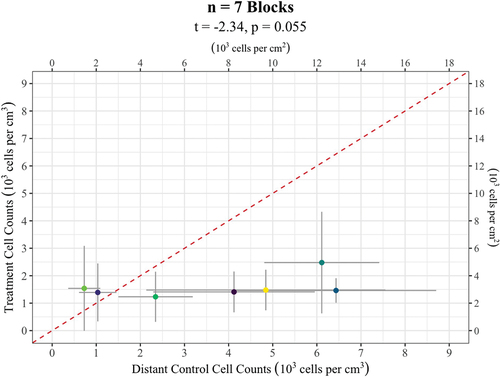
At the scale of the experimental block, over 90 percent of treatment samples were less than their paired sample of proximal control (, paired samples t-test, t = 10.6, n = 56, p < .001). We found no significant difference in mean abundance among experimental blocks (Supplementary Figure 1, linear mixed-effects model, block variance approximately zero). Moreover, the mean alga abundance within the treatment plots was relatively constant across blocks and approximately equal to the mean abundance of the lowest 10 percent of the proximal controls (1,574 ± 256, n = 5). The mean abundance of algae in distal controls was over two-and-a-half times the mean abundance in treatment plots (), a significant difference (independent samples t-test, t = 2.34, n = 7, p = .055). There was no significant difference between the mean abundances of treatment controls and distal controls (independent samples t-test, t = 0.9, n = 7, p = .4). Taken together, these observations suggest that approximately 1,500 cells per cm3 were derived from passive dispersal and double that derived from active resurfacing, suggesting that 2/3 of the algae on the surface of the glacier here represented active resurfacing and 1/3 represented passive dispersing cells.
Figure 4. Algal densities from plots above barrier (treatments) plotted against densities from matched controls. Title gives statistics of paired samples t-test matching controls with treatments. Symbol colors indicate block ID as in . Red dashed line indicates line of identity (1:1) relationship between treatment and control algal abundance, such that points below the line line indicate treatment abundances less than controls.
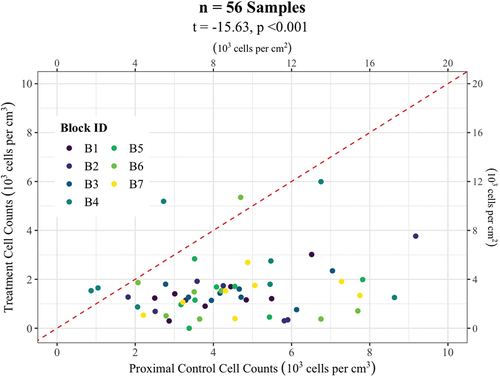
Figure 5. Algal densities of treatments (mean ± se) averaged by experimental block plotted against densities of nearest distal controls (mean ± se). Title gives statistics of independent samples t-test. Symbol colors indicate block ID as in . Red dashed line indicates line of identity (1:1) relationship between treatment and control algal abundance, such that points below line indicate treatment abundances less than controls.
We found little evidence for horizontal migration across the treatment blocks. Using the linear mixed-effects model, we found no effect (fixed factor, t = 0.37) of sample location (experimental block center vs. block margin) on algal abundance (Supplementary Figure 2). Mean snow algal abundance for treatment margin samples (1,589 ± 161, n = 56) and treatment center samples (1,420 ± 215, n = 7) were similar, showing no significant difference (independent samples t-test, t = 0.63, n = 7, p = .54).
Discussion
Here we have shown experimentally that significant differences in surface abundance of red snow algae on Alaska’s Harding Icefield exist between treatments with physical barriers and controls without, indicating that perhaps 2/3 of sampled seasonal surface abundance in 2023 derived from active resurfacing cells. This study provides direct experimental evidence supporting the hypothesis that resurfacing of snow algae can represent the predominant form of seasonal colonization in locations of persistent bloom occurrence. However, those years of very deep snowfall, may have a smaller fraction of resurfacing cells, whereas years of shallower snowfall may have a larger fraction. Precipitation in the nearby city of Seward from October 1, 2022 through April 30, 2023 was 56.4 cm (https://akclimate.org/data/data-portal/), a winter of below average precipitation.
Numerous field and remote sensing observations have shown that snow algae often persist in the same locations annually, providing circumstantial spatiotemporal support for resurfacing (Müller, Leya, and Fuhr Citation2001; Ganey et al. Citation2017; Engstrom and Quarmby Citation2023). The hypothesized motile vegetative cells of resurfacing are elusive in field samples of Sanguina and, although laboratory cultivation has been reported (Raymond, Engstrom, and Quarmby Citation2022), a complete lifecycle description including all distinct morphological stages remains largely unresolved. Flagellated life stages have been readily observed in other species of the order Chlamydomonadales (Hoham and Duval Citation2001; Remias Citation2012; Hoham, Roemer, and Mullet Citation1979). These previous observations have prompted phylogenetic comparisons to support the resurfacing hypothesis of snow algae (Procházková et al. Citation2019; Brown and Tucker Citation2020; Hoham and Remias Citation2020; Tucker and Brown Citation2022; Ezzedine et al. Citation2023; Soto, Gómez, and Huovinen Citation2023). More recently, a report of a green biciliate culture conspecific with Sanguina aurantia provides the first known morphological support for resurfacing within the genus (Raymond, Engstrom, and Quarmby Citation2022).
The ultimate origin of algal cells observed in treatment plots is uncertain. However, because the abundance of algae varied little between the center and margins of the treatment barriers, horizontal migration from nearby resurfacing populations appears unlikely. Further, sample concentrations among treatment blocks were consistently similar, as well as similar to the tenth percentile of proximal control samples. Taken together, these results suggest passive dispersal accounted for approximately 1,500 cells per cm3. Thus, near the peak of the growing season (July 18) about 35 percent of total average abundance of algae in control plots (4,214 ± 212, n = 128) derived from passive dispersal. The proportions of surface colonization derived from active resurfacing and passive dispersal likely vary over a single growing season and across climactic regions. The proportions reported here are representative of the area around the equilibrium line of a temperate glacier environment at the relative peak growing season in a year of below-average winter precipitation.
Observed treatment plot populations may derive from actively resurfacing propagules that were passively deposited during winter or dispersed horizontally within snowpack layers during the melt season. Alternatively, treatment plot populations may derive from passively deposited populations in summer from outside the sample area, originating within the local glacial landscape or from global atmospheric currents. A similar study with higher temporal sampling resolution integrated with population genetics would illuminate the primary source of passively dispersed algae. Further, a concurrent assessment of aerial sample abundance and the spatial variability of algal abundance between years would provide insight to the relative contributions of various passive dispersal vectors.
The experimental approach presented here does not describe the morphological stages of the snow algae lifecycle, nor offer conclusions about specific species. However, Fiołka et al. (Citation2020) collected samples two kilometers from the study site and found that 80 percent of the sampled algae were cysts of Sanguina nivaloides/aurantia, 17 percent were star morphotypes of Rosetta spp. (Engstrom et al. Citation2024), formerly often identified as Chloromonas spp., and the remaining 3 percent were hypnozygote cells of Chloromonas nivalis. While species-specific life cycle conclusions are beyond the scope of this study, we speculate that Sanguina spp. and Rosetta spp. most likely made up the majority of snow alga cells at the experimental site. A study of dispersal that considers variation in cell morphotype between treatment conditions could provide further insight into the snow algae life cycle.
If resurfacing is a primary mode of seasonal colonization, snow alga distribution may be limited by interannual snowpack characteristics. Deep snowpacks attenuate both light and melt water germination cues and present a physical barrier to active resurfacing. However, there is little consensus on the limiting threshold depth of snowpacks (Hoham Citation1975; Bischoff Citation2007; Müller, Leya, and Fuhr Citation2001; Curl, Hardy, and Ellermeier Citation1972). It is likely that other snowpack characteristics (snow grain size, moisture content, horizontal ice layers, density, and percolation rates) affect the resurfacing process directly, such that snowpack depth is a proxy for these characteristics. Therefore, the limiting threshold depth of snowpack likely varies among regions based on climate and latitude. At the time of sampling the remaining firn at the study site averaged 1.2 m depth, significantly deeper than previously hypothesized resurfacing maximums (but see Box 1 in Hotaling et al. (Citation2021) and supplementary Figures 1B and 2 in Ganey et al. (Citation2017) suggesting approximately 2 m). Further research is needed to fully understand the mechanistic effects of snowpack characteristics on active resurfacing.
Bass Becking’s hypothesis of cosmopolitan microbial distribution, “Everything is everywhere but the environment selects,” prosaically defines a general maximum for microbial dispersion (De Wit and Bouvier Citation2006). Indeed, biogeographic-genomic studies have revealed the presence of cosmopolitan snow alga species (Lutz et al. Citation2016; Segawa et al. Citation2018). However, the environmental limitations of snow algae are not yet well defined, leaving local environmental selection of snow algae largely unresolved. Previous studies have shown that algal blooms on Alaskan glacier snow surfaces are often distributed near mid-elevations and are conspicuously absent at higher elevations (Takeuchi et al. Citation2006; Takeuchi Citation2013). The characteristic saturated and ablating snowpack near the glacier equilibrium line likely facilitates cell resurfacing, promoting recolonization of glacier surfaces there. Remote sensing studies of North American snow alga populations have indicated that snow algae are less likely to be present adjacent to marine coasts, coinciding with areas of higher annual precipitation (Takeuchi et al. Citation2006; Takeuchi Citation2013; Engstrom and Quarmby Citation2023). While passive dispersal may allow for the broad geographic distribution of snow algae, local interannual snowpack dynamics could inhibit propagules from developing into persistent perennial blooms.
Supplemental Material
Download Zip (3.1 MB)Acknowledgments
We would like to acknowledge the contributions of Dany Davis, Malcolm Herstand, and Joey Smith who assisted in field work. Alaska Mountain Rescue Group who loaned the use of their RECCO detector. Deborah Kurtz who provided valuable field site knowledge. Christina Kriedeman and the National Park Service which permitted the research. Sylvia Taylor, Russell Wong, and Josh Clark who provided valuable input on early drafts of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15230430.2024.2370905.
Additional information
Funding
References
- Barnett, T. P., J. C. Adam, and D. P. Lettenmaier. 2005. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438 (7066):303–10. doi:10.1038/nature04141.
- Bischoff, Y. 2007. Diversité et mobilité des algues de neige dans les Alpes suisses. Geneva, Switzerland: Université de Genève. doi:10.13097/archive-ouverte/unige:516.
- Brown, S. P., and A. E. Tucker. 2020. Distribution and biogeography of Sanguina snow algae: Fine‐scale sequence analyses reveal previously unknown population structure. Ecology and Evolution 10 (20):11352–61. doi:10.1002/ece3.6772.
- Cook, J. M., A. J. Hodson, A. S. Gardner, M. Flanner, A. J. Tedstone, C. Williamson, T. D. L. Irvine-Fynn, J. Nilsson, R. Bryant, and M. Tranter. 2017. Quantifying bioalbedo: A new physically based model and discussion of empirical methods for characterising biological influence on ice and snow albedo. The Cryosphere 11 (6):2611–32. doi:10.5194/tc-11-2611-2017.
- Curl, H., Jr., J. T. Hardy, and R. Ellermeier. 1972. Spectral absorption of solar radiation in alpine snowfields. Ecology 53, no. 6: 1189–94. doi:10.2307/1935433.
- De Wit, R., and T. Bouvier. 2006. ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environmental Microbiology 8 (4):755–8. doi:10.1111/j.1462-2920.2006.01017.x.
- Dial, R. J., G. Q. Ganey, and S. M. Skiles. 2018. What color should glacier algae be? An ecological role for red carbon in the cryosphere. FEMS Microbiology Ecology 94 (3):fiy007. doi:10.1093/femsec/fiy007.
- Dove, A., J. Heldmann, C. McKay, and O. B. Toon. 2012. Physics of a thick seasonal snowpack with possible implications for snow algae. Arctic, Antarctic, and Alpine Research 44 (1):36–49. doi:10.1657/1938-4246-44.1.36.
- Engstrom, C. B., and L. M. Quarmby. 2023. Satellite mapping of red snow on North American glaciers. Science Advances 9 (47):eadi3268. doi:10.1126/sciadv.adi3268.
- Engstrom, C. B., B. B. Raymond, J. Albeitshawish, A. Bogdanovic, and L. M. Quarmby. 2024. Rosetta gen. nov. (Chlorophyta): Resolving the identity of red snow algal rosettes. Journal of Phycology 60 (2):275–98. doi:10.1111/jpy.13438.
- Engstrom, C. B., S. N. Williamson, J. A. Gamon, and L. M. Quarmby. 2022. Seasonal development and radiative forcing of red snow algal blooms on two glaciers in British Columbia, Canada, summer 2020. Remote Sensing of Environment 280:113164. doi:10.1016/j.rse.2022.113164.
- Ezzedine, J. A., C. Uwizeye, G. Si Larbi, G. Villain, M. Louwagie, M. Schilling, P. Hagenmuller, et al. 2023. Adaptive traits of cysts of the snow alga Sanguina nivaloides unveiled by 3D subcellular imaging. Nature Communications 14 (1). doi:10.1038/s41467-023-43030-7.
- Fiołka, M. J., N. Takeuchi, W. Sofińska-Chmiel, S. Mieszawska, and I. Treska. 2020. Morphological and physicochemical diversity of snow algae from Alaska. Scientific Reports 10 (1). doi:10.1038/s41598-020-76215-x.
- Ganey, G. Q., M. G. Loso, A. B. Burgess, and R. J. Dial. 2017. The role of microbes in snowmelt and radiative forcing on an Alaskan icefield. Nature Geoscience 10 (10):754–9. doi:10.1038/ngeo3027.
- Hoham, R. W. 1975. The life history and ecology of the snow alga chloromonas pichinchae (Chlorophyta, Volvocales). Phycologia 14 (4):213–226. doi:10.2216/i0031-8884-14-4-213.1.
- Hoham, R. W., and B. Duval. 2001. Microbial ecology of snow and freshwater ice with emphasis on snow algae. In Snow Ecology. An Interdisciplinary Examination of Snow-Covered Ecosystems, ed. H. G. Jones, J. W. Pomeroy, D. A. Walker, and R. W. Hoham, 168–228. Cambridge: Cambridge University Press.
- Hoham, R. W., and D. Remias. 2020. Snow and glacial algae: A review. Journal of Phycology 56 (2):264–82. doi:10.1111/jpy.12952.
- Hoham, R. W., S. C. Roemer, and J. E. Mullet. 1979. The life history and ecology of the snow alga Chloromonas brevispina comb. Nov. (Chlorophyta, Volvocales). Phycologia 18 (1):55–70. doi:10.2216/i0031-8884-18-1-55.1.
- Hotaling, S., S. Lutz, R. J. Dial, A. M. Anesio, L. G. Benning, A. G. Fountain, J. L. Kelley, et al. 2021. Biological albedo reduction on ice sheets, glaciers, and snowfields. Earth-Science Reviews 220:103728. doi:10.1016/j.earscirev.2021.103728.
- Intergovernmental Panel on Climate Change (IPCC). (Ed.). 2022. High mountain areas. In The Ocean and Cryosphere in a Changing Climate: Special Report of the Intergovernmental Panel on Climate Change, 131–202. Cambridge: Cambridge University Press. doi:10.1017/9781009157964.004.
- Leya, T., A. Rahn, C. Lütz, and D. Remias. 2009. Response of Arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiology Ecology 67 (3):432–43. doi:10.1111/j.1574-6941.2008.00641.x.
- Lutz, S., A. M. Anesio, S. E. Jorge Villar, and L. G. Benning. 2014. Variations of algal communities cause darkening of a Greenland glacier. FEMS Microbiology Ecology 89 (2):402–14. doi:10.1111/1574-6941.12351.
- Lutz, S., A. M. Anesio, R. Raiswell, A. Edwards, R. J. Newton, F. Gill, and L. G. Benning. 2016. The biogeography of red snow microbiomes and their role in melting Arctic glaciers. Nature Communications 7 (1). doi:10.1038/ncomms11968.
- Müller, T., T. Leya, and G. Fuhr. 2001. Persistent snow algal fields in Spitsbergen: Field observations and a hypothesis about the annual cell circulation. Arctic, Antarctic, and Alpine Research 33 (1):42–51. doi:10.1080/15230430.2001.12003403.
- Procházková, L., T. Leya, H. Křížková, and L. Nedbalová. 2019. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): The taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow. FEMS Microbiology Ecology 95 (6):fiz064. doi:10.1093/femsec/fiz064.
- Raymond, B. B., C. B. Engstrom, and L. M. Quarmby. 2022. The underlying green biciliate morphology of the orange snow alga Sanguina aurantia. Current Biology 32 (2): R68–R69. doi:10.1016/j.cub.2021.12.005.
- Remias, D. 2012. Cell structure and physiology of alpine snow and ice algae. In Plants in Alpine Regions: Cell physiology of adaption and survival strategies, ed. C. Lütz, 175–85. Vienna: Springer. doi:10.1007/978-3-7091-0136-0_13.
- Remias, D., M. Pichrtová, M. Pangratz, C. Lütz, and A. Holzinger. 2016. Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow. FEMS Microbiology Ecology 92 (4):fiw030. doi:10.1093/femsec/fiw030.
- Segawa, T., R. Matsuzaki, N. Takeuchi, A. Akiyoshi, F. Navarro, S. Sugiyama, T. Yonezawa, and H. Mori. 2018. Bipolar dispersal of red-snow algae. Nature Communications 9 (1). doi:10.1038/s41467-018-05521-w.
- Soto, D. F., I. Gómez, and P. Huovinen. 2023. Antarctic snow algae: Unraveling the processes underlying microbial community assembly during blooms formation. Microbiome 11 (1):200. doi:10.1186/s40168-023-01643-6.
- Takeuchi, N. 2001. The altitudinal distribution of snow algae on an Alaska glacier (Gulkana Glacier in the Alaska Range). Hydrological Processes 15 (18):3447–59. doi:10.1002/hyp.1040.
- Takeuchi, N. 2013. Seasonal and altitudinal variations in snow algal communities on an Alaskan glacier (Gulkana glacier in the Alaska range). Environmental Research Letters 8, no. 3: 035002. doi:10.1088/1748-9326/8/3/035002.
- Takeuchi, N., R. Dial, S. Kohshima, T. Segawa, and J. Uetake. 2006. Spatial distribution and abundance of red snow algae on the Harding Icefield, Alaska derived from a satellite image. Geophysical Research Letters 33 (21). doi:10.1029/2006GL027819.
- Tesson, S. V. M., C. A. Skjøth, T. Šantl-Temkiv, and J. Löndahl. 2016. Airborne microalgae: Insights, opportunities, and challenges. Applied and Environmental Microbiology 82 (7):1978–91. doi:10.1128/AEM.03333-15.
- Tucker, A. E., and S. P. Brown. 2022. Sampling a gradient of red snow algae bloom density reveals novel connections between microbial communities and environmental features. Scientific Reports 12 (1). doi:10.1038/s41598-022-13914-7.
