Abstract
Cellular uptake of clinically important deoxynucleoside analogs is mediated by nucleoside transporters including the human equilibrative nucleoside transporter 1 (hENT1) and the concentrative nucleoside transporter-1 (hCNT1). These transporters are responsible for influx of cytarabine and reduced hENT1 expression is a major resistance mechanism in acute myeloid leukemia. We determined hENT1 and hCNT1 protein expression by immunocytochemistry in 50 diagnostic pediatric acute myeloid leukemia patient samples. All samples expressed hENT1 [9/43 (21%) low; 26/43 (60%) medium and 8/43 (19%) high] and hCNT1 [2/42 (5%) low; 35/42 (83%) medium and 5/42 (12%) high] at the cell membrane and cytoplasm. Statistical analysis showed a non-significant relationship between survival and transporter expression and in vitro drug sensitivity. In conclusion, the nucleoside transporters hENT1 and hCNT1 are broadly expressed in pediatric acute myeloid leukemia at diagnosis.
1. Introduction
The human equilibrative nucleoside transporter 1 (hENT1; or SLC29A1) and concentrative nucleoside transporter-1 (hCNT1; SLC28A) are transmembrane proteins involved in nucleoside homeostasis and cellular uptake of deoxynucleoside analogs (NAs) by facilitated diffusion or concentrative uptake.[Citation1–4] In leukemic cells, hENT1 is responsible for the uptake of around eighty-percent of cytarabine (Ara-C), a cornerstone NA in the treatment of acute myeloid leukemia (AML).[Citation1,Citation5–7] In contrast, gemcitabine, which is used extensively for the treatment of solid tumors, is transported into the cell by both hCNT1 and hENT1.[Citation4,Citation8,Citation9]
Nucleoside transporters play a role in NA cytotoxicity and chemoresistance. Recent Cox regression analysis of AML patients (n = 28) showed that patients with high or baseline hENT1 expression prior to treatment, had a 50% longer median OS (p = 0.012) suggesting that hENT1 expression profiling might be of clinical importance.[Citation10] Other studies show inconclusive results, for example, a survival study reported that hENT1 expression did not significantly contribute to Ara-C resistance in acute lymphoblastic leukemia (ALL) patients older than 15 years of age and analysis of 79 B-cell precursor ALL cell lines found no correlation between hENT1 gene expression and Ara-C resistance. Unexpectedly, higher hENT1 expression was seen in cell lines with Ara-C resistance, when compared with cell lines with low expression.[Citation11,Citation12] Apparently other parameters also play a role in ara-C resistance.
However, hENT1 mRNA levels in pediatric and adult AML samples are related to resistance and outcome, suggesting that low transporter expression is a relevant mechanism of Ara-C resistance.[Citation13–15] Micro-array studies performed on Ara-C resistant human ALL cells demonstrated that a decrease in hENT1 expression was linked to lower Ara-C activity and decreased intracellular drug metabolism.[Citation16] Reconstitution of the hENT1 gene by transfection reversed Ara-C resistance 600-fold in human lymphoid H9 cells.[Citation17] It has been shown that indirect modulation of hENT1 from the cell membrane of leukemic cells resulted in a significant decrease in nucleoside transporter activity and increased Ara-C resistance in AML cells in vitro.[Citation18] Reversal of resistance due to decreased transporter activity has been attempted by bypassing the transporter, e.g., by lipophilic pro-drugs.[Citation19]
In addition, elevated hENT1 mRNA, which has been reported previously in infants with MLL gene-rearranged ALL (ALL-MLL+) explained the remarkable Ara-C sensitivity in this patient population.[Citation20,Citation21] It is plausible that elevated levels of transporter lead to increased Ara-C influx across the cellular membrane, resulting in increased intracellular drug accumulation and enhanced cytotoxicity.[Citation22] Furthermore, in pediatric ALL-MLL + indirect modulation of hENT1 expression affects significantly Ara-C cytotoxicity in pediatric AML in vitro.[Citation21] Interestingly, certain genetic variants of hENT1, which may magnify genetic expression, are also associated with treatment outcome in AML.[Citation2,Citation23,Citation24]
The aim of our present study was to relate the immunohistochemical detection of protein expression of hENT1 and the pyrimidine-preferring hCNT1 transporter, and ex vivo sensitivity to nucleoside analogs.
2. Materials and methods
2.1. Patient material
Bone marrow or peripheral blood samples were collected from 50 untreated children diagnosed with de novo AML. The patient population consisted of 32 boys and 18 girls with a median age of 10.7 years (range 0.1–16.8 years). The median white blood cell count (WBC) was 83.8 (range 2.1–524 × 106/ml) and all FAB-types were represented (2 FAB-M0; 5 FAB-M1; 8 FAB-M2, 4 FAB-M3, 18 FAB-M4, 10 FAB-M5, 1 RAEB and 2 unknown). A summary of treatment protocols for these patients (DCOG AML 87, 97, all including ara-C) is given elsewhere.[Citation15]
2.2. Cell isolation and immunocytochemistry staining
Mononuclear cells were separated by density gradient centrifugation (Lymphoprep, density 1.077 g/ml; Nycomed Pharma, Oslo, Norway) and where necessary the percentage of malignant cells was enriched as described previously.[Citation25] All samples contained >80% blasts as determined morphologically by May-Grünwald-Giemsa staining. Immunocytochemical staining was performed on (cryo-preserved) cytospins standard alkaline phosphatase/anti-alkaline phosphatase (APAAP) method. Cells were fixed with acetone (10 minutes at room temperature). Slides were washed with Phosphate-buffered saline (PBS) twice, for 5 minutes) and incubated overnight with the primary antibodies, rabbit-anti human hENT1 and hCNT1 diluted 1:100 and 1:150, respectively, in PBS with 1% bovine serum albumin (BSA) and 0.1% Na-azide as described elsewhere.[Citation21,Citation26] The following day, slides were washed (2 × 5 minutes in PBS) and incubated with the secondary antibody, biotinylated swine-anti-rabbit (Dako, Glostrup, Denmark) diluted 1:300 in PBS with 1% BSA and 0.1% Na-azide for one hour. After the wash steps, slides were incubated with the alkaline-phosphatase conjugated streptavidine (1:100 in PBS with 1% BSA and 0.1% Na-azide) for 30 minutes (Dako, Glostrup, Denmark). Visualization of AP was performed by incubation in New Fuchsin/naphtol ASBI phosphate solution supplemented with levamisole. Cells were counterstained with using Mayer’s Hematoxylin Solution (Merck, Darmstadt, Germany) and embedded in Aquamount. hENT1 and hCNT1 protein expression was evaluated by two independent investigators by scoring the intensity of the staining as either low, medium or high. When compared to positive controls, “low” or weak staining was defined as less than one third of the intensity (<0.3) and “high” staining was defined as the upper fifth of the intensity (>0.8) and medium staining was in between these two intensities.[Citation26]
2.3. MTT assay
For assessment of drug sensitivity, the MTT colorimetric assay was performed on cells suspended in 96-well round bottom plates at a concentration of 2 × 106 cells/ml in RPMI-1640 cell culture medium containing 20% fetal calf serum (FCS), 200 µg/ml of gentamycin, 2 mM glutamine, and 0.5 ml ITS (insulin 5 µg/ml, transferrin 5 µg/ml, sodium selenite 5 ng/ml.[Citation27] Treatment wells contained 80 µl of cell suspension and 20 µl of the drugs of interest at different concentrations (refer to supplementary table A.4 for concentrations). Control wells contained 20 µl of RPMI-1640 medium and 80 µl of cell suspension, while blank controls had 100 µl of RPMI-1640 medium. MTT was performed in duplicates and plates were incubated at 37 °C, in a humidified 5% CO2 incubator for 4 days. At the end of the incubation period, 10 µl of MTT dye (5 mg/ml) was added to each well and placed in the 37 °C incubator for an additional 6 hours, followed by the addition of 100 µl isopropanol. After 5 minutes, the optical density (OD) was measured at 562 nm. For the analysis, the average OD of the blank wells is subtracted from the average OD of the control wells or the treatment wells. The lethal dose 50 (LC50) was then determined by calculating cell survival with the following equation: mean OD treatment well (minus blank)/mean OD control well (minus blank) x100, as described elsewhere.[Citation27]
2.4. mRNA extraction
Total mRNA from these primary pediatric AML cells was extracted previously[Citation15] using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions and reverse transcribed to cDNA using random hexamers as described elsewhere.[Citation15] For measurement of hENT1 and hCNT1 mRNA expression probes and primers have been described earlier as well.[Citation15]
2.5. Statistical analysis
Statistical analysis was done using SPSS Version 24.0 (IBM Corp., Armonk, NY, US) software. Overall Survival (OS) analysis was evaluated by constructing Kaplan-Meier curves. For comparisons between means we used Student’s T test and Spearman’s Rho for non-parametric correlations (hENT protein level and outcome). In order to evaluate any significant differences between categorical sets of data (FAB types and immunohistochemistry intensity scores) we used Pearson Chi-square test.
3. Results
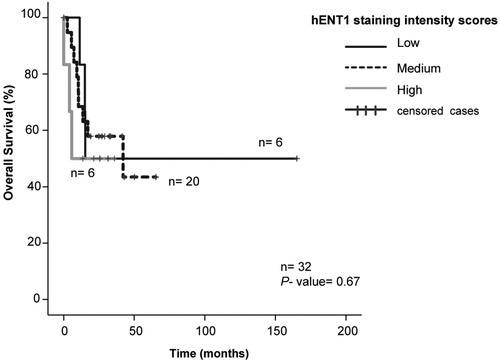
HL60 consistently stained positive for both hENT1 and hCNT1 in the cytoplasm (, and ). Negative controls were performed by omitting the first antibody and did not have a staining (). Seven samples were non-evaluable due to poor morphology and one sample was lost during the procedure. hENT1 and hCNT1 were localized at the membrane and in the cytoplasm in all AML patient samples (). There was a relatively uniform staining within one patient, while there was no significant difference between samples obtained from peripheral blood or bone marrow. hENT1 staining intensity was low in 9/43 (21%) samples, medium in 26/43 (60%) samples and high in 8/43 (19%) samples, and appeared to be slightly granular. hCNT1 staining intensity was low in 2/42 (5%) samples, medium in 35/42 (83%) samples and high in 5/42 (12%) samples ( and ). In 8/42 (19%) AML patient samples hCNT1 was located predominantly at the cellular membrane. hENT1 and hCNT1 expression were not associated with age or WBC at diagnosis. Both hENT1 and hCNT1 expression appeared to be higher in (myelo) monocytic (FAB M4/5) AML blasts compared to poorly differentiated/myeloblastic (FAB M0-3) AML blasts () but there was no significant difference (Pearson Chi-square= 10.8, P = 0.37). There was no correlation between mRNA expression and immunocytochemistry staining intensity scoring (Spearman’s rho= 0.19, two-tailed). Additionally, hENT1 mRNA[Citation15] and corresponding immunohistochemistry intensities, were significantly different from each other (two tailed Student T-test, P = 0.044) (). There was no significant correlation with cytogenetic markers such as chromosomal rearrangements.
Figure 1. Immunocytochemistry for hENT1 and hCNT1 proteins, as scored by two independent observers in several representative patient samples. Negative control in HL-60 cells was done by omitting one antibody (1 A). Positive controls (1B, 1C) are compared with AML samples of different color intensity (1 D, E, F, G). Low staining was defined as less than one third of the intensity (<0.3) and high staining was defined as the upper fifth of the intensity (>0.8) and medium staining was in between these two intensities (- +).
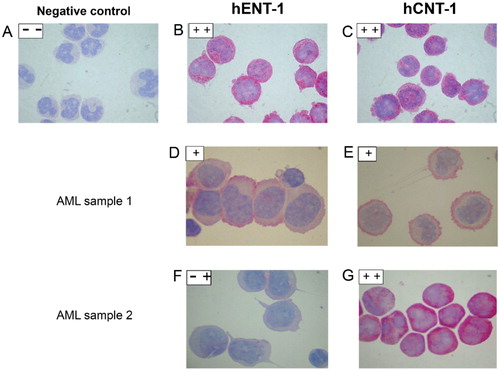
Figure 2. A: Frequencies (count) of samples in low (- -), medium (- +) and high (+ +) staining intensity for hENT1 and (2B) hCNT1. 2C: hENT1 mRNA levels of samples (from ref[Citation15]) allocated to score groups low, medium and high.
![Figure 2. A: Frequencies (count) of samples in low (- -), medium (- +) and high (+ +) staining intensity for hENT1 and (2B) hCNT1. 2C: hENT1 mRNA levels of samples (from ref[Citation15]) allocated to score groups low, medium and high.](/cms/asset/309f03f5-acc9-4bb0-aa01-2323823268e6/lncn_a_1746803_f0002_b.jpg)
Table 1. hENT1 and hCNT1 staining in different AML FAB types.
Table 2. Frequencies of hCNT and hENT samples and staining intensities.
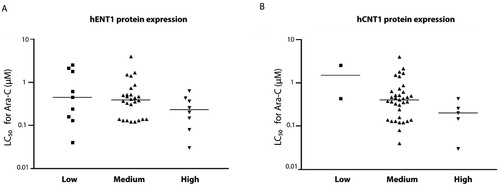
The sensitivity of primary AML samples to Ara-C in the high hENT1 and hCNT protein expression groups tended to be higher (lower LC50) than in the low hENT and hCNT group, but this was not significant (). We did not observe a difference for the other deoxynucleoside analogs cladribine, gemcitabine, fludarabine and decitabine. The immunocytochemistry staining intensity did not correlate with OS (Spearman’s Rho, P = 0.67) () and survival analysis for hCNT1 was difficult to determine due to the low number of samples allocated to low and high staining intensity scores (data not shown).
4. Discussion
The intracellular uptake of antimetabolites such as ara-C, is transporter dependent. Both hENT1 and hCNT1 are essential for ara-C uptake.[Citation21] In this study, we find that hENT1 and the pyrimidine-preferring hCNT1 transporters are broadly expressed in the blasts of pediatric AML patients. Since the majority of patients scored medium levels of expression, a potential relation with in vitro sensitivity to deoxynucleoside analogs such as Ara-C was difficult to establish in this study. In addition, it is important to note that the effective concentrations of drugs used in the MTT assay were already so high that Ara-C would be taken up by diffusion, bypassing the transporters. Furthermore, hENT1 and hCNT1 expression are regulated at the posttranslational level, which may explain the lack of correlation between immunocytochemistry protein detection and mRNA levels.[Citation2,Citation13,Citation18] Furthermore, this lack of correlation between hENT1 and hCNT1 expression with OS of these patients, may be related to the multidrug regimens which are commonly used to treat these patients.[Citation28]
The cells’ functional capacity is characterized by the enzyme activity within its cellular pathways. Proteomic profiling, (i.e., through immunohistochemistry) could be a useful tool to get a glimpse into the cells functional state. Further ex vivo cytotoxicity testing (i.e., MTT) can reveal the chemoresistance state of primary AML blasts and can therefore redirect and personalize treatment. In future studies, functional measurements in conjunction with immunocytochemistry might elucidate the precise role of hENT1 and hCNT1 in AML. Recently, a collaborative EORTC-GIMMEA trial has reported increased survival and a positive response rate with high-dose Ara-C in very high-risk setting patients, including AML-FLT3-ITD + cases, which have notoriously high levels of hENT1 expression levels.[Citation21,Citation29] This supports the notion that pretreatment analysis of patient genetic profile might be a useful tool to classify treatment options for patients.
5. Conclusions
The nucleoside transporters hENT1 and hCNT1, which are crucial for the transport of clinically important deoxynucleoside analogs (e.g., cytarabine), can be visualized by immunocytochemistry showing that they are broadly expressed in almost all AML patients.
References
- Clarke, M. L.; Mackey, J. R.; Baldwin, S. A.; Young, J. D.; Cass, C. E. The Role of Membrane Transporters in Cellular Resistance to Anticancer Nucleoside Drugs. Cancer Treat. Res. 2002, 112, 27–47.
- Cao, H. X.; Miao, C. F.; Yan, L.; Tang, P.; Zhang, L. R.; Sun, L. Polymorphisms at microRNA Binding Sites of Ara-C and Anthracyclines-Metabolic Pathway Genes Are Associated with Outcome of Acute Myeloid Leukemia Patients. J. Transl. Med. 2017, 15, 235. DOI: 10.1186/s12967-017-1339-9.
- Hummel-Eisenbeiss, J.; Hascher, A.; Hals, P. A.; Sandvold, M. L.; Muller-Tidow, C.; Lyko, F.; et al. The Role of Human Equilibrative Nucleoside Transporter 1 on the Cellular Transport of the DNA Methyltransferase Inhibitors 5-Azacytidine and CP-4200 in Human Leukemia Cells. Mol. Pharmacol. 2013, 84, 438–450. DOI: 10.1124/mol.113.086801.
- Pastor-Anglada, M.; Perez-Torras, S. Emerging Roles of Nucleoside Transporters. Front. Pharmacol. 2018, 9, 606. DOI: 10.3389/fphar.2018.00606.
- Peters, G. J.; van der Wilt, C. L.; van Moorsel, C. J.; Kroep, J. R.; Bergman, A. M.; Ackland, S. P. Basis for Effective Combination Cancer Chemotherapy with Antimetabolites. Pharmacol. Ther. 2000, 87, 227–253. DOI: 10.1016/S0163-7258(00)00086-3.
- Tsukimoto, I.; Tawa, A.; Horibe, K.; Tabuchi, K.; Kigasawa, H.; Tsuchida, M.; et al. Risk-Stratified Therapy and the Intensive Use of Cytarabine Improves the Outcome in Childhood Acute Myeloid Leukemia: The AML99 Trial from the Japanese Childhood AML Cooperative Study Group. J. Clin. Oncol. 2009, 27, 4007–4013. DOI: 10.1200/JCO.2008.18.7948.
- Murphy, T.; Yee, K. Cytarabine and Daunorubicin for the Treatment of Acute Myeloid Leukemia. Expert Opin. Pharmacother. 2017, 18, 1765–1780. DOI: 10.1080/14656566.2017.1391216.
- Ciccolini, J.; Serdjebi, C.; Peters, G. J.; Giovannetti, E. Pharmacokinetics and Pharmacogenetics of Gemcitabine as a Mainstay in Adult and Pediatric Oncology: An EORTC-PAMM Perspective. Cancer Chemother. Pharmacol. 2016, 78, 1–12. DOI: 10.1007/s00280-016-3003-0.
- Momparler, R. L. Optimization of Cytarabine (ARA-C) Therapy for Acute Myeloid Leukemia. Exp. Hematol. Oncol. 2013, 2, 20. DOI: 10.1186/2162-3619-2-20.
- Candelaria, M.; Corrales-Alfaro, C.; Gutierrez-Hernandez, O.; Diaz-Chavez, J.; Labardini-Mendez, J.; Vidal-Millan, S.; et al. Expression Levels of Human Equilibrative Nucleoside Transporter 1 and Deoxycytidine Kinase Enzyme as Prognostic Factors in Patients with Acute Myeloid Leukemia Treated with Cytarabine. Chemotherapy 2016, 61, 313–318. DOI: 10.1159/000445370.
- Rizzieri, D.; Vey, N.; Thomas, X.; Huguet-Rigal, F.; Schlenk, R. F.; Krauter, J.; et al. A Phase II Study of Elacytarabine in Combination with Idarubicin and of Human Equilibrative Nucleoside Transporter 1 Expression in Patients with Acute Myeloid Leukemia and Persistent Blasts after the First Induction Course. Leuk. Lymphoma 2014, 55, 2114–2119. DOI: 10.3109/10428194.2013.867489.
- Huang, M.; Inukai, T.; Miyake, K.; Tanaka, Y.; Kagami, K.; Abe, M.; et al. Clofarabine Exerts Antileukemic Activity against Cytarabine-Resistant B-Cell Precursor Acute Lymphoblastic Leukemia with Low Deoxycytidine Kinase Expression. Cancer Med. 2018, 7, 1297–1316. DOI: 10.1002/cam4.1323.
- Galmarini, C. M.; Thomas, X.; Calvo, F.; Rousselot, P.; El Jafaari, A.; Cros, E.; et al. Potential Mechanisms of Resistance to Cytarabine in AML Patients. Leuk. Res. 2002, 26, 621–629. DOI: 10.1016/S0145-2126(01)00184-9.
- Galmarini, C. M.; Thomas, X.; Calvo, F.; Rousselot, P.; Rabilloud, M.; El Jaffari, A.; et al. In Vivo Mechanisms of Resistance to Cytarabine in Acute Myeloid Leukaemia. Br. J. Haematol. 2002, 117, 860–868. DOI: 10.1046/j.1365-2141.2002.03538.x.
- Hubeek, I.; Stam, R. W.; Peters, G. J.; Broekhuizen, R.; Meijerink, J. P.; van Wering, E. R.; et al. The Human Equilibrative Nucleoside Transporter 1 Mediates in Vitro Cytarabine Sensitivity in Childhood Acute Myeloid Leukaemia. Br. J. Cancer 2005, 93, 1388–1394. DOI: 10.1038/sj.bjc.6602881.
- Takagaki, K.; Katsuma, S.; Kaminishi, Y.; Horio, T.; Nakagawa, S.; Tanaka, T.; et al. Gene-Expression Profiling Reveals down-Regulation of Equilibrative Nucleoside Transporter 1 (ENT1) in Ara-C-Resistant CCRF-CEM-Derived Cells. J. Biochem. 2004, 136, 733–740. DOI: 10.1093/jb/mvh180.
- Sarkar, M.; Han, T.; Damaraju, V.; Carpenter, P.; Cass, C. E.; Agarwal, R. P. Cytosine Arabinoside Affects Multiple Cellular Factors and Induces Drug Resistance in Human Lymphoid Cells. Biochem Pharmacol 2005, 70, 426–432. DOI: 10.1016/j.bcp.2005.05.014.
- Macanas-Pirard, P.; Broekhuizen, R.; Gonzalez, A.; Oyanadel, C.; Ernst, D.; Garcia, P.; et al. Resistance of Leukemia Cells to Cytarabine Chemotherapy is Mediated by Bone Marrow Stroma, Involves Cell-Surface Equilibrative Nucleoside Transporter-1 Removal and Correlates with Patient Outcome. Oncotarget 2017, 8, 23073–23086. DOI: 10.18632/oncotarget.14981.
- Bergman, A. M.; Adema, A. D.; Balzarini, J.; Bruheim, S.; Fichtner, I.; Noordhuis, P.; et al. Antiproliferative Activity, Mechanism of Action and Oral Antitumor Activity of CP-4126, a Fatty Acid Derivative of Gemcitabine, in in Vitro and in Vivo Tumor Models. Invest New Drugs 2011, 29, 456–466. DOI: 10.1007/s10637-009-9377-7.
- Stam, R. W.; den Boer, M. L.; Meijerink, J. P.; Ebus, M. E.; Peters, G. J.; Noordhuis, P.; et al. Differential mRNA Expression of Ara-C-Metabolizing Enzymes Explains Ara-C Sensitivity in MLL Gene-Rearranged Infant Acute Lymphoblastic Leukemia. Blood 2003, 101, 1270–1276. DOI: 10.1182/blood-2002-05-1600.
- Catala, A.; Pastor-Anglada, M.; Caviedes-Cardenas, L.; Malatesta, R.; Rives, S.; Vega-Garcia, N.; et al. FLT3 is Implicated in Cytarabine Transport by Human Equilibrative Nucleoside Transporter 1 in Pediatric Acute Leukemia. Oncotarget 2016, 7, 49786–49799. DOI: 10.18632/oncotarget.10448.
- Winters, A. C.; Bernt, K. M. MLL-Rearranged Leukemias-An Update on Science and Clinical Approaches. Front. Pediatr. 2017, 5, 4. DOI: 10.3389/fped.2017.00004.
- Kim, J. H.; Lee, C.; Cheong, H. S.; Koh, Y.; Ahn, K. S.; Kim, H. L.; et al. SLC29A1 (ENT1) Polymorphisms and Outcome of Complete Remission in Acute Myeloid Leukemia. Cancer Chemother. Pharmacol. 2016, 78, 533–540. DOI: 10.1007/s00280-016-3103-x.
- Amaki, J.; Onizuka, M.; Ohmachi, K.; Aoyama, Y.; Hara, R.; Ichiki, A.; et al. Single Nucleotide Polymorphisms of Cytarabine Metabolic Genes Influence Clinical Outcome in Acute Myeloid Leukemia Patients Receiving High-Dose Cytarabine Therapy. Int. J. Hematol. 2015, 101, 543–553. DOI: 10.1007/s12185-015-1766-4.
- Kaspers, G. J.; Veerman, A. J.; Pieters, R.; Broekema, G. J.; Huismans, D. R.; Kazemier, K. M.; et al. Mononuclear Cells Contaminating Acute Lymphoblastic Leukaemic Samples Tested for Cellular Drug Resistance Using the Methyl-Thiazol-Tetrazolium Assay. Br. J. Cancer 1994, 70, 1047–1052. DOI: 10.1038/bjc.1994.446.
- Hubeek, I., Giovannetti, E., Broekhuizen, A. J. F., Pastor-Anglada, M., Kaspers, G. J. L., Peters, G. J. Immunocytochemical Detection of hENT1 and hCNT1 in Normal Tissues, Lung Cancer Cell Lines and NSCLC Patient Samples. Nucleosides, Nucleotides & Nucleic Acids. 2008, 27, 787–793. DOI: 10.1080/15257770802145942.
- van Meerloo, J.; Kaspers, G. J.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. DOI: 10.1007/978-1-61779-080-5_20.
- Hubeek, I.; Kaspers, G. J.; Ossenkoppele, G. J.; Peters, G. J. Cytosine Arabinoside: Metabolism, Mechanisms of Resistance and Clinical Pharmacology. In Cancer Drug Discovery and Development: Deoxynucleoside Analogs in Cancer Therapy; Humana Press: Totowa, NJ, 2006; pp 119–151.
- Willemze, R.; Suciu, S.; Meloni, G.; Labar, B.; Marie, J. P.; Halkes, C. J.; et al. High-Dose Cytarabine in Induction Treatment Improves the Outcome of Adult Patients Younger than Age 46 Years with Acute Myeloid Leukemia: results of the EORTC-GIMEMA AML-12 Trial. J. Clin. Oncol. 2014, 32, 219–228. DOI: 10.1200/JCO.2013.51.8571.