This study describes polycyclic aromatic hydrocarbon (PAH) concentrations, patterns, and possible sources from atmospheric filter samples collected from three different areas in the city of Nairobi, Kenya. Total median concentrations for the 25 PAHs detected were higher in the traffic area (201 ng/m−3), followed by the residential area (141 ng/m−3), and lowest in the industrial area (128 ng/m−3). Results from the three sampled areas show that the percentage contributions of carcinogenic PAHs were approximately 30% of the total PAH concentrations reported. Some PAH isomer ratios differentiated traffic sources from non-traffic sources. Principal component analysis showed four significant principal components accounting for 82% of the variance. The first principal component (35%) was associated with fuel burning. The second principal component (27%) was associated with traffic emissions (diesel and gasoline). The other two principal components, which accounted for 12% and 8%, could not be interpreted with certainty. In order to interpret all the principal components in relation to sources, further collection of data is needed. More data points would have helped in further resolving the sources because data analysis models recommend more than 30 data sets.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants formed during the incomplete combustion of organic matter (CitationHarrison et al., 1996; CitationMackay and Hickie, 2000). A number of high-molecular-weight PAHs are known carcinogens, mutagens, and some are suspected endocrine disrupters (CitationBoström et al., 2002). To control their emission, source identification methods have been studied. Certain PAH concentration ratios to benzo[e]pyrene (B[e]P) have been used to differentiate PAH sources successfully (CitationOhura et al., 2004; CitationSanderson et al., 2004). Different sources of PAHs (e.g., gasoline and diesel engine cars, oil combustion from power plants, and domestic fuel burning) have been shown to generate different PAH profiles (CitationBzdusek et al., 2004; CitationLi et al., 2003; CitationSimcik et al., 1999). Profiles obtained from environmental monitoring might differ from actual source data due to such factors as mixing of PAHs from different sources, degradation during transport, weather conditions, and type of fuel used in a particular source (CitationDachs et al., 2002; CitationDickhut et al., 2000; Simcik et al., 1997). Therefore, site-specific PAH signatures help relate the compositional profiles from suspected sources to local and regional atmospheric conditions.
In Nairobi, Kenya, factors such as industrial activities, motor vehicles, and population have been on the rise recently, making air pollution a high priority. However, the lack of data on major air environmental pollutants that affect human health is an obstacle for source identification. PAHs in Nairobi could originate from different sources including industrial activities, motor vehicles, and household burning of fuel. The main types of fuel used in industrial area include industrial diesel, fuel oil, sawdust, pulverized coke, and used oil (CitationUNEP, 1999), whereas power generation uses diesel. Motor vehicles in the city do not have catalytic converters because of the use of leaded fuel but this fuel will be phased out in 2006. The main types of petroleum products used in Nairobi are liquefied petroleum gas (LPG), premium and regular gasoline, automotive gas oil (diesel), industrial diesel, fuel oil, and bitumen. Crude oil imported in Kenya is low in sulphur and the refinery has the capability of reducing the sulphur content (CitationUNEP, 1999). In the sampled areas, trash burning is limited because most of the solid waste is collected and dumped in the only solid waste dumpsite in Nairobi. Some waste in this site is openly burned; however, this could be a major PAH source because the open burning was located far from the sampled areas. Residential area samples were collected in a heavily populated area where most households use kerosene, charcoal, wood, and, to a lesser extent, LPG for cooking.
Due to the stability and health concerns of particle-bound PAHs, particulate matter in air was collected using quartz filter and analyzed. The aim for the present research was to report the concentration levels and patterns of these PAHs, including the high-molecular-weight fractions, which are potentially toxic and lacking in many PAH studies. Therefore, this study analyzed 30 different PAHs from atmospheric particle samples, collected for 2 to 3 hours from three different areas covering residential, industrial, and heavily trafficked areas during the period of August 25 to September 12, 2003, in Nairobi, Kenya. To the best knowledge of these authors, little or no data is available on PAH levels in Nairobi.
Materials and Methods
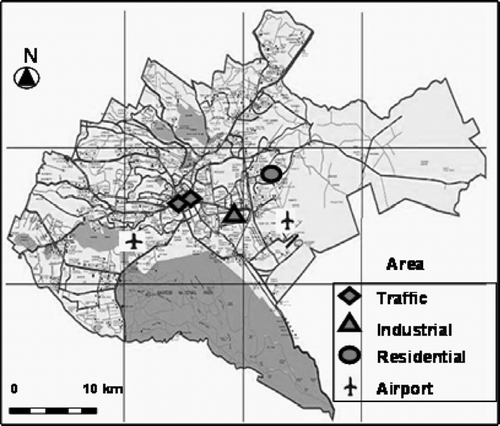
Sampling sites were located in traffic, industrial, and residential areas in Nairobi, Kenya (). There were two traffic area sampling points—one was located near the junction of a major highway (Uhuru Highway) that links the port city of Mombasa to the city of Nairobi and other neighboring countries. The other traffic area was located near a junction where traffic from residential areas enters the central business district. Major activities in the vicinity where the industrial samples were collected include food factories, wood processing, tire manufacturing, and power generation. The residential area was located in a densely populated area where the major types of domestic fuel used are charcoal, wood, and kerosene. These fuels are used for both cooking and heating. During the month of September, the nights are cold and therefore some households heat their homes with charcoal.
shows the sampling information during air data collection in Nairobi. Atmospheric particle samples were collected on quartz filters (QR100, Advantec, Japan; cut to 3.2-cm diameter and preheated to 450°C for 4 hours), using a mini-pump (MP-∑500, Sibata, Tokyo, Japan) at 5 L· min−1 from the three different areas. Among the total of 24 samples, the volumes of air collected ranged from 0.47 to 0.93 m3. The filters sampled were folded and stored in aluminum foil under 4°C until analysis. The traffic area filters darkened a few minutes after start of sampling. The intensity of blackness was highest in traffic area samples. The industrial area filters were not as dark as traffic area samples whereas the residential area filters were light with a tinge of brown to black.
Table 1 Sampling information in Nairobi, Kenya
The sampling time was enough to quantify PAHs collected in the filters, the concentration of PAH seems to be high due to speed at which the filters turned black. In addition, sampling was carried out during high traffic time, industrial production, and peak cooking time. Therefore, the samples were representative of the sampling area.
The PAH analysis was adapted from previously published methods (CitationMandoulet et al., 2000; CitationPleil et al., 2004; CitationShu et al., 2003; CitationUS EPA, 1996). Briefly, the filter samples were spiked with 100 μL of 1.25 μg/mL of deuterated PAH mixture containing naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, perylene-d12, and benzo[ghi]perylene-d12 prior to extraction (EPA 8270 surrogate mix, Sigma-Aldrich, USA). Automated Soxhlet equipment (Soxtherm, Gerhardt, Germany) was used in extracting the PAHs from the filter samples, using a 1:1 mixture of acetone:hexane solution (140 mL) at 140°C programmed for 2 hours and 30 minutes. The extract was cleaned up by a Sep-Pak silica cartridge (Waters, MA, USA) with 10–12 mL of 20% acetone:hexane mixture. The purified extract was then concentrated to 0.9 mL by N2 purge at room temperature and 100 μL of 1 μg/mL internal standard (pyrene-d10) was added to the vial. The sample was then stored in the freezer below 4°C until analysis with gas chromatography/mass spectometry (GC/MS). Two μL of the extracted sample in the vial was injected into a GC/MS (Agilent HP6890 series GC and HP5973 series MS) equipped with a DB-5 capillary column (60 m × 0.25 mm i.d.; 2.5 μm film thickness, J&W Scientific, Folsom, CA) and detected by selected ion monitoring (SIM) mode. The temperature program was operated as follows: initial temperature of 90°C, held for 2 minutes, ramped at 30°C·min−1 to 200°C, then 5°C·min−1 to 320°C and held isothermally at 320°C for 16 minutes. Injector and auxiliary transfer line temperatures were 280°C and 290°C, respectively. Carrier gas (helium 99.99% purity) was maintained at a constant flow rate of 1.4 mL/min−1 throughout the analysis. The overall program lasted for 46 minutes and was able to analyze 30 different PAHs.
All target PAHs were calibrated using 30 PAH native standards (Quebec Ministry of Environmental PAH mix, Sigma-Aldrich, USA, and Accustandards, USA), and six surrogate standards. Internal standard (pyrene-d10) was spiked in all the extracts before GC/MS analysis. Quality assurance program was carried out by: 1) setting up a six-point calibration curve from six different concentrations of the native and surrogate matrix (0.04, 0.1, 0.2, 0.5, 1.0, 1.5 μg/mL), 2) spiking the samples with a known concentration of the six surrogate standards before extraction, 3) running a 0.2 μg/mL matrix mixture of all the PAH standards in each GC/MS run to check calibration curve, and 4) running two filter blanks using the method previously described, which and showed very low or no peaks for the target PAHs. Detection limits for the PAHs calculated from S/N ratio multiplied by 3 ranged from 0.04 to 0.1 ng/m−3. Good correlation coefficients of over 0.99 were obtained between standard concentrations and peak areas for all the native and surrogate standards. The percentage recoveries for the surrogate standards were used to correct the concentration of the PAHs. Average recoveries for the surrogate standards were 52%, 54%, 82%, 74% and 77% for acenaphthene-d10, phenanthrene-d10, chrysene-d12, perylene-d12, and benzo[ghi]perylene-d12, respectively. Recoveries of naphthalene-d8 were below 30% due to its volatility.
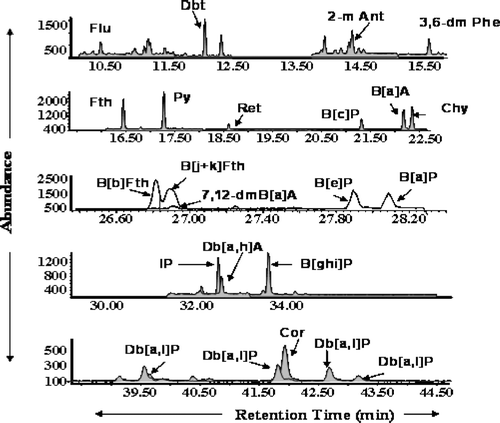
Of the 30 target analytes, 25 were reportable in aerosol samples (). Namely, five PAHs, naphthalene, 2,6-dimethyl naphthalene, acenaphthylene, and acenaphthene concentrations, were not reported because of the low recoveries of naphthalened8 surrogate standard. The concentration of 2-methyl anthracene was not reported because of matrix interference. Internal standard method and Agilent special ChemStation programs, such as retention time locking (RTL), were used to determine PAH concentration of the samples. PAHs were deemed detected when retention time and qualifier ion ratio of the standard matched that of a sample exactly. A deviation of less than or around 5 seconds for retention time and less than 10% of the qualifier ion ratio was allowed for some PAHs. A chromatogram of some of the PAHs that were measured in one of the samples is shown in .
Table 2 Ranges and median concentrations of 25 PAHs (ng·m −3) in Nairobi, Kenya
Statistical analysis was performed using statistical software (StatSoft Inc, Tulsa, USA). PAH concentration levels were reported for their range, median, and Pearson's correlation coefficients. The data were analyzed for sources by isomer ratio analysis and principal component analysis (PCA). Nonparametric tests, such as the Kruskal-Wallis test, were used to detect inherent differences in PAH concentration data obtained from the sampled areas.
Results and Discussion
Concentration Levels
Range and median concentrations of each of the PAH species and their totals from 24 filter samples in Nairobi are shown in . In the traffic area the most abundant PAHs were: Py, B[b]Fth, B[ghi]P, and Cor. Their concentrations ranged from 2.7–34.0 ng/m−3, 0.8–35.8 ng/m−3, 1.9–43.1 ng/m−3, and 1.2–29.2 ng/m−3, respectively. In the industrial area the same PAHs were abundant, but their concentrations were lower. They ranged from 5.5–23.2 ng/m−3 (Py), 3.8–31 ng/m−3 (B[b]Fth), 9–24.6 ng/m−3 (B[ghi]P), and 5.2–14.7 ng/m−3 (Cor). In the residential area Py, B[a]A, and Chy were more dominant and their concentrations ranged from 5–26.6 ng/m−3, 4.9–22.5 ng/m−3, and 4.8–19 ng/m−3, respectively. Concentrations of B[b]Fth, 5.3–9.1 ng/m−3, B[ghi]P, 7.4–13.8 ng/m−3, and Cor, 2.8–8.1 ng/m−3 in residential areas were lower compared with traffic and industrial areas. Total median concentrations for the 25 PAHs were higher in the traffic area (200.9 ng/m−3), followed by the residential area (140.9 ng/m−3), and lowest in the industrial area (128.2 ng/m−3). These results are expected because traffic emissions have been shown to be a major source of PAHs in urban areas (CitationPark et al., 2002; CitationGuo et al., 2003). For specific PAH markers for traffic emissions such as B[ghi]P and Cor, median concentrations were approximately two and four times higher in trafficked areas than in industrial and residential areas, respectively.
Median concentrations of carcinogenic PAHs such as B[a]P were high in the trafficked areas (10.1 ng/m−3) and lower in the industrial (5.0 ng/m−3) and residential areas (8.1 ng/m−3). These concentrations were higher than that recommended by World Health Organization (WHO) guidelines for Europe of 0.1 ng/m−3 (CitationBoström et al., 2002) and a newly established annual average value of 1.0 ng/m−3 for PM10-associated B[a]P (CitationEuropean Parliament, 2004). However, it should be kept in mind that direct comparison of PAH concentrations among the three sites is not allowed because sampling in the traffic area was conducted during the rush morning hours, whereas in the industrial and in the residential areas, sampling was carried out during different time periods with possibly lower PAH emissions and/or with atmospheric conditions more favorable for pollutant dispersion. PAHs that had average concentrations lower than 0.7 ng/m−3 included 7,12-dmB[a]A, 3-Mchol, Db[a,i]P, and Db[a,h]P. Concentration levels of Db[a,l]P were reported, but it should be noted that a number of other dibenzopyrene isomers elute at the same retention time, complicating the certainty of detecting this PAH. Concentration data in this study for PAHs such as Fth, Py, B[b]Fth, B[e]P), and Cor were comparable with those presented by CitationFang et al. (2004) in Taiwan and CitationSmith et al. (1996) in Pakistan.
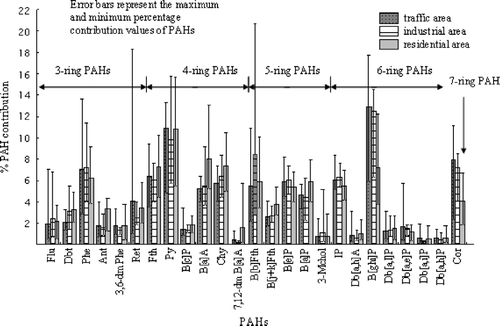
Average percentage contributions of individual PAHs to the total of 25 PAHs measured are presented in . Statistical analysis (Kruskal-Wallis test) showed no significant differences between the sampling areas for three-ring (H = 0.64, df = 2, P < 0.01), four-ring (H = 2.15, df = 2, P < 0.01), five-ring (H = 1.87, df = 2, P < 0.01), and six-ring PAHs (H = 7.63, df = 2, P < 0.01). This could be partly due to mixing of the PAH sources or a strong influence of one source in the sampled areas.
Carcinogenic PAHs
PAHs that have been classified as possible carcinogens include: B[a]A, B[b]Fth, 7,12-dmB[a]A, benzo[j+k]fluoranthene (B[j+k]Fth), B[a]P, indeno[1,2,3-cd]pyrene (IP), dibenz[a,h]anthracene (Db[a,h]A), Db[a,l]P, dibenzo[a,e]pyrene (Db[a,e]P), Db[a,i]P, and Db[a,h]P (CitationBoström et al., 2002; CitationTsai et al., 2004; CitationWHO/IPCS, 1998). Results from the three sampled areas show that the percentage contribution of carcinogenic PAHs is approximately 30% of the total PAHs concentrations reported. This percentage is slightly higher than that reported by CitationTsai et al. (2004) for medium- and high-molecular-weight PAHs from samples collected in a trafficked area.
Source Analysis
Isomer Ratios
Sources of PAHs have been explained using isomer ratios (CitationGuo et al., 2003; CitationManoli et al., 2004; CitationOhura et al., 2004; CitationPark et al., 2002). and show the range and average isomer ratios observed in this study, and source isomer ratios reported in literature, respectively. The average ratios for Fth/(Fth+Py), B[e]P/(B[e]P+B[a]P), and IP/(IP+B[ghi]P) ranged between 0.37–0.41, 0.48–0.58, and 0.33–0.45, respectively. These values are comparable with those reported by CitationKavouras et al. (1999) of 0.41 ± 0.1, 0.45 ± 0.27, and 0.32 ± 0.22, for vehicle emissions (gasoline and diesel engines). The ratios calculated were almost constant in the three different areas sampled indicating that a single source or multiple constant sources which had the constant isomer ratio might have influenced the three sampling areas. The average ratio of B[ghi]P/B[a]P was 2.89 in industrial area, 2.81 traffic area, and 1.39 in residential area. The high values obtained in industrial and heavily trafficked areas indicate that gasoline emissions were major sources in these areas (CitationRogge et al., 1993).
Table 3a Summary of isomer ratios of selected PAHs
Table 3b Reference isomer ratios of selected PAHs from previous research
The average ratio for Cor/B[e]P was low in residential (0.75) relative to industrial (1.20) and heavily trafficked (1.35) area, indicating that sources other than traffic influenced PAH levels in residential area. These results can be compared with the one presented by CitationNielsen (1996) who reported a value of 1.54 ± 0.16 for traffic emissions. CitationLarsen and Baker (2003) reported a higher ratio of 1.8 than the values recorded in this study. Samples that recorded a value > 1 for the Cor/B[e]P ratio were interpreted as influenced by traffic sources. Within this region were 80% of the traffic area samples, 62% of industrial area samples, and 16% of residential area samples. Samples that recorded a value < 1 for the same ratio were considered to be from other sources than traffic since five out of six residential samples and three out of eight industrial samples were within this region. However, one must take into account the extensive use of diesel in industrial area as a contributor to the high percentage observed and PAH weathering as a contributing factor to the percentage shown in residential area.
A plot of B[a]A/(B[a]A+Chy) versus IP/(IP+B[ghi]P) ratios shows differences among the sampling areas (). The plot shows an approximate positive linear relationship for residential area samples (R = 0.93, P < 0.005) an indication that both pyrogenic and petrogenic combustion influence the area (CitationTsapakis and Stephanou, 2005; CitationYunker et al., 2002). For the latter ratio, seven traffic samples were within the range of 0.25–0.35, whereas three were within the range of 0.35–0.70; they were interpreted as gasoline and diesel engine emissions, respectively, based on literature values. For example, CitationKhalili et al. (1995) reported a range of 0.21–0.22 for gasoline engine vehicles, whereas CitationManoli et al. (2004) reported a range of 0.16–0.39 for the same ratio in ambient air in Thessaloniki, Greece, suggesting a stronger contribution from gasoline than diesel engine emissions. Three residential area samples recorded values of between 0.49–0.77, which CitationYunker et al. (2002) associated with wood combustion.
PAH Weathering
A brief report on the meteorological conditions of Nairobi indicates that during the months of August and September it is mostly cloudy and sunshine occurs between 4.2 and 5.9 hours in a day. Also in May through September, east and southeast winds of 10–15 mph are dominant. The winds pass through industrial and traffic areas located south of the residential area (approximately 10–15 km from industrial and traffic areas). It is expected that weathering will affect PAHs as they move through air from traffic and industrial areas to residential area. Correlation analysis showed that two PAHs (Cor and B[ghi]P, source markers for diesel and gasoline engines) had a high ratio in all the sampled areas, suggesting a common source. These authors therefore expect weathering of these two PAHs as they move through air from industrial and traffic areas to residential area. Four ratios, namely IP/(IP+B[e]P), IP/(IP+B[ghi]P), Cor/B[e]P, and B[ghi]P/B[e]P, were used in interpretation of weathering. The average ratio of IP/(IP+B[e]P) was the same in the all the areas sampled areas, probably because these PAHs have sources in the three areas and/or they are more stable, therefore undergoing less weathering, whereas the average ratio for IP/(IP+B[ghi]P) was almost the same in traffic (0.33) and industrial areas (0.34) relative to residential area (0.45), which was slightly higher. This result is probably due to B[ghi]P degradation from industrial and traffic area sources to the residential area. The Cor/B[e]P ratio was low in the residential area (0.75) compared with industrial (1.20) and traffic (1.35) areas. Coronene is a marker of traffic pollution; it is probable that this PAH was degraded as it moved from the traffic area to residential area, given the low ratio in residential area. The average B[ghi]P/B[e]P ratio between traffic and industrial area relative to residential areas was higher than that of Cor/B[e]P due to the stability of Cor relative to B[ghi]P in terms of photochemical reaction (CitationEsteve et al., 2004). CitationBaymer and Hites (1988) indicated that dark (i.e., high carbon content) substrates stabilize PAHs to photolytic breakdown because they absorb more light, making less light available for photolysis. The intensity of blackness of the filter samples was highest for the traffic area followed by industrial area samples but the residential area samples were less dark. The main routes of PAH removal from the atmosphere could be through dilution, dry deposition, and photochemical degradation (CitationDachs et al., 2002). Therefore, it is probable that dilution of PAHs occurs as they move in the air mass followed by photochemical loss by OH, NO2 scavenging (CitationDachs et al., 2002; CitationEsteve et al., 2004; CitationHayakawa et al., 2002; Simcik et al., 1997).
Correlation Analysis
Correlation between different PAHs has been used to explain the sources of PAHs (CitationOhura et al., 2004, CitationSchauer et al., 2003). CitationMiguel et al. (1998) and CitationLarson and Baker (2003) showed high correlation existed between Cor and B[ghi]P for diesel and gasoline emissions sources. shows that the correlation coefficient between Cor and B[ghi]P was high (R = 0.97, n = 10) for the trafficked area, suggesting diesel and gasoline engine emissions as major PAH sources in the area. High correlations between B[a]A and Py (R = 0.98, n = 8) and between B[a]P and Fth (R = 0.92, n = 8) were recorded for industrial area samples. These PAHs were associated with oil combustion (Larsen and CitationBaker, 2003; CitationHarrison et al., 1996; CitationYunker et al., 1996). In residential area samples, high correlations were recorded between chrysene (Chy) and B[a]A (R = 0.93, n = 6); Py and Fth (R = 0.93, n = 6); and Chy and Fth (R = 0.90, n = 6). These PAHs have been associated with domestic fuel burning (CitationHays et al., 2003; CitationLee et al., 2004; CitationSimcik et al., 1999; CitationRogge et al., 1993).
Table 4 Correlations of selected PAHs in the sampling areas
Generally, all samples from different areas showed a high correlation for six- and seven-ring PAHs, suggesting diesel or gasoline contribution. The differences in were observed because traffic area samples did not show higher correlations for four- and five-ring PAHs, whereas industrial area samples showed better correlations between four- and five-ring PAHs, relative to residential area samples that showed good correlations for the four-ring PAHs.
Principal Component Analysis (PCA)
PCA was employed to distinguish PAH sources and describe inherent differences in PAH profiles. To avoid bias in PCA, concentrations of fluoranthene to coronene were used because recovery standards used to correct their concentrations were high. Concentrations of 7,12-dmB[a]A, 3-Mchol, Db[a,l]P, B[c]P, Db[a,i]P, and Db[a,h]P were low and therefore not included in PCA because spurious effects could be introduced. Preceding PCA, individual PAH concentrations were transformed to individual PAH percentages, divided by the total PAH concentrations measured in each sample and multiplied by 100 (CitationJohnson et al., 2002; CitationKim et al., 2004). The obtained matrix of 14 individual PAHs by 24 samples was then used for PCA.
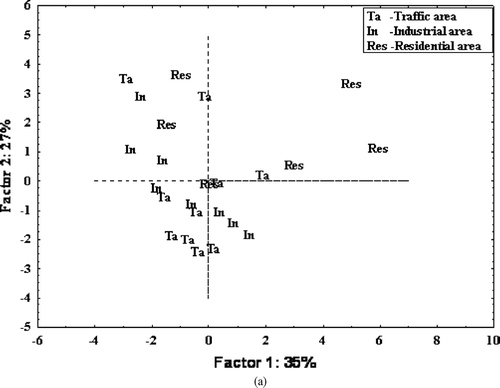
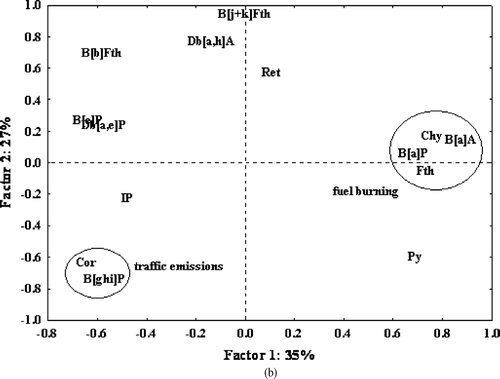
PCA extracted four factors with eigenvalues > 1, which accounted for 82% of the variance in total. Varimax rotation did not further improve the resolution of components The first principal component accounting for 35% of the variance had a high loading for B[a]A (0.87), Chy (0.76), Fth (0.73), Py (0.69), and B[a]P (0.68) (); these PAHs have been associated with domestic fuel burning (CitationRogge et al., 1993; CitationLee et al., 2004) and stationary sources in industrial areas (CitationFang et al., 2004). This principal component was interpreted as domestic and industrial fuel burning. The second principal component had a positive moderate loading for B[b]Fth (0.65) and Db[a,h]A (0.73) high loading for B[j+k]Fth (0.91), high negative loading for B[ghi]P (0.78), and moderate negative loading for Cor (0.68). Collectively, this pattern was attributed to traffic emissions. These PAHs are markers of diesel and gasoline engine vehicles (CitationMiguel et al., 1998; CitationSanderson et al., 2004; CitationSchauer et al., 2003). The third and fourth principals accounting for 12% and 8% could not be interpreted fully. However, the factors may be linked to wood combustion because of the positive loadings for Ret (0.62), and moderate loadings for IP (0.45) (CitationDidyk et al., 2000; CitationLi and Kamens, 1993; CitationLehndorff and Schwark, 2004). The result would have been more conclusive if other wood source markers such as 1,7-dimethylphenanthrene and 2,6-dimethylphenanthrene were measured (CitationLarsen and Baker, 2003). and show the score and loading plots of the first versus the second PC. These plots show the influence of fuel burning source markers (B[a]A, Chy, Py, B[a]P, and Fth) in the residential area. This is probably due to influence from industrial sources in addition to residential area sources, a fact reinforced by the extensive use of fuel in the industrial area in addition to the residential areas where charcoal is used both for cooking and space heating.
Table 5 PCA loadings for particle bound PAHs in Nairobi
Varimax-rotated PCA of log-transformed PAH concentrations that have been Z-score normalized were similar to percentage normalized results. Four principal components accounting for 82% of the variance were obtained from both methods. PC2 and PC3 explaining 40% and PC1 27% of the variance were interpreted as fuel burning and traffic emissions, respectively. These values correspond with PC1 and PC2 from the previously calculated results. Therefore, from PCA of the major sources of PAHs in Nairobi are fuel burning and traffic emissions with moderate inputs from other sources.
Conclusions
In summary, PAH concentrations in the traffic areas were higher than in the other two sampling areas. Specific PAH markers such as B[ghi]P and Cor concentrations were higher in trafficked areas. B[a]P concentration was higher than the recommended value of WHO guidelines in Europe. Contribution of carcinogenic PAHs to the 25 total PAHs reported in Nairobi was approximately 30%. PAH profile data showed no statistically significant differences between the sampling areas. Isomer ratio results such as Cor/B[e]P were able to differentiate traffic sources from non-traffic sources, whereas the B[ghi]P/B[a]P ratio showed that traffic and industrial areas were influenced more by gasoline and diesel engines relative to residential areas. Environmental weathering of the PAHs was probably through wind dilution followed by photochemical loss, a fact shown from the difference in the previously mentioned isomer ratios from traffic, industrial, and residential areas. High correlations of some PAHs such as Cor and B[ghi]P in trafficked areas, B[a]A and Py in industrial areas, and B[a]A and Chy in residential areas were associated with traffic emissions, oil, and fuel combustion, respectively. Overall source analysis from isomer ratios and PCA indicate that fuel burning and traffic emissions were the major sources of PAH levels in the three sampled areas in Nairobi and other sources were not outstanding or could not be explained because of PAH source mixing.
Acknowledgement
This work was partly supported by the 21st Century COE Program “Environmental Risk Management for Bio/Eco-system” of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Notes
j Neilsen (1996)
References
- Behymer , T. D. and Hites , R. A. 1988 . Photolysis of polycyclic aromatic hydrocarbons adsorbed on fly ash . Environmental Science and Technology , 22 : 1311 – 1319 . [CSA]
- Boström , C. -E. , Gerde , P. , Henberg , A. , Jernstrom , B. , Johansson , C. , Kyrklund , T. , Rannug , A. , Tornqvist , M. , Victorin , K. and Westerholm , R. 2002 . Cancer risk assessment, indicators and guidelines for polycyclic aromatic hydrocarbons in the ambient air . Environmental Health Perspectives Supplement , 110 : 451 – 488 . [CSA]
- Bucheli , T. D. , Blum , F. , Desaules , A. and Gustafsson , O. 2004 . Polycyclic aromatic hydrocarbons, black carbon, and molecular markers in soils of Switzerland . Chemosphere , 56 : 1061 – 1076 . [INFOTRIEVE] [CROSSREF] [CSA]
- Bzdusek , P. A. , Christensen , E. R. , Li , A. and Zou , Q. 2004 . Source apportionment of sediment PAHs in Lake Calumet, Chicago: Application of factor analysis with nonnegative constraints . Environmental Science and Technology , 38 : 97 – 103 . [INFOTRIEVE] [CROSSREF] [CSA]
- Christensen , E. R. and Bzdusek , P. A. 2005 . PAHs in sediments of the Black River and the Ashtabula River, Ohio: Source apportionment by factor analysis . Water Research , 39 : 511 – 524 . [INFOTRIEVE] [CROSSREF] [CSA]
- Dachs , J. , Glen , T. R. , Gigliotti , C. L. , Brunciak , P. , Totten , L. A. , Nelson , E. D. , Franz , P. T. and Eisenreich , S. J. 2002 . Process driving the short-term variability of polycyclic aromatic hydrocarbons in the Baltimore and northern Chesapeake Bay atmosphere, USA . Atmospheric Environment , 36 : 2281 – 2295 . [CROSSREF] [CSA]
- Dickhut , R. M. , Canuel , E. A. , Gustafson , K. E. , Liu , K. , Arzayus , K. M. , Walker , S. E. , Edgecombe , G. , Gaylor , M. O. and Macdonanld , E. H. 2000. Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region . Environmental Science and Technology , 36 4635 – 4640 . [CSA]
- Didyk , B. M. , Simoneit , B. R.T. , Pezoa , L. A. , Riveros , M. L. and Flores , A. A. 2000. Urban aerosol particles of Santiago, Chile: Organic content and molecular characterization . Atmospheric Environment , 34 1167 – 1179 . [CROSSREF] [CSA]
- Esteve , W. , Budzinski , H. and Villenave , E. 2004. Relative rate constants for the heterogeneous reactions of OH, NO2 and NO radicals with polycyclic aromatic hydrocarbons adsorbed on carbonaceous particles. Part 1: PAHs adsorbed on 1–2 mm calibrated graphite particles . Atmospheric Environment , 38 6063 – 6072 . [CROSSREF] [CSA]
- European Parliament . 2004 . http://www.milieu.be/news/legislation_0501.pdfDirective 2004/107/EC of the European Parliament and of the Council of 13 October relating to the arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Available at:
- Fang , G -C. , Wu , Y. -S. , Chen , M. -H. , Ho , T. -T. , Huang , S. -H. and Rau , J. -Y. 2004 . Polycyclic aromatic hydrocarbons study in Taichung, Taiwan, during 2002–2003 . Atmospheric Environment , 38 : 3385 – 3391 . [CROSSREF] [CSA]
- Guo , H. , Lee , S. C. , Ho , K. F. , Wang , X. M. and Zou , S. C. 2003 . Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong-Kong . Atmospheric Environment , 37 : 5307 – 5317 . [CROSSREF] [CSA]
- Harrison , R. M. , Smith , D. J. T. and Luhana , L. 1996 . Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK . Environmental Science and Technology , 30 : 825 – 832 . [CROSSREF] [CSA]
- Hayakawa , K. , Tang , N. , Akutsu , K. , Murahashi , T. , Kakimoto , H. , Kizu , R. and Toriba , A. 2002 . Comparison of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in airborne particulates collected in downtown and suburban Kanazawa, Japan . Atmospheric Environment , 36 : 5535 – 5541 . [CROSSREF] [CSA]
- Hays , M. D. , Smith , N. D. , Kinsey , J. , Dong , Y. and Kariher , P. 2003 . Polycyclic aromatic hydrocarbon size distributions in aerosols from appliances of residential wood combustion as determined by direct thermal desorption-GC/MS . Aerosol Science , 34 : 1061 – 1084 . [CROSSREF] [CSA]
- Johnson , G. W. , Ehrlich , R. and Full , W. 2002 . “ Principal components analysis and receptor models in environmental forensics ” . In Environmental forensics , Edited by: Murphy , B. L. and Morrison , R. D. London : Academic Press .
- Kavouras , I. G. , Koutrakis , P. , Tsapakis , M. , Lagoudaki , E. , Stephanou , E. G. , Von Baer , D. and Oyola , P. 2001 . Source apportionment of urban particulate aliphatic and polynuclear aromatic hydrocarbons (PAHs) using multivariate methods . Environmental Science and Technology , 35 : 2288 – 2294 . [INFOTRIEVE] [CROSSREF] [CSA]
- Kavouras , I. G. , Lawrence , J. , Koutrakis , P. , Stephanou , E. G. and Oyola , P. 1999 . Measurement of particulate aliphatic and polynuclear aromatic hydrocarbons in Santiago de Chile: Source reconciliation and evaluation of sampling artifacts . Atmospheric Environment , 33 : 4977 – 4986 . [CROSSREF] [CSA]
- Khalili , N. R. , Scheff , P. A. and Holsen , T. M. 1995 . PAH source fingerprints for coke ovens, diesels and gasoline engines, highway tunnels and wood combustion emissions . Atmospheric Environment , 29 : 533 – 542 . [CROSSREF] [CSA]
- Kim , K. S. , Hirai , Y. , Kato , M. , Urano , K. and Masunaga , S. 2004 . Detailed PCB congener patterns in incinerator flue gas and commercial PCB formulations (Kanechlor) . Chemosphere , 55 : 539 – 553 . [INFOTRIEVE] [CROSSREF] [CSA]
- Larsen , R. K. and Baker , J. E. 2003 . Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods . Environmental Science and Technology , 37 : 1873 – 1881 . [INFOTRIEVE] [CROSSREF] [CSA]
- Lee , J. H. , Gigliotti , C. L. , Offenberg , J. H. , Eisenreich , J. S. and Turpin , B. J. 2004 . Sources of polycyclic aromatic hydrocarbons to the Hudson River airshed . Atmospheric Environment , 38 : 5971 – 5981 . [CROSSREF] [CSA]
- Lehndorff , E. and Schwark , L. 2004 . Biomonitoring of air quality in the Cologne Conurbation using pine needles as a passive sampler-part II: polycyclic aromatic hydrocarbons (PAH) . Atmospheric Environment , 38 : 3793 – 3808 . [CROSSREF] [CSA]
- Li , A. , Jang , J. -K. and Scheff , P. A. 2003 . Application of EPA CMB8.2 Model for source apportionment of sediment PAHs in Lake Calumet, Chicago . Environmental Science and Technology , 37 : 2958 – 2965 . [INFOTRIEVE] [CSA]
- Li , C. K. and Kamens , R. M. 1993 . The use of polycyclic aromatic hydrocarbons as source signatures in receptor modeling . Atmospheric Environment , 27 : 523 – 532 . [CSA]
- Mackay , D. and Hickie , B. 2000 . Mass balance model of source apportionment, transport and fate of PAHs in Lac Saint Louis, Quebec . Chemosphere , 41 : 681 – 692 . [INFOTRIEVE] [CROSSREF] [CSA]
- Mandoulet , A. J. , Abarnou , A. , Le Guellec , A. -M. , Loizeau , V. and Leboulenger , F. 2000 . Validation of an analytical procedure for polychlorinated biphenyls, coplanar polychlorinated biphenyls and polycyclic aromatic hydrocarbons in environmental samples . Journal of Chromatography A. , 886 : 153 – 173 . [CROSSREF] [CSA]
- Manoli , E. , Kouras , A. and Samara , C. 2004 . Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece . Chemosphere , 56 : 867 – 878 . [INFOTRIEVE] [CROSSREF] [CSA]
- Miguel , A. H. , Kirchstetter , T. W. , Harley , R. A. and Hering , S. 1998 . On-road emissions of particulate polycyclic aromatic hydrocarbons and black carbon from gasoline and diesel vehicles . Environmental Science and Technology , 32 : 450 – 455 . [CROSSREF] [CSA]
- Nielsen , T. 1996 . Traffic contribution of polycyclic aromatic hydrocarbons in the center of a large city . Atmospheric Environment , 30 : 3481 – 3490 . [CROSSREF] [CSA]
- Ohura , T. , Amagai , T. , Fusaya , M. and Matsushita , H. 2004 . Spatial distributions and profiles of atmospheric polycyclic aromatic hydrocarbons in two industrial cities in Japan . Environmental Science and Technology. , 38 : 49 – 55 . [INFOTRIEVE] [CROSSREF] [CSA]
- Park , S. S. , Kim , Y. J. and Kang , C. H. 2002 . Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea . Atmospheric Environment , 36 : 2917 – 2924 . [CROSSREF] [CSA]
- Pleil , J. D. , Vette , A. F. and Rappaport , S. M. 2004 . Assaying particle bound polycyclic aromatic hydrocarbons from archived PM2.5 filters . Journal of Chromatography A , 1033 : 9 – 17 . [INFOTRIEVE] [CROSSREF] [CSA]
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1993 . Sources of fine organic aerosol. 2. Noncatalyst-equipped automobiles and heavy-duty diesel trucks . Environmental Science and Technology , 27 : 636 – 651 . [CROSSREF] [CSA]
- Sanderson , E. G. , Raqbi , A. , Vyskocil , A. and Farant , J. -P. 2004 . Comparison of particulate polycyclic aromatic hydrocarbon profiles in different regions of Canada . Atmospheric Environment , 38 : 3417 – 3429 . [CROSSREF] [CSA]
- Schauer , C. , Niessner , R. and Poschl , U. 2003 . Polycyclic aromatic hydrocarbons in urban air particulate matter: decadal and seasonal trends, chemical degradation, and sampling artifacts . Environmental Science and Technology , 37 : 2861 – 2868 . [INFOTRIEVE] [CROSSREF] [CSA]
- Shu , Y. Y. , Tey , S. Y. and Wu , D. K. S. 2003 . Analysis of polycyclic aromatic hydrocarbons in airborne particles using open-vessel focused microwave-assisted extraction . Analytica Chemic Acta , 495 : 99 – 108 . [CROSSREF] [CSA]
- Sicre , M. A. , Marty , J. C. , Saliot , A. , Aparicio , X. , Grilmat , J. and Albaiges , J. 1987 . Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: occurrence and origin . Atmospheric Environment , 21 : 2247 – 2259 . [CROSSREF] [CSA]
- Simick , M. F. , Huixiang , Z. and Eisenreich , S. J. 1997 . Urban contamination of the Chicago/coastal Lake Michigan atmosphere by PCBs and PAHs during AEOLOS . Environmental Science and Technology , 31 : 2141 – 2147 . [CROSSREF] [CSA]
- Simcik , M. F. , Eisenreich , S. J. and Lioy , P. J. 1999 . Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan . Atmospheric Environment , 33 : 5071 – 5079 . [CROSSREF] [CSA]
- Smith , D. J. T. , Harrison , R. M. , Luhana , L. , Pio , A. C. , Castro , M. L. , Tariq , N. M. , Hayat , S. and Quraishi , T. 1996 . Concentrations of particulate airborne polycyclic aromatic hydrocarbons and metals collected in Lahore, Pakistan . Atmospheric Environment , 30 : 4031 – 4040 . [CROSSREF] [CSA]
- Tsai , P. -J. , Shih , T. -S. , Chen , H. -L. , Lee , W. -J. , Lai , C. -H. and Liou , S. -H. 2004 . Assessing and predicting the exposures of polycyclic aromatic hydrocarbons (PAHs) and their carcinogenic potencies from vehicle engine exhaust to highway toll station workers . Atmospheric Environment , 38 : 333 – 343 . [CROSSREF] [CSA]
- Tsapakis , M. and Stephanou , E. G. 2005 . Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: study of sources and ambient temperature effect on the gas/particle concentration and distribution . Environmental Pollution , 133 : 147 – 156 . [INFOTRIEVE] [CROSSREF] [CSA]
- WHO/IPCS (World Health Organization/ International Programme on Chemical Safety) . 1998 . Environmental health criteria 202, selected non-heterocyclic polycyclic aromatic hydrocarbons , Geneva : World Health Organization .
- UNEP (United Nations Environment Programme)/UNDP/DUTCH joint project on environmental law and Institutions in East Africa . 1999 . Report on the development and harmonization of environmental standards in East Africa http://www.unep.org/padelia/publications/VOLUME2preface.htmAvailable at:
- USEPA (U.S. Environmental Protection Agency) . 1996 . http://www.epa.gov/SW-846Method 3541 and 3630C. Available at:
- Yunker , M. B. , Macdonald , R. W. , Vingarzan , R. , Mitchell , R. H. , Goyette , D. and Sylvestre , S. 2002 . PAH in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source composition . Organic Geochemistry , 33 : 489 – 515 . [CROSSREF] [CSA]
- Yunker , M. B. , Snowdon , L. R. , Macdonald , R. W. , Smith , J. N. , Fowler , M. G. , Skibo , D. N. , Mclaughlin , F. A. , Danyushevskaya , A. I. , Petrova , V. I. and Ivanov , G. I. 1996 . Polycyclic aromatic hydrocarbon composition and potential sources for sediment samples from the Beaufort and Barents Seas . Environmental Science and Technology , 30 : 1310 – 1320 . [CROSSREF] [CSA]
![Figure 4 Plot of isomer ratios B[a]A/(B[a]A + Chy) versus IP/(IP + B[ghi]P).](/cms/asset/ae3c1a32-4da8-44cf-8a7f-c74aaee0595b/uenf_a_166684_o_f0004g.gif)