This research aimed to locate and characterize emission sources for airborne volatile organic compounds (VOCs) inside a petrochemical plant in Chiayi, Taiwan. Air was sampled with canisters at 20 sites inside this plant, twice per quarter, and analyzed by gas chromatography/mass spectrometry following the TO–14 method. Data were interpreted by means of a database and contour maps. Alkanes were most abundant species, followed by aromatics, and both accounted for 98% of total airborne VOCs. Two emission sources were located around the filling unit and the aromatic unit. The characterization of VOCs from emission sources were elucidated in table after cross-analyzing and statistical calculation using Microsoft Excel.
Introduction
Vehicle exhaust, especially in the urban area, was the greatest emission source of volatile organic compounds (VOCs), accounting for about one-half to two-thirds of airborne VOCs (CitationFujita, 2001). However, VOCs emitted from petroleum refineries and petrochemical industries, second to the vehicle exhaust, were another concern of increasing importance. The VOCs from a petrochemical complex was reported to be 4- to 20-fold higher in concentration than those at suburban sites (CitationCetin et al., 2003; CitationKalabokas et al., 2001; CitationNa et al., 2001; CitationÖstermark, 1995; CitationTsai et al., 1996). CitationSiegell (1998) pointed out that more than 80% of VOCs came from 20% of equipment components. Therefore, locating emission sources is the first step to abate VOC emissions.
In general, a widely used method to detect possible leakage and the rate of VOC emission is monitoring all components one by one; however, it is uneconomical in terms of labor and money (CitationBrandão, 1994). To locate the emission sources more quickly, the downwind concentrations were inverted through an atmospheric dispersion model to reconstruct the VOCs distribution (CitationLehning et al., 1994). Point Origin of Nuisance Gases (PONG)-2, a Gaussian plume modeling technique, has been used to map possible odor sources (CitationTapper et al., 1991). The contour map was employed to locate emission sources and a large number of data were managed and interpreted by using database or Geographic Information System (GIS) (CitationLi et al., 1995; CitationPuliafito et al., 2003; CitationSiegell, 1996).
A chemical mass balance model using multiple linear least-square regressions has been widely used for identification and source apportionment of VOCs. It was observed that evaporative emission from refueling stations, hot soak vehicles, and asphalt roads, dominated in Mumbai, India; in contrast, emissions from diesel internal combustion engines were found to be the dominant source of VOCs in Delhi, India (CitationSrivastava, 2004; CitationSrivastava et al., 2005). Based on chemical mass balance simulation, the source apportionment of airborne VOCs in the ozone non-attainment region in southern Taiwan were passenger cars (21–58%), motorcycles (9–24%), industrial sources (14–33%), and solvent application (13–46%) (CitationTsai et al., 2004). The principal component analysis/absolute principal component score (PCA/APCA) receptor model is another source appointment technique that requires a minimum of inputs regarding source characteristics but provides quantitative information regarding both source profiles and their impacts; this model has been applied to estimate source contributions to ambient VOCs in Hong Kong and eastern China, (CitationGuo et al., 2004a, Citation2004b; CitationSo and Wang, 2004).
This article discusses a survey of airborne VOCs inside a large petrochemical plant in Chiayi, Taiwan. This plant produces various organic solvents, such as hexane, benzene, toluene, xylene, petroleum ether, and solvents for varnish and rubber industries. Because this plant is situated within a densely populated city, its VOCs emission has been drawing nearby residents' attention over the years. To characterize and locate its possible VOC emission sources, the air was sampled with canisters at 20 selected sites inside the plant and all possible emitted VOCs were identified and quantified by gas chromatography/mass spectrometry (GC/MS). To elicit and interpret the data, the data was consolidated into a database using Microsoft Excel, by which data could be retrieved, sorted, arranged, cross-analyzed, and statistically calculated. The distribution of VOCs inside this plant was presented using Surfer Software (Golden Software, Inc., Golden, CO, USA) in terms of contour maps, which served to locate possible emission sources.
Materials and Methods
Sampling
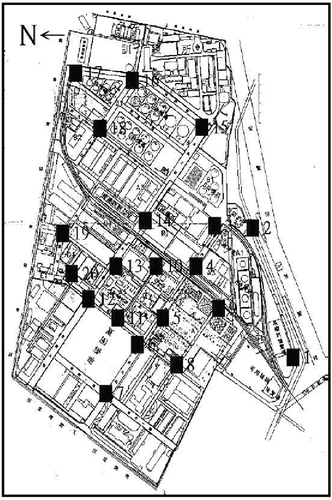
All units in this plant were categorized into process unit, tank farm, waste-disposal section, surroundings, and utility. Each unit was deployed with one sampling site, which remained unchanged over the survey period. A total of 20 sampling sites were situated inside the plant, as shown in and . Sampling height was about 1.5 meters above ground level, equivalent to the height of a human nose. The survey period was about 1 year, extending from July 2001 to June 2002. The sampling campaign was conducted twice per quarter (on August 31, September 20, November 12, and December 17, 2001, and February 19, March 13, April 17, and May 29, 2002). The weather on each sampling day was clear, and the sampling times were all within 10:30–2:30.
Table 1 Sampling sites in this study
All procedures followed the TO-14 method recommended by United States Environmental Protection Agency (US EPA). Samples were collected with 6-liter stainless steel canisters (Enteck, Inc., Simi Valley, CA, USA), with fused silica interior to ensure inertness to VOCs. Sampling duration was 3 hours. During sampling, each canister was fitted at the inlet with a flow restrictor (CS 1200E Passive Sampler, Enteck, Inc., USA) to maintain a constant sampling rate of 33 mL/min−1. In this way, it could level out the interference due to the fluctuating wind speed and wind direction.
Analysis
The setup for analysis consisted of a device called AUTOCAN (Tekmar, Inc., Cincinnati, OH, USA), which could autosample, cryoconcentrate, thermodesorb, and cryofocuse, and an HP 5890 gas chromatograph (GC) equipped with 5971 mass selective (MS) detector (Hewlett Packard, Inc., Palo Alto, CA, USA). In cryoconcentration, a sample of 400 mL was drawn at rate of 60 mL min−1 regulated by a mass flow regulator, and trapped in a stainless steel cartridge packed with Carbotrap C and Carbosieve maintained at –50°C by liquid nitrogen. The trapped VOCs were then thermally desorbed at 275°C into a cryofocuser maintained at −180°C, followed by heating to 200°C to vaporize the condensed analytes into GC/MS for separation and identification.
The separation was performed on a DB-1 fused-silica capillary column (60 m × 0.32 mm × 1 μm; J & W Scientific, Inc., Folsom, CA, USA). The GC oven was initially held at 5°C for 4 minutes, then programmed to 200°C at 6°C min−1 and held for 5 minutes. MS was operated in electron impact (EI), scan mode, with its scan range within 34–200 amu. Each species of the sampled VOCs was identified by comparing its mass spectrum with the corresponding one in the National Institute of Standards and Technology (NIST) mass spectra library. Precision, in terms of relative standard deviation, was within 12% from seven replicate analyses of the calibration gas mixture at 10 ppb, and the method detection limit was about 0.1 ppb for the trapped volume of 400 mL. More detail is provided in the report by CitationSin et al. (2001).
The certified gas standards, for TO-14 method and ozone precursor with each species at nominal concentration of 1 ppm, were purchased from Resteck, Inc., Bellefonte, PA, USA, and were used to prepare the calibration gas mixture by static dilution. The response factor for each species was determined by analyzing five calibration gas mixtures at concentrations of 5, 10, 20, 50, 200 ppb, respectively, for a trapped volume of 400 mL and taking their average. Concentration = peak area × response factor ÷ trapped volume (mL).
Database
The data were consolidated into an Excel database. The database contained fields such as plant name, sampling site, site number, site coordinate, sampling date, species, carbon number (Cn), type, and concentration. Excel provides some indigenous functions, such as sorting, arranging, simple retrieval, conditional retrieval, cross-analysis, mathematical computation, statistical calculation, and statistical plotting. The data could be retrieved through simple retrieval or conditional retrieval according to the condition set by a user and then through cross-analysis and statistical calculation, the result could be presented in table or graph such as bar chart or pie chart. The characterization of airborne VOCs from the emission source were depicted in table in terms of minimum, maximum, mean, standard deviation, and 90% confidence interval after each VOC species was arranged by type and subsequently by carbon number. The worst case could also be predicated in terms of the sum of maximums. The indigenous functions in Excel are powerful enough to serve this purpose. Validation was unnecessary because no macroprogramming was conducted.
Estimation of Unknown Values
It is impossible to obtain exhaustive data at every desired location in a plant because of practical constraints; thus, interpolation is necessary. Kriging is a popular regression technique to interpolate data, which predicts unknown values from data observed at known locations. This method uses a variogram to express the spatial variation and minimizes the error of predicted values, which are estimated by spatial distribution of the predicted values. Kriging is based on the assumption that the parameter being interpolated can be treated as a regionalized variable. A regionalized variable is intermediate between a truly random variable and a completely deterministic variable in that it varies in a continuous manner from one location to the next and therefore points that are near each other have a certain degree of spatial correlation, but points that are widely separated are statistically independent. Kriging is a set of linear regression routines that minimize estimation variance from a predefined covariance model (CitationIsaaks and Srivastava, 1989; CitationOliver and Webster, 1990).
The first step in kriging is to construct a variogram from the scattered point set to be interpolated. A variogram consists of two parts: an experimental variogram and a model variogram. In the following example, the value to be interpolated is denoted as F. The experimental variogram is found by calculating the variance of each point in the set with respect to each of the other points and plotting the variances versus distance between the points. Once the experimental variogram is computed, the next step is to define a model variogram. A model variogram is a simple mathematical function that models the trend in the experimental variogram. Once the model variogram is constructed, it is used to compute the weights used. The basic equation used is as follows: F(X,Y) = ∑ i = 1 n W i F i , in which n is the number of scatter points in the set, F i represents the values of the scatter points, and W i represents the weights assigned to each scatter point. For example, to interpolate at a point P based on the surrounding points P 1, P 2, and P 3, the weights W 1, W 2, and W 3 must be found. The weights are found through the solution of the simultaneous equations:
The equations are then solved for the weights W 1,W 2, and W 3. The F value of the interpolation point is then calculated as: F p = W 1 F 1+W 2 F 2+W 3 F 3. By using the variogram in this fashion to compute the weights, the expected estimation error is minimized in a least-squares sense. For this reason, kriging is sometimes said to produce the best linear, unbiased estimate (CitationIsaaks and Srivastava, 1989; CitationOliver and Webster, 1990).
Contour Map
The data, including abscissa, ordinate of each sampling site, and concentration, were retrieved from the database, then copied onto a Surfer worksheet and subsequently transformed into a grid-file by the kriging method, supported in Surfer. After a grid-file was processed to produce contour lines, the map of the plant was loaded into Surfer and then overlapped exactly under the contour lines to form a contour map, which could be used not only to map out the VOC distribution inside the plant, but also to locate the emission sources.
Results and Discussion
Abundant VOCs
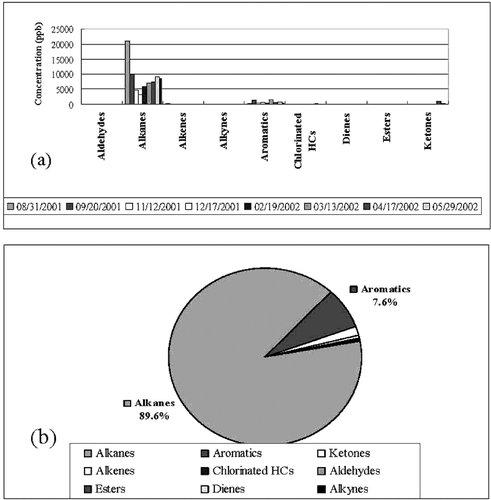
The VOCs emitted from this plant could be categorized into various types: alkanes, aromatics, alkenes, dienes, aldehydes, ketones, esters, and chlorinated hydrocarbons. The abundant VOCs were alkanes (89.6%), followed by aromatics (7.6%) (). The average concentration of alkanes was approximately ten times as high as that of aromatics, both of which accounted for more than 97% of total VOCs whereas the remainder accounted for less than 3% of total VOCs ().
Locating Emission Sources
By arranging the average concentrations of alkanes and aromatics at different sites, the dominant emission sources of alkanes were located around the filling unit and the aromatic unit (). The average concentration of alkanes was six times higher in the filling unit than in the aromatic unit. In contrast, the 18 other sites accounted for 6% of total alkanes. The primary emission sources of aromatics were also situated around the filling unit and the secondary source was around the aromatic unit, as was the same case with alkanes. The filling unit, however, were merely about 2.5 times as much in average concentration as the aromatic unit, and the other 18 sites contributing 32% to total aromatics. Obviously, the variance in the concentration for aromatics was much less than that for alkanes.
Table 2 Average concentration of alkanes and aromatics at each site
Although the possible VOC emission sources had been tracked down, it was unclear whether they were the same. A contour map for pollutants was an available tool to unravel this question, because it could show the geographic relation of pollutants with positions inside the plant. In terms of contour maps, the possible area for the emission source(s) to hide could be mapped out. Conversely, the chemical mass balance model or PCA/APCS receptor model was an improper approach in such a case because definite emission sources were unknown from the surveyed data; these models could not be used until definite emission sources were identified.
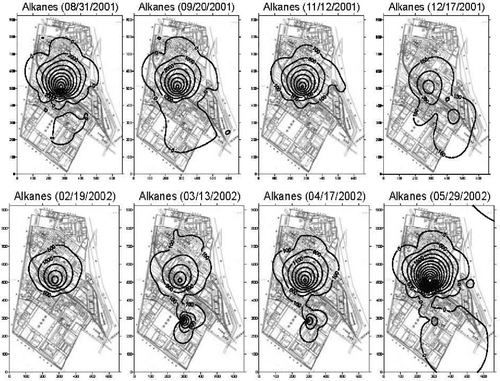
From eight contour maps for alkanes, the primary emission source usually lay around the filling unit (). The filling unit, through a field survey, consisted of two subunits: one outdoor filling station for filling solvent products into tank trunks and another indoor filling chain for bottling products. The secondary emission source sometimes appeared around the aromatic unit, only three times in eight surveys. The secondary emission source, without enough surveys, might have been missed.
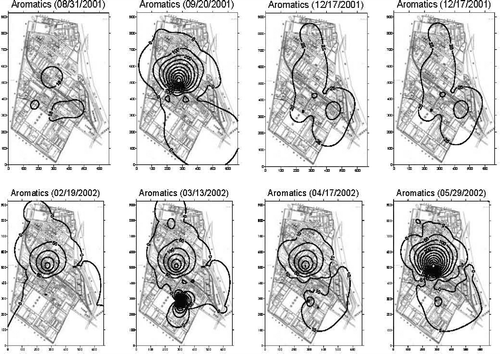
The contour maps for aromatics also show a similar tendency (). Even though the contour profile varied slightly with the sampling date, possibly due to the fluctuation in wind direction, wind speed, temperature, and humidity, the potential emission sources almost remained unchanged around the same place. Judging from these contours, these two emission sources were not the same and had highly different emission intensities; this difference could be further confirmed by the characterization of emitted VOCs.
Characterization of VOCs Emitted from Filling Unit
From the environmental and hygienic standpoints, the characterization of VOCs emitted was imperative either to take measures for reclamation or to estimate adverse impacts on the health of workers. Through these eight surveys, the authors of this article could gain an insight into the characterization of VOCs. By means of cross-analysis and statistical calculation, the information, such as minimum, maximum, average concentration, and standard deviation could be determined, as listed in . As many as 150 species were detected during eight surveys. The data for VOCs with a maximum concentration less than 20 ppb were excluded due to limitation in content, but benzene, toluene, ethylbenzene, and xylenes (BTEX) were retained in light of their great toxicity. presents the range and confidence interval for each species. Taking the benzene concentration around the filling unit as an example, the range of concentration was between minimum and maximum, namely, 3–39 ppb, and the 90% confidence interval was Average of ± 1.645 S.D./√8, namely, 12 ± 7.6 ppb. The emission in the worst case could be predicted through the sum of maximum values. For example, at the filling unit, the heaviest emission for alkanes might peak at about 24,000 ppb and aromatics at 1,000 ppb; at the aromatic unit, alkanes might peak at 5,000 ppb and aromatics at 770 ppb.
Table 3 Characterization of VOCs emitted from the primary pollution source (filling unit) and secondary pollution source (aromatic unit)
Included in the miscellaneous category were only three alkenes, including pentene, 3-hexene, 3-methyl-2-hexene; one ketone, acetone; one ester, ethyl acetate; and one chlorinated hydrocarbon, methylene chloride (). The VOCs were eight types, including alkanes, aromatics, alkenes, dienes, aldehydes, ketons, esters, and chlorinated hydrocarbons. Alkanes represented 94% of total VOCs and aromatics 5%, whereas the remainder was only about 1% (). Most alkanes were branched members, with a few linear and cyclic members. The most abundant alkanes were C6 members, accounting for approximately 58% of total alkanes, the second were C7 members, about 31%, and the third were C5 members, about 8%. The heaviest alkane was undecane.
As for aromatics, the maximum concentrations of benzene, toluene, and xylenes were 39 ppb, 150 ppb, 47 ppb, respectively, which were far below the local regulatory standards set by Taiwan EPA (i.e., benzene regulated at 500 ppb, toluene at 2,000 ppb, and xylenes at 2,000 ppb). C8 Aromatic isomers included ethylbenzene, styrene, and xylenes. In contrast, C9 and C10 aromatics included only alkylbenzene isomers, but no benzyl alkenes such propenylbenzene. C9 aromatics contributed to roughly one-half of total aromatics, C7 to one-fourth, and C9 to one-seventh (). Nevertheless, as far as a single aromatic was concerned, toluene was most abundant and accounted for about one-fourth of total aromatics.
The top ten abundant species () in decreasing order were: 2-methylpentane, hexane, methylcyclopentane, 3-methylpentane, 3-methylhexane, heptane, 2-methylhexane, 2,3-dimethylbutane, 2,4-dimethylpentane, and 2,2,3-trimethylbutane. They all were alkanes, representing about 70% of total VOCs.
Characterization of VOCs Emitted from Aromatic Unit
As previously discussed, the aromatic unit had also been identified as the secondary emission source. Although data for as many as 100 species were initially found, only about 40 species were left after omitting those with maximum concentration below 20 ppb (). The VOCs included eight types: alkanes, aromatics, alkenes, dienes, aldehydes, ketones, esters, and chlorinated hydrocarbons. Only a ketone, namely acetone, is listed. Alkanes represent 85% of total VOCs and aromatics represent 14%, whereas, the rest represent less than 1%. Alkanes were mostly composed of branched members, in addition to cyclic and a few linear members. The most abundant alkanes were C8 species (33% of total alkanes), the second most abundant were C7 species (30%), and the third were C6 species (23%).
As for aromatics, the maximum concentrations of benzene, toluene, and xylenes were 8 ppb, 414 ppb, 170 ppb, respectively, which were still far below local regulatory standards; this was the same case with the filling unit. All aromatic isomers were alkylbenzene isomers. C7 Aromatics contributed about one-half of total aromatic; C8, one-fourth; and C9, one-fifth, whereas for aromatics, toluene was the most abundant and approximated one-half.
The top ten abundant species in decreasing order were: octane, heptane, 2-methylbutane, 3-methylhexane, toluene, 1-ethyl-3-methylcyclopentane, 2-methylpentane, 2-methylheptane, 1,3-dimethylcyclohexane, and 2,2-dimethylpentane. Except toluene, all were alkanes, accounting about 44% of total VOCs.
Effect of Process and Vehicle Exhaust on Airborne VOCs
Regarding the processes in this plant, naphtha fractionated from crude oil is catalytically reformed in a refinery in this company to increase the content of aromatics up to 20–30% and then the reformed product, termed reformate, is sent to this plant as raw materials. The reformate consists of 70–80% aliphatics and 20–30% aromatics, without alkenes present. At the aromatic unit in this plant, aromatics are extracted from the reformate by diethyl glycol ether, and, subsequently, aliphatic moiety are left in raffinate. The extract, containing more aromatics and less aliphatics, is used as feedstock for production of BTEX, and high aromatic solvents for varnishes and insecticides. This fact was responsible for the reason why toluene was one of the top ten abundant species around the aromatic unit.
The raffinate with more aliphatics and less aromatics is pumped to the aliphatic unit and used as feedstock for production of pentane, hexane, heptane, cleaning naphtha, multi-purpose mineral spirit, dry-cleaning solvent, petroleum ether, and solvents for varnish, paint, and rubber. Because only physical treatments such as separation and blending involved in all processes, these researches inferred that airborne VOCs were similar in composition to the feedstock. As indicated in , VOCs emitted from around the filling unit consisted of 89% alkanes, 9% aromatics, and 2% miscellaneous species, and VOCs from the aromatic unit were 84% alkanes, 14% aromatics, and 1% miscellaneous species, which was in fair agreement with the feedstock in terms of composition.
Because this plant is situated in the middle of a densely populated city, vehicle exhaust is likely to interfere with the result of sampling outside the plant. To avoid this interference, the sampling sites were all deployed inside in this plant. In addition, as shown in and , both emission sources were located inside the plant, about 80–100 m from the nearest road. Obviously, the emission sources originated from this plant, not from outside vehicle exhaust. In contrast, comparison of airborne VOCs with vehicle exhaust in Taiwan also showed that they were distinctly different in composition from each other (CitationTsai et al., 2004). As shown in , the vehicle exhaust comprises less alkanes (41–63%) and more aromatics (29–50%), but VOCs emitted from this plant include more alkanes (85–94%) and less aromatics (5–15%). Moreover, there were scarcely alkenes in VOCs emitted from this plant versus 5–10% alkenes in vehicle exhaust. These differences further suggest that the airborne VOCs did come from emission sources inside this plant.
Table 4 Comparison of airborne VOCs from emission sources with those from vehicle exhaust
Conclusions
This article elucidated an approach to locate VOC emission sources and to characterize airborne VOCs emitted from these sources by database and contour maps. Consolidated into a database, the data became easily retrieved, statistically analyzed, and clearly presented in tables and graphs. Through cross-analysis and statistical computation, data showed that the most abundant VOCs were alkanes and the second most abundant were aromatics; both of these accounted for more than 98% of total VOCs. Results from the statistical analysis suggested that there were two emission sources, the filling unit and the aromatic unit. The contour maps of alkanes and aromatics further confirmed this fact. The VOCs were characterized to gain an insight into the attribute of each emission source. According to both the locations of emission sources and the comparison with vehicle exhaust, in terms of composition the airborne VOCs did originate from inside this plant.
Acknowledgements
The authors are grateful to Chinese Petroleum Corporation (CPC) in Taiwan for providing financial assistance and experimental apparatus. The authors also thank the staff of the Department of Environmental and Biotechnology in CPC for their cooperation in sampling and analysis.
Notes
*A) Process unit, B) Tank farm, C) Waste disposal section, D) Surroundings, E) Utility. CPI-corrugated plate interceptor.
*Benzene, toluene, ethyl benzene, and xylenes are all included in table due to their toxicity and significance.
References
- Brandão , R. F. 1994 . Monitoring of fugitive emissions in petrochemical plant . Water Science and Technology , 29 : 125 – 133 . [CSA]
- Cetin , E. , Odabasi , M. and Seyfioglu , R. 2003 . Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery . Science of the Total Environment , 312 : 103 – 112 . [INFOTRIEVE] [CROSSREF] [CSA]
- Fujita , E. M. 2001 . Hydrocarbon source apportionment for the 1996 Paso del Norte Ozone study . Science of the Total Environment , 276 : 171 – 184 . [INFOTRIEVE] [CROSSREF] [CSA]
- Guo , H. , Wang , T. and Louie , P. K.K. 2004a . Source apportionment of ambient non-methane hydrocarbons in Hong Kong: Application of a principal component analysis/absolute principal component scores (PCA/APCA) receptor model . Environmental Pollution , 129 : 489 – 498 . [INFOTRIEVE] [CROSSREF] [CSA]
- Guo , H. , Wang , T. , Simpson , I. J. , Blake , D. R. and Yu , X. M. 2004b . Source contributions to ambient VOCs and CO at a rural site in eastern China . Atmospheric Environment , 38 : 4551 – 4560 . [CROSSREF] [CSA]
- Isaaks , E. H. and Srivastava , R. H. 1989 . An Introduction to Applied Geostatistics , Oxford University Press .
- Kalabokas , P. D. , Hatzianestis , J. , Bartzis , J. G. and Papagiannakopoulos , P. 2001 . Atmospheric concentrations of saturated and aromatic hydrocarbons around a Greek oil refinery . Atmospheric Environment , 5 : 2545 – 2555 . [CROSSREF] [CSA]
- Lehning , M. , Chang , P. Y. , Shonnard , D. R. and Bell , R. L. 1994 . An inversion algorithm for determining area-source emissions from downwind concentration measurements . Journal of the Air and Waste Management Association , 44 : 1204 – 1213 . [INFOTRIEVE] [CSA]
- Li , R. , Karell , M. and Boddy , J. W. 1995 . “ Develop a plant-wide air emissions inventory ” . In Chemical Engineering Progress 96 – 103 . March
- Na , K. , Kim , Y. P. , Moon , K. C. , Moon , I. and Fung , K. 2001 . Concentrations of volatile organic compounds in an industrial area of Korea . Atmospheric Environment , 35 : 2747 – 2756 . [CROSSREF] [CSA]
- Oliver , M. A. and Webster , R. 1990 . Kriging: A method of interpolation for geographical information system . International Journal of Geographical Information Systems , 4 : 313 – 332 . [CSA]
- Östermark , U. 1995 . Characterization of volatile hydrocarbons emitted to air from a cat-cracking refinery . Chemosphere , 30 : 1813 – 1817 . [CROSSREF] [CSA]
- Puliafito , E. , Guevara , M. and Puliafito , C. 2003 . Characterization of urban air quality using GIS as a management system . Environmental Pollution , 122 : 105 – 117 . [INFOTRIEVE] [CROSSREF] [CSA]
- Siegell , J. H. 1996 . “ Solve plant odor problems ” . In Chemical Engineering Progress 35 – 41 . January
- Siegell , J. H. 1998 . “ Monitor your fugitive emissions correctly ” . In Chemical Engineering Progress 33 – 38 . November
- Sin , D. W. , Wong , Y. C. , Sham , W. C. and Wang , D. 2001 . Development of an analytical technique and stability evaluation of 143 C3–C12 volatile organic compounds in summa canisters by gas chromatography/mass spectrometry . Analyst , 126 : 310 – 321 . [INFOTRIEVE] [CROSSREF] [CSA]
- Srivastava , A. 2004 . Source apportionment of ambient VOCS in Mumbai city . Atmospheric Environment , 38 : 6829 – 6843 . [CROSSREF] [CSA]
- Srivastava , A. , Sengupta , B. and Dutta , S. A. 2005 . Source apportionment of ambient VOCS in Delhi city . The Science of the Total Environment , 343 : 207 – 220 . [INFOTRIEVE] [CROSSREF] [CSA]
- So , K. L. and Wang , T. 2004 . C3-C12 Non-methane hydrocarbons in subtropical Hong Kong: Spatial-temporal variations, source-receptor relationships and photochemical reactivity . Sci. of the Tol. Environ. , 328 : 161 – 174 . [CROSSREF] [CSA]
- Tapper , N. J. and Sudbury , A. W. 1991 . Mapping odor sources from complaint statistics I: Identifying the major source . Journal of the Air and Waste Management Association , 41 : 433 – 441 . [CSA]
- Tsai , J. H. , Hsu , Y. C. and Yang , J. Y. 2004 . The relationship between volatile organic profiles and emission sources in ozone episode region—A case study in southern Taiwan . Science of the Total Environment , 328 : 131 – 142 . [INFOTRIEVE] [CROSSREF] [CSA]
- Tsai , J. H. , Sheu , Y. C. , Lee , D. Z. and Lin , S. J. 1996 . Airborne aromatic volatile organics in the vicinity of a refinery complex during operation and hot-standby modes . Journal of Environment Science and Health, Part A Environmental Science , 31 : 463 – 477 . [CSA]