Abstract
Background: The resistance profiles for patients on first-line antiretroviral therapy (ART) regimens after viremia have not been well studied in community clinic settings in the modern treatment era.
Objective: To determine time to viremia and the ART resistance profiles of viremic patients.
Methods: HIV-positive patients aged ≥16 years initiating a three-drug regimen were retrospectively identified from 01/01/06 to 12/31/12. The regimens were a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs) and a third agent: a protease inhibitor (PI), non-nucleoside reverse transcriptase inhibitor (NNRTI), or an integrase inhibitor (II). Time to viremia was compared using a proportional hazards model, adjusting for demographic and clinical factors. Resistance profiles were described in those with baseline and follow-up genotypes.
Results: For 653 patients, distribution of third-agent use and viremia was: 244 (37%) on PIs with 80 viremia, 364 (56%) on NNRTIs with 84 viremia, and 45 (7%) on II with 11 viremia. Only for NNRTIs, time to viremia was longer than PIs (p = 0.04) for patients with a CD4 count ≥200 cells/mm3. Of the 175 with viremia, 143 (82%) had baseline and 37 (21%) had follow-up genotype. Upon viremia, emerging ART resistance was rare. One new NNRTI (Y181C) mutation was identified and three patients taking PI-based regimens developed NRTI mutations (M184 V, M184I, and T215Y).
Conclusions: Time to viremia for NNRTIs was longer than PIs. With viremia, ART resistance rarely developed without PI or II mutations, but with a few NRTI mutations in those taking PI-based regimens, and NNRTI mutations in those taking NNRTI-based regimens.
Introduction
Antiretroviral therapy (ART) has greatly changed the course of HIV infection over the past 25 years. The evolution from the era of mono- and dual-therapy to triple combination therapy in 1996 has significantly reduced AIDS-related morbidity and mortality,Citation1−Citation5 perinatal transmission,Citation6 and onward HIV transmission.Citation7−Citation11 Current guidelines for the use of antiretrovirals (ARVs) in adults and adolescents recommend the use of a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs) and a third agent: either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor boosted with ritonavir (PI/r), or an integrase inhibitor (II).Citation12, 13
Primary concerns for clinicians and patients are viremia, the development of drug resistance, and the possible transition from the state of viremia to virologic failure. The causes of viremia for patients on first-line ART regimens include the periodic release of HIV from latently infected cells or sites inadequately accessed by ART Citation14, 15 as well as through immune activation due to immunization, for example. Citation16, 17 Other factors, although with conflicting evidence reviewed by Ryscavage et al. (2006), Citation18 are related to ART treatment and adherence and include suboptimal adherence,Citation18, 19 treatment initiation during late stage diagnosis,Citation18, 20, 21 the type of ARV regimen particularly in those with high baseline plasma viral load, Citation22 and the presence of baseline drug resistance.Citation22−Citation28 Screening for baseline ARV resistance is a standard of care today and a determinant of successful first-line therapies.Citation12, 29−Citation31 The association between adherence and the development of drug resistance differs dramatically across ARV classes.Citation24, 25, 28, 32−Citation34 For example, slight differences in the chemical structure of PIs lead to primary mutations conferring cross-resistance to several PIs.Citation35 Interestingly, such mutations contribute rarely to viremia resulting in virologic failure and few or no mutations are detected in patients failing their first PI-based regimen.Citation36 This high genetic barrier translates into a requirement for the accumulation of multiple mutations before their antiviral activity is negatively affected by resistance.Citation37, 38 Ritonavir-boosted lopinavir requires three to four mutations for resistance and even more mutations for ritonavir-boosted darunavir.Citation30, 39−Citation43 Thus, PIs offer long-term viral suppression and in the event of poor adherence, rarely lead to the development of resistance and maintain a wide selection of options for salvage therapies.Citation40
In contrast to PIs, first-generation NNRTIs have a low genetic barrier to resistance such that a single-point mutation often confers a high degree of cross-resistance within the NNRTI class.Citation44, 45 The long half-life of NNRTIs could result in viral replication in the presence of suboptimal plasma ARV levels, if all drugs are stopped enabling the selection of NNRTI-resistant viruses.Citation35, 46−Citation48 In standard first-line NNRTI-based regimens, the NNRTI mutations, K103 N and Y181C have been linked to 50–70% of virologic failure.Citation49 The K103 N mutation emerging most commonly in response to efavirenz confers cross-resistance to efavirenz, nevirapine, and delavirdine.Citation50, 51 In contrast, Y181C developed in response to nevirapine and confers cross-resistance to nevirapine and delavirdine and in some cases, efavirenz.Citation52−Citation54 Another commonly emerging resistance mutation, E138 K, has been shown to confer cross-resistance to other NNRTIs (i.e. etravirine, efavirenz, and nevirapine) in half of the individuals receiving rilpivirine.Citation55−Citation57 Minority NNRTI-resistant variants, not detected by standard genotypic testing, were also present in treatment-experienced individuals with virologic failure.Citation58
II mutations have emerged with evidence of variable levels of cross-resistance, whereby raltegravir mutations have cross-resistance with elvitegravir and with some also having an impact on dolutegravir. Specifically, the common resistance-associated mutations were: Y143C/H/R, N155H, and Q148H/K/R in the absence of secondary II mutations.Citation59−Citation62 The genetic barrier of the IIs, raltegravir and elvitegravir, to resistance have been shown to be lower than that of PIs and a bit more than NNRTIs, as two mutations are often required for resistance. Dolutegravir, on the other hand, has a higher genetic barrier with R263 K (clade B) or G118R (clade C) and H51Y being the only unique mutations.Citation59−Citation61
The purpose of this retrospective cohort study was to determine whether time to viremia for patients differed between the three standard first-line ART regimens, which included a backbone of two NRTIs, with either a (1) NNRTI, (2) PI, or (3) II. This cohort study also assessed if the resistance profiles of those experiencing viremia differed.
Methods
Study design and setting
The Maple Leaf Medical Clinic (MLMC), founded in 2001, is an urban multi-specialty community clinic in Toronto, Ontario, Canada providing primary and specialty care to approximately 6,900 persons including 2,677 HIV-positive patients. A retrospective clinical chart review of HIV-positive patients was done using information collected and retained within the MLMC Electronic Medical Record system (EMR). The EMR contains demographic and clinical characteristics of MLMC patients collected since 1 July 2005. Using the MLMC EMR, HIV-positive patients were retrospectively selected for analysis.
Participants
Patients were included if they: were aged 16 or over; started a three-drug first-line ART regimen (with two NRTIs and a third agent being either a NNRTI, PI, or II) between 1 January, 2006 and 31 December 2012;and remained on ART for at least 14 days and had at least one viral load test within 6 months of starting ART. Patients were excluded if: baseline viral load was less than 40 copies/mL (as some patients could have been transferred from other clinics on ART); ARV was within a clinical trial; or having discontinued a third-agent ARV class or added a new ARV prior to day 14 on their regimens.
Variables
Demographic variables collected from the MLMC EMR were: age, gender, and HIV risk factors. Clinical variables collected were: time since HIV diagnosis, baseline viral load, baseline CD4 count, ART regimen (i.e. NRTI backbone and a third agent, NNRTI, PI, or II), hepatitis B and C serostatus, and the number of viral load test from the first day of ARV up to and including the last day of follow-up. Viral load testing is typically carried out at MLMC monthly, after the start of ART until the viral load is undetectable, then every three–four months for two years, and then every four–six months, as per guidelines.Citation12, 13 Viral load testing in Ontario is done using Abbott’s m2000 RealTime™ System and RealTime™ HIV-1 assay (Abbott Molecular Inc., Mississauga, Ontario, Canada) as of 16 August 2010 and Siemens’ Versant® HIV-1 bDNA 3.0 Assay (Siemens, Oakville, Ontario, Canada) prior to this date. The primary outcome of viremia was defined for this study as either (1) no suppression by 6 months or (2) after suppression, two consecutive viral loads greater than 40 and below 200 copies/mL at least 14 days apart or one viral load greater than 200 copies/mL. Virologic suppression was defined as a viral load less than or equal to 50 copies/mL or 40 copies/mL (after 1 January 2011). The secondary outcome of interest is the resistance profile of viremic patients. Resistance testing at MLMC is carried out at the British Columbia Centre for Excellence in HIV/AIDS (Vancouver, British Columbia, Canada) using validated “in-house” methods.Citation28 Pre-treatment resistance testing has become standard for patients prior to starting ART; however, some are missed due to being transferred from another clinic. Follow-up resistance testing is conducted on any sample with a viral load greater than 200 copies/mL. The resistance profiles were described for each ARV class and further classified by drugs within the classes. Emergent mutations per patient by third agent and definition of viremia were also described.
Statistical methods
Descriptive analyses were reported as means and standard deviations or medians and interquartile ranges (IQR) for continuous variables and compared using the Kruskal–Wallis test or analysis of variance (ANOVA). Categorical variables were summarized using frequencies and proportions and Fisher’s exact test or the C test was used for comparisons. The log rank test was used to compare time to viremia between the PI, II, and NNRTI groups, and each pairwise comparison. There was no adjustment made for multiple comparisons since this is an explorative analysis. Proportional hazards models were used to evaluate if treatment group was associated with time to viremia. A multivariable proportional hazards model was adjusted for age, sex, baseline viral load, and count of viral load tests during follow-up. Variables with an unadjusted p-value < 0.05 were included in the multivariable model. Patients were censored if they: (1) were viremic by 31 December 2013; and if between 1 January 2006 to 31 December 2012 they: (2) died; (3) were lost to follow-up; (4) discontinued or switched third agent ARV class after 14 days of initiating treatment; or (5) did not have a second viral load available to determine if viremia occurred. Resistance profiles for NRTIs and third agents, NNRTI, PI, or II, were described in patients with viremia who had both baseline and follow-up genotypes. Variables in each analysis were stratified by CD4 count less than and greater or equal to 200 cells/mm3, where the former was suggestive of advanced HIV disease. Stratification by CD4 cell count was done to address channeling bias with patients with more advanced HIV disease more frequently starting a PI-based regimen. SAS version 9.3 (SAS Institute Inc., Cary, NC) was used for all analyses.
Missing data consisting of no dates and viral load were excluded from the analysis. Data from individuals who were loss to follow-up were included in the analysis up to the date of their last visit.
Results
Patient characteristics
There were 10,784 patients in the MLMC database with 3,801 being HIV positive. Of the 2,560 active patients living with HIV (i.e. not transferred, not dead, or loss to follow-up), 716 patients started on a standard first-line ART regimen containing two backbone NRTIs with an NNRTI, PI and II between 1 January 2006 and 31 December 2012 and stayed on it for more than 14 days. Fifty-six patients did not meet the inclusion criteria of having at least one follow-up viral load. Seven additional patients were excluded because they did not have a baseline CD4 cell count.
Of the 653 patients who met the study inclusion criteria, 91.5% with a CD4 count less than 200 cells/mm3 and 93.7% with a CD4 count greater or equal to 200 cells/mm3 were male with a mean age of 39.7 (SD = 8.8) and 38.8 (SD = 9.3), respectively, and a median follow-up period of 35.3 (IQR: 32.2–39.3) months. Distribution of third-agent use was: NNRTI 40.1% (n = 71), PI 57.6% (n = 102), and II 2.3% (n = 4) for patients with a CD4 count less than 200 cells/mm3. Distribution was: NNRTI 61.6% (n = 293), PI 29.8% (n = 142), and II 8.6% (n = 41) for those with a CD4 count greater or equal to 200 cells/mm3. Frequency of viral tests per year of follow-up (p = .004) and hepatitis B serostatus (p = .01) for patients with a CD4 cell count greater or equal to 200 cells/mm3 was significantly different between third-agent groups (Table ). The initial backbone medications were: emtricitabine with tenofovir, lamivudine with abacavir or zidovudine or tenofovir, or stavudine, and abacavir with tenofovir. Patient characteristics are summarized in Table .
Table 1 Baseline demographic and clinical characteristics stratified by CD4 counts less than and equal or greater than 200 cells/mm3
The majority of the patients received an efavirenz-based regimen (45.6%), next a ritonavir-boosted lopinavir-based regimen (15.0%), and thirdly, an atazanavir-based regimen (11.6%) (Table ). Third ARV agents and viremia distribution are summarized in Table . In brief, raltegravir was the only II investigated as a third agent; first-and-second generation NNRTIs (i.e. efavirenz, etravirine, nevirapine, and rilpivirine) were among the third agents studied and lastly, six PIs (i.e. atazanavir- and ritonavir-boosted atazanavir, ritonavir-boosted darunavir, ritonavir-boosted saquinavir, ritonavir-boosted lopinavir, nelfinavir,and ritonavir-boosted fosamprenavir) (Table ).
Table 2 Third antiretroviral agents and viremia distributions
Viremia and resistance analysis
The viremia rate was 23% (n = 84/364) for patients taking an NNRTI-based regimen, 33% (n = 80/244) for patients taking a PI-based regimen, and 24% (n = 11/45) for the II-based regimen (Table ). In the patients who experienced viremia, median time to viremia (months) was longer with NNRTIs (point estimate [PE]: 37.4, 95% CI: 32.5–43.4) compared to PIs (PE: 33.1, 95% CI: 25.1–39.3) and IIs (PE: 35.4, 95% CI: 25.1–42.5). There was no evidence that time to viremia with NNRTIs was different from IIs and IIs from PIs (data not shown). There was no difference in time to viremia between NNRTI and PI for patients with a CD4 cell count less than 200 cells/mm3 (p = .08) (Figure A), whereas there was a significant difference in time to viremia between NNRTI and PI (p = .016) (Figure B) and between NNRTI, PI, and II (p = .04) (Figure C) for patients with a CD4 cell count greater or equal to 200 cells/mm3. Using PI treatment as the reference group and adjusting for age, sex, baseline CD4 count, hepatitis B serostatus, baseline viral load, and count of viral load test during follow-up, the significant aHRs for viremia were: 2.11 [95% CI: 1.32–3.37] for baseline viral (Log10) for patients with a CD4 count less than 200 cells/mm3; and for patients with a CD4 count greater or equal to 200 cells/mm3, 1.43 [95% CI: 1.06–1.93] for baseline viral load (Log10) and 0.61 [95% CI: 0.41–0.91] for NNRTI. The unadjusted and adjusted Cox proportional hazards models are presented in Table .
Figure 1 Time to viremia compared by antiretroviral classes; integrase inhibitor (II), non-nucleoside reverse transcriptase inhibitor (NNRTI), and protease inhibitor (PI) for patients with a CD4 count less than 200 cells/mm3 (A) and greater or equal to 200 cells/mm3 (B and C).
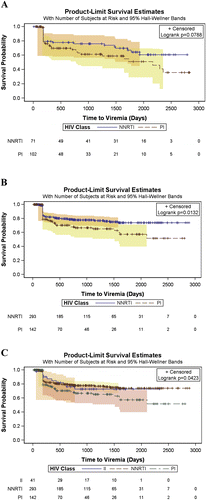
Table 3 Unadjusted and adjusted Cox proportional hazard models for viremia
Of the 653 patients, 86% (n = 565) had a baseline genotype. Among these patients, 175 had viremia of which 82% (n = 143) had a baseline genotype and 21% (n = 37) had a follow-up genotype (Table ). Among those with a follow-up genotype, 46% (n = 17) were on a PI-based regimen, 49% (n = 18) on an NNRTI-based regimen, and 5% (n = 2) on an II-based regimen (Table ). The NNRTI resistance mutation, Y181C, was detected in one person on an NNRTI-based regimen who experienced no virologic suppression at 6 months and had CD4 count greater or equal to 200 cells/mm3. Three persons on a PI-based regimen had the following NRTI mutation patterns each; M184 V + T215Y after viremia with one viral load greater than 200 copies/mL and a CD4 count greater or equal to 200 cells/mm3 and M184 V and M184 V/I with no virologic suppression at 6 months in patients with a CD4 count less than or greater or equal to 200 cells/mm3, respectively (Table ).
Table 4 New mutations per patient experiencing viremia by class and type of viremia
Discussion
Among patients accessing care in an urban community clinic setting, time to viremia was significantly delayed among persons who started a first-line NNRTI-based regimen compared to those who started a first-line PI-based regimen. In those experiencing viremia, ART resistance rarely developed with no PI mutations, but a few NRTI mutations in those taking PI-based regimens, and NNRTI mutations in those taking an NNRTI-based regimen.
Our results show that time to viremia was significantly longer among persons with a CD4 cell count greater or equal to 200 cells/mm3, following an NNRTI-based regimen compared to those on a PI-based regimen. Other studies have similarly shown longer time to viremia and specifically virologic failure for persons on an NNRTI-based regimen compared to a ritonavir-boosted PI-based regimen and that low level rebound occurred less frequently in patients on an NNRTI-based regimen compared to other regimens.Citation63−Citation70 Also, 45.8% of the patients in our study were on an efavirenz-based regimen and the literature has shown that efavirenz-based regimen exhibits improved immunological and virological outcomes as well as greater adherence compared to nevirapine, boosted PI, and single PI-based regimens.Citation70 Other reasons may also include that NNRTIs have a longer half-life than PIs and in the event of less than perfect adherence, levels of NNRTI may still be adequate.Citation35, 46−Citation48, 71 An example of this has been demonstrated in the FOTO pilot study where a short cycle treatment interruption strategy (i.e. five-days-on (FO) and two-days-off (TO)) demonstrated that virologic suppression was durable for patients on an efavirenz- and nevirapine-based regimens.Citation72, 73 Also, patients with lower CD4 count presumed poorer adherence and higher viral load was placed on a PI-based regimen due to the high genetic barrier and the multiple mutations that are required for resistance. As such, we stratified our findings by baseline CD4 counts less than 200 cells/mm3 as suggestive of advanced HIV disease and greater than or equal to 200 cells/mm3 to account for this channeling bias. In our study, only a few patients were on an II-based regimen (n = 45) and all of them restricted to raltegravir precluding us from drawing comparisons regarding time to viremia with patients on either a PI- or NNRTI-based regimen.
Several factors have previously been described as leading to viremia and include as previously mentioned release of HIV from latently infected cells or sites not readily accessed by ART, immune activation, suboptimal adherence, type of ARV regimen, stage of infection prior to initiating ART, and baseline drug resistance.Citation14, 15, 18−Citation28 We showed an association between viremia and increasing baseline viral load for both patients with a CD4 count less than 200 cells/mm3 and patients with CD4 counts greater or equal to 200 cells/mm3. Careful monitoring of viremia is required since several studies have also demonstrated persistent low-level viremia is associated with increased risk of virologic failure.Citation63, 74, 75
In our study, major mutations were not reported in HIV-positive patients following an II-based regimen. In contrast, in patients following NNRTI- and PI-based regimens, NRTI resistance mutations were described. The discriminatory NRTI resistance mutations, M184 V and M184 V/I, were each identified in two patients following a PI-based regimen. The NRTIs mutations, M184 V/I, are the most common NRTI mutations and are selected by lamivudine and emtricitabine, which confer cross-resistance to abacavir and didanosine and susceptibility to tenofovir and zidovudine.Citation35, 37, 76−Citation84 Lamivudine in the MLMC cohort is part of four of the five backbone regimens. The NRTI thymidine analog mutation T215Y was also identified among a HIV-positive patient taking a PI-based regimen who also had a M184 V mutation. T125Y is selected only by zidovudine- and stavudine-based regimens and confer cross-resistance to tenofovir, abacavir, and didanosine.Citation82−Citation84 Few patients in the MLMC cohort are on a regimen, which includes stavudine (n = 4) and zidovudine (n = 21). The mutational pattern of M184 V and T215Y is one of the most common combinations and is associated with a high level of resistance against NRTIs.Citation35, 37, 76−Citation84 Also in this cohort, a HIV-positive patient who experienced viremia after taking an NNRTI-based regimen had the NNRTI Y181C mutation found. The latter mutation develops in response to nevirapine with cross-resistance to delavirdine and in some cases, efavirenz.Citation35, 54, 84 Three out of the four mutations described were present in patients with a CD4 count greater or equal to 200 cells/mm3 and on a PI-based regimen. A previous study in British Columbia with treatment of naïve patients without baseline resistance showed that 19% (38/196) of patients had a least one drug resistance mutation at their first low-level viremia affecting NRTIs and NNRTIs Citation85 and other studies have similarly showed low risk of PI-resistant mutations at low-level viremiaCitation86, 87 and important major NRTI (e.g. M184 V and T215Y) and NNRTI (e.g. Y181C) resistance mutations at the first low-level viremia of individuals primarily on PI-based regimens.Citation88
Virologic suppression at six months was not attained in a person taking the NNRTI-based regimen with an Y181C mutation and in two patients following PI-based regimens exhibiting M184 V/I mutations. As such, major resistance mutations and viremia in these patients are suggestive of a failure of the ART regimen to suppress HIV. In a patient with a viral load greater or equal to 200 copies/mL, the mutational pattern of M184 V and T215Y was described. Viremia and major resistance mutations in these patients may be indicative of low-level viremia, requiring further monitoring to prevent transition to virologic failure. There were few major mutations present among the patients. Despite this, viremia was reported for 2 (18.2%) patients on an II-based regimen, 18 (20.7%) patients on an NNRTI-based regimen, and 17 (21.0%) patients following a PI-based regimen. Identified causes for viremia such as the existence of minority variants not detected by genotypic testing, poor adherence, or other variables were not characterized in our study.
A previous cohort study in British Columbia (1996–1999) detected ARV resistance mutations in approximately 25% (n = 298/1191) of their cohort, where the majority of patients were receiving nevirapine (n = 288, 96.6%) followed by efavirenz (n = 8, 2.7%).Citation28 Factors associated with drug resistance mutations included high baseline viral load and high (80–89%) to perfect (≥95%) refill prescriptions.Citation28 In our study, the majority of the patients were taking an efavirenz-based regimen (45.8%), followed by a ritonavir-boosted lopinavir-based regimen (15.0%), and thirdly an atazanavir-based regimen (12.1%). Both nevirapine (Y181C) and efavirenz (K103 N) are associated with major resistance mutations. The lower rate of resistance mutations in our study may reflect the fact that in contrast to the BC cohort, which took place at the beginning of the new treatment era consisted of both nevirapine and efavirenz mostly versus the current era with a shift toward efavirenz. In addition, modern day regimens as in our study may be more resistant to the development of resistance mutations.
A major study limitation is that the data were derived from a convenience sample. One possible source of selection bias is that patients are required to have a viral load within six months and to be engaged in care. The higher frequency of men in the analysis is reflective of the population in care at MLMC, which consists primarily of a gay male patient population. The results may also only be generalizable to viremia and viral suppression in persons living with HIV who are accessing care in specialized HIV clinics in urban areas. Possible unobserved confounding variables shown in other studies to influence viremia and viral suppression are substance use and ART adherence which were not reported for our cohort.Citation89, 90 Other possible unobserved confounders are factors involving the selection of a regimen by the patient and physician. Persons initiating PI-based regimens may have different characteristics from those initiating an NNRTI-based regimen prior to the start of treatment. Also, channeling bias may be an issue since patients with presumed poorer adherence and higher viral load are placed on a PI-based regimen which we addressed by stratifying our population by CD4 cell counts less than and greater or equal to 200 cells/mm3. Most patients with a CD4 cell count less than 200 cells/mm3 in this study were on a PI-based regimen (n = 102, 57.6%), followed by a NNRTI-based regimen (n = 71, 40.1%), and lastly only a few were on an II-based regimen (n = 4, 2.3%). In contrast, most patients with a CD4 count greater or equal to 200 cells/mm3 were on a NNRTI-based regimen (n = 293, 61.6%), followed by a PI-based regimen (n = 142, 29.8%), and lastly an II-based regimen (n = 41, 8.6%). Findings regarding patients on an II-based regimen were restricted to those on raltegravir. Another important study limitation is that the resistance profile is limited to a small number of patients experiencing viremia with both baseline and follow-up genotype testing. Lastly, our study did not differentiate between intermittent versus persistent low-level viremia. This is important to further understand the clinical significance of our findings since the evidence implicates persistent low-level viremia among those on treatment to have an increased risk of virologic failure.Citation21, 91, 92
Evidence from our retrospective chart review suggests that among HIV-positive patients accessing specialized HIV care in an urban setting, ART resistance rarely develops after starting first-line therapy. Despite only few cases of ART resistance, it is important to consider the clinical significance of resistance on viremia and salvage therapies. Future research needs to investigate the newer ART regimens and agents and an assessment of their virologic and resistance outcomes in cases where viremia occurs in the clinic setting.
References
- Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2(4):a007161.
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–1730.
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860.10.1056/NEJM199803263381301
- Vittinghoff E, Scheer S, O’Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination Antiretroviral Therapy and Recent Declines in AIDS Incidence and Mortality. J Infect Dis. 1999;179(3):717–720.10.1086/jid.1999.179.issue-3
- Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293.
- Mofenson LM, Lambert JS, Stiehm ER, Bethel J, Meyer WA 3rd, Whitehouse J, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341(6):385–393.10.1056/NEJM199908053410601
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365(6):493–505.10.1056/NEJMoa1105243
- Dieffenbach CW, Fauci AS. Universal Voluntary Testing and Treatment for Prevention of HIV Transmission. JAMA. 2009;301(22):2380–2382.10.1001/jama.2009.828
- Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–536.10.1016/S0140-6736(06)69162-9
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929.10.1056/NEJM200003303421303
- Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649.10.1136/bmj.b1649
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed June 23, 2015.
- Battegay M, Mulcahy F, Geretti AM. European AIDS Clinical Society (EACS) Guidelines - ART of HIV-positive persons. http://www.eacsociety.org/files/guidelines-7.1-english.pdf. Accessed May 18, 2015.
- Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–3884.10.1073/pnas.0800050105
- Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, et al. Differences in HIV Burden and Immune Activation within the Gut of HIV‐Positive Patients Receiving Suppressive Antiretroviral Therapy. J Infect Dis. 2010;202:1553–1561.10.1086/653022
- Staprans SI, Hamilton BL, Follansbee SE, Elbeik T, Barbosa P, Grant RM, et al. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med. 1995;182:1727–1737.10.1084/jem.182.6.1727
- Stanley SK, Ostrowski MA, Justement JS, Gantt K, Hedayati S, Mannix M, et al. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334:1222–1230.10.1056/NEJM199605093341903
- Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. 2014;58:3585–3598.10.1128/AAC.00076-14
- Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis. 2007;196(12):1773–1778.10.1086/527364
- Di Mascio M, Markowitz M, Louie M, Hurley A, Hogan C, Simon V, et al. Dynamics of Intermittent viremia during highly active antiretroviral therapy in patients who initiate therapy during chronic versus acute and early human immunodeficiency virus type 1 infection. J Virol. 2004;78(19):10566–10573.10.1128/JVI.78.19.10566-10573.2004
- Sungkanuparph S, Groger RK, Overton ET, Fraser VJ, Powderly WG. Persistent low-level viraemia and virological failure in HIV-1-infected patients treated with highly active antiretroviral therapy. HIV Med. 2006;7(7):437–441.10.1111/hiv.2006.7.issue-7
- Jones LE, Perelson AS. Transient viremia, plasma viral load, and reservoir replenishment in HIV-infected patients on antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;45(5):483–493.10.1097/QAI.0b013e3180654836
- Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353(9156):863–868.
- Gallego O, de Mendoza C, Perez-Elias MJ, Guardiola JM, Pedreira J, Dalmau D, et al. Drug resistance in patients experiencing early virological failure under a triple combination including indinavir. AIDS. 2001;15(13):1701–1706.10.1097/00002030-200109070-00014
- Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, Perry S, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17:1925–1932.10.1097/00002030-200309050-00011
- Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37:1112–1118.10.1086/378301
- Cheung PK, Wynhoven B, Harrigan PR. 2004: which HIV-1 drug resistance mutations are common in clinical practice? AIDS Rev. 2004;6:107–116.
- Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, et al. Predictors of HIV drug‐resistance mutations in a large antiretroviral‐naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339–347.10.1086/jid.2005.191.issue-3
- Clotet B. Strategies for overcoming resistance in HIV-1 infected patients receiving HAART. AIDS Rev. 2004;6:123–130.
- Di Giambenedetto S, Bacarelli A, Pinnetti C, Colafigli M, Prosperi M, Gatti G, et al. Genotypic resistance to lopinavir and fosamprenavir with or without ritonavir of clinical isolates from patients failing protease inhibitors-containing HAART regimens: Prevalence and predictors. Scand J Infect Dis. 2007;39:813–818.10.1080/00365540701367728
- Scherrer AU, Boni J, Yerly S, Klimkait T, Aubert V, Furrer H, et al. Long-Lasting protection of activity of nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) by boosted pi containing regimens. PLoS ONE. 2012;7:e50307.10.1371/journal.pone.0050307
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366.10.1097/00002030-200003100-00008
- Walsh JC, Pozniak AL, Nelson MR, Mandalia S, Gazzard BG. Virologic rebound on HAART in the context of low treatment adherence is associated with a low prevalence of antiretroviral drug resistance. J Acquir Immune Defic Syndr. 2002;30(3):278–287.10.1097/00126334-200207010-00003
- Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004;53(5):696–699.10.1093/jac/dkh162
- Antiretroviral Resistance in Clinical Practice. In: Geretti AM, ed. London: Mediscrit; 2006.
- Andreoni M, Perno CF. Positioning of HIV-protease inhibitors in clinical practice. Eur Rev Med Pharmacol Sci. 2012;16(1):10–18.
- Geretti AM, Easterbrook P. Antiretroviral resistance in clinical practice. Int J STD AIDS. 2001;12(3):145–153.10.1258/0956462011916938
- Harrigan PR, Alexander CS. Selection of drug-resistant HIV. Trends Microbiol. 1999;7:120–123.10.1016/S0966-842X(99)01467-5
- Arribas JR. Drugs in traditional drug classes (nucleoside reverse transcriptase inhibitor/nonnucleoside reverse transcriptase inhibitor/protease inhibitors) with activity against drug-resistant virus (tipranavir, darunavir, etravirine). Curr Opin HIV AIDS. 2009;4(6):507–512.10.1097/COH.0b013e328331b911
- Prosperi MC, Zazzi M, Punzi G, Monno L, Colao G, Corsi P, et al. Low rate of virological failure and maintenance of susceptibility to HIV-1 protease inhibitors with first-line lopinavir/ritonavir-based antiretroviral treatment in clinical practice. J Med Virol. 2010;82(12):1996–2003.10.1002/jmv.v82:12
- Poveda E, de Mendoza C, Martin-Carbonero L, Corral A, Briz V, Gonzalez-Lahoz J, et al. Prevalence of darunavir resistance mutations in HIV-1-infected patients failing other protease inhibitors. J Antimicrob Chemother. 2007;60:885–888.10.1093/jac/dkm276
- Pulido F, Arribas J, Hill A, Moecklinghoff C. No evidence for evolution of protease inhibitor resistance from standard genotyping, after three years of treatment with darunavir/ritonavir, with or without nucleoside analogues. AIDS Res Hum Retroviruses. 2012;28(10):1167–1169.10.1089/aid.2011.0256
- Wensing AM, van Maarseveen NM, Nijhuis M. Fifteen years of HIV Protease Inhibitors: raising the barrier to resistance. Antiviral Res. 2010;85(1):59–74.10.1016/j.antiviral.2009.10.003
- Perrin L, Telenti A. HIV treatment failure: testing for HIV resistance in clinical practice. Science. 1998;280:1871–1873.10.1126/science.280.5371.1871
- Sluis-Cremer N. Review the emerging profile of cross-resistance among the nonnucleoside HIV-1 reverse transcriptase inhibitors. Viruses. 2014;6:2960–2973.10.3390/v6082960
- Kunz A, Frank M, Mugenyi K, Kabasinguzi R, Weidenhammer A, Kurowski M, et al. Persistence of nevirapine in breast milk and plasma of mothers and their children after single-dose administration. J Antimicrob Chemother. 2009;63:170–177.
- Cunningham CK, Chaix ML, Rekacewicz C, Britto P, Rouzioux C, Gelber RD, et al. Development of resistance mutations in women receiving standard antiretroviral therapy who received intrapartum nevirapine to prevent perinatal human immunodeficiency virus type 1 transmission: a substudy of pediatric AIDS clinical trials group protocol 316. J Infect Dis. 2002;186(2):181–188.10.1086/jid.2002.186.issue-2
- Jackson JB, Becker-Pergola G, Guay LA, Musoke P, Mracna M, Fowler MG, et al. Identification of the K103 N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14:F111–115.10.1097/00002030-200007280-00001
- Miller MD, Margot NA, McColl DJ, Tran S, Coakley D, Cheng A. Genotypic and phenotypic characterisation of virologic failure through 48 weeks among treatment-naive patients taking tenofovir DF (TDF) or stavudine (d4T) in conjunction with lamivudine (3TC) or efavirenz (EFV). Paper presented at: Sixth International Congress on Drug Therapy in HIV Infection; November 17-21, 2002; Glasgow, United Kingdom.
- Deeks SG. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. J Acquir Immune Defic Syndr. 2001;26(Suppl 1):S25–33.10.1097/00126334-200103011-00004
- Delaugerre C, Rohban R, Simon A, Mouroux M, Tricot C, Agher R, et al. Resistance profile and cross-resistance of HIV-1 among patients failing a non-nucleoside reverse transcriptase inhibitor-containing regimen. J Med Virol. 2001;65:445–448.10.1002/(ISSN)1096-9071
- Casado JL, Moreno A, Hertogs K, Dronda F, Moreno S. Extent and importance of cross-resistance to efavirenz after nevirapine failure. AIDS Res Hum Retroviruses. 2002;18:771–775.10.1089/08892220260139503
- Hanna GJ, Johnson VA, Kuritzkes DR, Richman DD, Brown AJ, Savara AV, et al. Patterns of resistance mutations selected by treatment of human immunodeficiency virus type 1 Infection with zidovudine, didanosine, and nevirapine. J Infect Dis. 2000;181:904–911.10.1086/jid.2000.181.issue-3
- Antinori A, Zaccarelli M, Cingolani A, Forbici F, Rizzo MG, Trotta MP, et al. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res Hum Retroviruses. 2002;18:835–838.10.1089/08892220260190308
- Picchio G, Vingerhoets J, Tambuyzer L, Coakley E, Haddad M, Witek J. Short communication prevalence of susceptibility to etravirine by genotype and phenotype in samples received for routine HIV type 1 resistance testing in the United States. AIDS Res Hum Retroviruses. 2011;27:1271–1275.10.1089/aid.2011.0049
- Tambuyzer L, Vingerhoets J, Azijn H, Daems B, Nijs S, de Bethune MP, et al. Characterization of genotypic and phenotypic changes in HIV-1-infected patients with virologic failure on an etravirine-containing regimen in the DUET-1 and DUET-2 clinical studies. AIDS Res Hum Retroviruses. 2010;26(11):1197–1205.10.1089/aid.2009.0302
- Cohen CJ, Molina JM, Cahn P, Clotet B, Fourie J, Grinsztejn B, et al. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naive HIV-1-infected patients: pooled results from the phase 3 double-blind randomized ECHO and THRIVE trials. J Acquir Immune Defic Syndr. 2012;60:33–42.10.1097/QAI.0b013e31824d006e
- Halvas EK, Wiegand A, Boltz VF, Kearney M, Nissley D, Wantman M, et al. Low frequency nonnucleoside reverse-transcriptase inhibitor-resistant variants contribute to failure of efavirenz-containing regimens in treatment- experienced patients. J Infect Dis. 2010;201:672–680.
- Blanco JL, Varghese V, Rhee SY, Gatell JM, Shafer RW. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis. 2011;203(9):1204–1214.10.1093/infdis/jir025
- Mesplede T, Quashie PK, Wainberg MA. Resistance to HIV integrase inhibitors. Curr Opin HIV AIDS. 2012;7(5):401–408.10.1097/COH.0b013e328356db89
- Llibre JM, Pulido F, Garcia F, Garcia Deltoro M, Blanco JL, Delgado R. Genetic barrier to resistance for dolutegravir. AIDS Rev. 2014;17(1):56–64.
- Miller MM, Liedtke MD, Lockhart SM, Rathbun RC. The role of dolutegravir in the management of HIV infection. Infect Drug Resist. 2015;8:19–29.10.2147/IDR
- Geretti AM, Smith C, Haberl A, Garcia-Diaz A, Nebbia G, Johnson M, et al. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13:927–936.
- Smith CJ, Phillips AN, Hill T, Fisher M, Gazzard B, Porter K, et al. The Rate of Viral Rebound after Attainment of an HIV Load <50 Copies/mL According to Specific Antiretroviral Drugs in Use: Results from a Multicenter Cohort Study. J Infect Dis. 2005;192(8):1387–1397.10.1086/jid.2005.192.issue-8
- Initio Trial International Co-ordinating Committee. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet. 2006;368:287–298.
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3):251–260.10.1056/NEJMoa051871
- MacArthur RD, Novak RM, Peng G, Chen L, Xiang Y, Hullsiek KH, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomised trial. Lancet. 2006;368:2125–2135.10.1016/S0140-6736(06)69861-9
- Tashima K, Staszewski S, Nelson M, Rachlis A, Skiest D, Stryker R, et al. Efficacy and tolerability of long-term efavirenz plus nucleoside reverse transcriptase inhibitors for HIV-1 infection. AIDS. 2008;22:275–279.10.1097/QAD.0b013e3282f21b9d
- Bartlett JA, Johnson J, Herrera G, Sosa N, Rodriguez A, Liao Q, et al. Long-term results of initial therapy with abacavir and lamivudine combined with efavirenz, amprenavir/ritonavir, or stavudine. J Acquir Immune Defic Syndr. 2006;43:284–292.10.1097/01.qai.0000243092.40490.26
- Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21:1579–1589.10.1097/QAD.0b013e3281532b31
- Kuritzkes DR, Ribaudo HJ, Squires KE, Koletar SL, Santana J, Riddler SA, et al. Plasma HIV‐1 RNA dynamics in antiretroviral‐naive subjects receiving either triple‐nucleoside or efavirenz‐containing regimens: ACTG A5166s. J Infect Dis. 2007;195:1169–1176.10.1086/522475
- Cohen C, Colson A. Durable suppression possible with FOTO treatment schedule in subjects on nevirapine-based regimens. HIV Clin Trials. 2007;8(4):256.10.1310/hct0804-256
- Cohen CJ, Colson AE, Sheble-Hall AG, McLaughlin KA, Morse GD. Pilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) Study. HIV Clin Trials. 2007;8:19–23.10.1310/hct0801-19
- Greub G, Cozzi-Lepri A, Ledergerber B, Staszewski S, Perrin L, Miller V, et al. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS. 2002;16:1967–1969.10.1097/00002030-200209270-00017
- Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57:1489–1496.10.1093/cid/cit529
- Zaccarelli M, Perno CF, Forbici F, Cingolani A, Liuzzi G, Bertoli A, et al. Using a database of HIV patients undergoing genotypic resistance test after HAART failure to understand the dynamics of M184V mutation. Antivir Ther. 2003;8:51–56.
- Eron JJ, Benoit SL, Jemsek J, MacArthur RD, Santana J, Quinn JB, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. North American HIV working party. N Engl J Med. 1995;333:1662–1669.10.1056/NEJM199512213332502
- Gu Z, Gao Q, Li X, Parniak MA, Wainberg MA. Novel mutation in the human immunodeficiency virus type 1 reverse transcriptase gene that encodes cross-resistance to 2’,3’-dideoxyinosine and 2’,3’-dideoxycytidine. J Virol. 1992;66(12):7128–7135.
- Tisdale M, Kemp SD, Parry NR, Larder BA. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3’-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci U S A. 1993;90(12):5653–5656.10.1073/pnas.90.12.5653
- Schuurman R, Nijhuis M, van Leeuwen R, Schipper P, de Jong D, Collis P, et al. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). Journal of Infectious Diseases. 1995;171(6):1411–1419.10.1093/infdis/171.6.1411
- Winters MA, Shafer RW, Jellinger RA, Mamtora G, Gingeras T, Merigan TC. Human immunodeficiency virus type 1 reverse transcriptase genotype and drug susceptibility changes in infected individuals receiving dideoxyinosine monotherapy for 1 to 2 years. Antimicrob Agents Chemother. 1997;41(4):757–762.
- Whitcomb JM, Parkin NT, Chappey C, Hellmann NS, Petropoulos CJ. Broad nucleoside reverse‐transcriptase inhibitor cross‐resistance in human immunodeficiency virus type 1 clinical isolates. The Journal of Infectious Diseases. 2003;188(7):992–1000.10.1086/jid.2003.188.issue-7
- Wolf K, Walter H, Beerenwinkel N, Keulen W, Kaiser R, Hoffmann D, et al. Tenofovir resistance and resensitization. Antimicrob Agents Chemother. 2003;47(11):3478–3484.10.1128/AAC.47.11.3478-3484.2003
- D’Aquila RT, International AS-USA, Schapiro JM, Brun-Vezinet F, Clotet B, Conway B, et al. Drug Resistance Mutations in HIV-1. Top HIV Med. 2002;10(5):21–25.
- Gonzalez-Serna A, Woods C, Li J, Harrigan R, Swenson L. Low-level HIV viremia and drug resistance. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA.
- Taiwo B, Gallien S, Aga E, Ribaudo H, Haubrich R, Kuritzkes DR, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis. 2011;204(4):515–520.10.1093/infdis/jir353
- Nettles RE, Kieffer TL, Simmons RP, Cofrancesco J Jr, Moore RD, Gallant JE, et al. Genotypic resistance in HIV‐1–infected patients with persistently detectable low‐level viremia while receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39(7):1030–1037.10.1086/cid.2004.39.issue-7
- Li JZ, Gallien S, Do TD, Martin JN, Deeks S, Kuritzkes DR, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother. 2012;56(11):5998–6000.10.1128/AAC.01217-12
- Cescon A, Kanters S, Brumme CJ, Lepik kJ, Forrest JI, Hull M, et al. Trends in plasma HIV-RNA suppression and antiretroviral resistance in British Columbia, 1997–2010. J Acquir Immune Defic Syndr. 2014;65(1):107–114.10.1097/QAI.0b013e3182a8efc3
- Rodríguez-Arenas MA, Jarrín I, Amo J, Iribarren JA, Moreno S, Viciana P, et al. Delay in the initiation of HAART, poorer virological response, and higher mortality among HIV-infected injecting drug users in Spain. AIDS Res Hum Retroviruses. 2006;22(8):715–723.10.1089/aid.2006.22.715
- Karlsson AC, Younger SR, Martin JN, Grossman Z, Sinclair E, Hunt PW, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18(7):981–989.10.1097/00002030-200404300-00005
- Havlir DV, Bassett R, Levitan D, Gilbert P, Tebas P, Collier AC, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA. 2001;286(2):171–179.10.1001/jama.286.2.171
