Abstract
Rilpivirine (RPV) is a non-nucleoside reverse transcriptase inhibitor, which has better lipid profiles than efavirenz (EFV) in treatment naïve patients. However, the data on treatment experience are limited especially in dyslipidemic HIV patients; thus, we aimed to assess the change of lipid profiles after switching from EFV to RPV in these patients. In this prospective, open-label, cohort study, we enrolled HIV-1 infected adults who had received at least 6 months of EFV-based regimen, with HIV RNA <50 copies/mL for ≥6 months prior to switching. The objectives of this study were to analyze lipid changes and to evaluate the efficacy, safety, tolerability at 24 weeks after switching therapy. Fifty-three patients were enrolled and completed the study. At week 24, a significant decrease in the mean (95% confident interval, CI) total cholesterol (−28.06 mg/dL, 95%CI −35.20 to −20.91, p < 0.0001), LDL-cholesterol (−20.96 mg/dL, 95%CI −28.12 to −13.80, p < 0.0001), high-density lipoprotein (HDL)-cholesterol (−5.11 mg/dL, 95%CI −7.79 to −2.44, p < 0.0001), and triglyceride (−29.79 mg/dL. 95%CI −52.39 to −7.19, p = 0.011) levels were observed. One patient had virologic rebound with HIV RNA of 114 copies/mL at week 24. Three (5.7%) patients had grade 2 elevations of liver enzymes. None of the patients discontinued RPV during the study. Switching from EFV-based therapy to RPV-based regimen improved lipid profiles in fully suppressed HIV patients with dyslipidemia. This treatment should be considered in these patients.
Recent treatment guidelines for human immunodeficiency virus (HIV)-1 which recommends early initiation of highly active antiretroviral therapy has dramatically reduced HIV-associated morbidity and mortality, and has transformed HIV disease into a chronic, manageable condition.Citation1 With immune reconstitution and viral suppression, patients have a “return to health,” generally accompanied by an increase in the total cholesterol, low-density lipoprotein (LDL)-cholesterol, and triglycerides, which are cardiovascular risk factors. Antiretroviral regimen usually consists of two nucleoside analog reverse transcriptase inhibitors (NRTI) plus a third agent, such as protease inhibitor (PI), non-nucleoside analog reverse transcriptase inhibitor (NNRTI), or integrase strand transfer inhibitors.Citation1 Among the NNRTIs, efavirenz (EFV), once daily, has high potency and tolerability and is recommended as the preferred third agent in some guidelines as its high efficacy has been demonstrated in many clinical trials.Citation2 However EFV is associated with adverse neurological and psychiatric events, rashes, and increased serum lipid levels.Citation3,4
Rilpivirine (RPV), 25 mg once daily, is an US FDA-approved NNRTI in 2011.Citation5 In pooled THRIVE (TMC278 against HIV, in a once daily Regimen versus Efavirenz) and ECHO (Early Capture HIV Cohort) studies, treatment naïve adults infected with HIV-1, RPV demonstrated non-inferior efficacy to EFV at 48 and 96 weeks for HIV-1 RNA <50 copies/mL.Citation6,7 RPV was associated with smaller mean changes from baseline in total cholesterol and triglyceride levels than EFV. However, there was a greater increase from baseline in levels of HDL-cholesterol in the EFV group, with no change in total cholesterol/HDL-cholesterol ratio between groups. Mills et al. conducted a 48-week, phase 2b, open-label, multicenter study evaluating the efficacy and safety of switching from EFV to RPV in HIV-infected patients using emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF)-based regimen in 49 patients.Citation8 At week 48, 93.9% of subjects remained suppressed and virological failure occurred with no emergence of resistance. At week 12, there were significant decreases in the fasting total cholesterol, LDL-cholesterol, and triglycerides, and these changes persisted through to week 48.
Management of dyslipidemia in HIV-infected people involves a comprehensive approach that includes lifestyle counseling (diet, exercise, and appropriate weight), selection of antiretroviral drugs that do not exacerbate dyslipidemia, modification of antiretroviral, and (when necessary) utilization of lipid-lowering agents such as statin agents and fibrates.Citation9 Currently, there is limited data on switching from EFV to RPV in dyslipidemic HIV patients.
The primary objective of this study was to analyze lipid changes in HIV patients with dyslipidemia at 24 weeks after switching from EFV to RPV. A secondary objective includes evaluating the efficacy of RPV in maintaining HIV-1 RNA <50 copies/mL after 24 weeks of treatment, and evaluating the safety and tolerability after switching from EFV to RPV.
Patients and Methods
This is a prospective, open-label, 24-week cohort study, conducted at Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand, from June 2014 through to February 2015. Eligible subjects included HIV-1 infected adults (≥18 years of age) who had received at least 6 months of EFV-based regimen therapy, and who had HIV RNA level of <50 copies/mL for ≥6 months prior to switching and were diagnosed dyslipidemia.
Dyslipidemia was defined as either (1) LDL-cholesterol ≥130 mg/dL in the presence of at least one of the coronary heart disease (CHD) risk factors: age > 45 years among males or age > 55 years among females, hypertension (BP ≥ 140/90 mm Hg or on antihypertensive medication), current cigarette smoking, family history of premature CHD and/or diabetes; (2) LDL-cholesterol ≥ 160 mg/dL regardless of CHD risk factors or; (3) previously diagnosed of dyslipidemia and on lipid-lowering drugs. Exclusion criteria were receiving ongoing therapy with any proton pump inhibitors, rifamycin (rifampicin or rifabutin), systemic steroid, anticonvulsants (carbamazepine, oxcarbamazepine, phenobarbital, phenytoin), St John’s wort, alanine aminotransferase (AST) and aspartate aminotransferase (ALT) > 3 upper normal limit, significant psychiatric disorder, pregnancy or breast feeding. Modification of the 2 NRTIs backbone and lipid-lowering drugs were not allowed during the study. All patients gave written informed consent before participating. The study was approved by the Khon Kaen University Ethics Committee.
Subject evaluation was performed at baseline, weeks 12 and 24. The clinical laboratory testing was performed at a local laboratory. Laboratory tests included HIV RNA, absolute CD4 cell count (% CD4), lipid profiles (including total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides), ALT, AST, glucose, creatinine, hemoglobin, leukocyte and platelet counts in the fasted state. The safety of the study regimens was assessed using patient’s interview, medical history, physical examination and clinical laboratory test results.
Sample Size Calculation
Assuming a difference in change of LDL-cholesterol level of 15 mg/dL and a standard deviation (SD) of ±36 mg/dL (SD extrapolated from the THRIVE studyCitation7) with 80% power, and a 1-sided type I error of 0.05, inclusion of 44 patients was necessary; however, we estimated 20% of patients may be loss to follow-up. Therefore, the 53 patients were included into the study.
Statistical Analysis
The data were analyzed using SPSS version 17. Categorical data were expressed by proportions. The continuous data were presented as means (SD, or 95% confidence interval) or median (range) as appropriate. Comparison between values before and after changing the drug were performed using the paired t-test for lipid profiles and the Wilcoxon signed-rank test for ALT, AST, and CD4 cell counts. P value <0.05 was considered significant.
Results
A total of 53 HIV-infected patients with dyslipidemia were enrolled in the study and completed the study visits through 24 weeks. Table summarizes the baseline characteristics for all subjects. Majority of subjects were male (71%), the mean age was 50 years, and mean body mass index was 23.5 kg/m2. Median (range) duration of diagnosis HIV infection was 93 (13–240) months. Subjects received EFV with the mean duration of 71.1 months before switching to RPV. The most common NRTI backbones was tenofovir/emtricitabine (TDF/FTC) 45 (84.9%), followed by TDF/lamivudine 4 (7.6%), zidovudine/lamivudine 3 (5.7%), and zidovudine/didanosine 1 (1.9%). Median (range) CD4 at baseline was 514 (129–1562) cells/μL, and HIV RNA <50 copies/mL in all subjects. CHD risks were hypertension (23.8%), diabetes mellitus (17%), and current smoking (13.2%).
Table 1 Demographics and baseline characteristics of subjects
Majority of the patients (69.8%) received lipid-lowering agents for their dyslipidemia before switching to RPV, which included simvastatin 20 (37.7%), atorvastatin 3 (5.7%), rosuvastatin 1 (1.9%), gemfibrozil 6 (11.3%), fenofibrate 2 (3.8%), and 5 (9.4%) subjects received a combination of simvastatin plus fenofibrate. Among 25 patients who were on simvastatin, most of them (64%) received only 10 mg/day. Sixteen subjects (30.2%) were on lifestyle modification without lipid-lowering medications.
Lipid Profiles
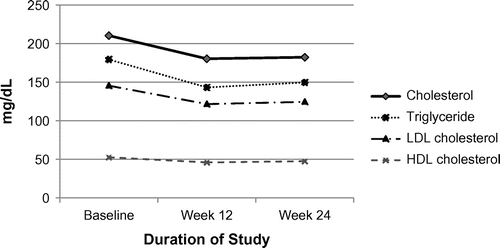
Lipid profile changes from baseline to weeks 12 and 24 are presented in Table . At week 24, significant decrease was seen in the means (95% confident interval, CI) of total cholesterol (−28.06 mg/dL, 95%CI −35.20 to −20.91, p < 0.0001), LDL-cholesterol (−20.96 mg/dL, 95% CI −28.12 to −13.80, p < 0.0001), HDL-cholesterol (−5.11 mg/dL, 95% CI −7.79 to −2.44, p < 0.0001), and triglyceride (−29.79 mg/dL. 95% CI −52.39 to −7.19, p = 0.011). The decrease in lipid profiles observed in week 12 was already significant. A decrease from baseline in the ratio of total cholesterol to HDL was observed at week 12 and week 24 but was not statistically significant (−0.19 mg/dL, 95%CI −0.39 to 0.01, p = 0.07). The changes in the means of total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides and cholesterol/HDL from baseline through to week 24 are shown in Figure .
Table 2 Mean change in fasting lipid parameters from baseline
Virologic and Immunological Outcomes
At baseline, all subjects had HIV RNA levels <50 copies/mL. Overall, 52 of 53 subjects (98.1%) showed HIV RNA levels <50 copies/mL after 24 weeks of switching. One patient had HIV RNA of 114 copies/mL, experienced virologic rebound under RPV therapy defined as an increase in HIV RNA levels above 50 copies/mL from baseline. This patient had a repeated HIV RNA test done on week 29 and showed 192 copies/mL. Median (range) CD4 at 24 weeks of study was 529 (152–1355) cells/μL. Median (range) CD4 changes from baseline to 24 weeks was 23 (−325 to 356) cells/μL, not statistically significant (p = 0.25).
Adverse Effects
ALT increased to grade 2 in 3 patients (5.7%) at week 24. One patient had normal baseline ALT and developed grade 2 at weeks 12 and 24. Another patient had grade 1 ALT at baseline, which increased to grade 2 at week 24. The third patient had grade 2 ALT at baseline, which sustained through to week 24. All of them were asymptomatic.
No subject developed rashes after switching to RPV. None of the patients discontinued RPV regimen.
Discussion
This study demonstrates the beneficial effects on lipid profiles in dyslipidemic HIV patients after switching from EFV to RPV in their treatment regimen. There were significant decreases in total cholesterol, LDL-cholesterol, and triglyceride levels in this study, which are risk factors for cardiovascular diseases. HDL-cholesterol was also significantly decreased, but the decrease in cholesterol to HDL-cholesterol ratio, also a risk factor for cardiovascular diseases, was not statistically significant. The changes in lipid profiles in our patients were not influenced by lipid-lowering agents since modification of medication was not allowed during study. Our findings were similar to other studies as virologically suppressed HIV patients showed favorable outcome of lipid parameters when switching to RPV-containing treatment regimen.Citation8,10–14 In those studies, the previous regimens before switching to RPV were EFV, nevirapine, PI, or integrase inhibitor-based regimens.
The major concern in switching therapies in virologically suppressed patients is virological failure. In prior clinical trials that evaluated the components of RPV/FTC/TDF among antiretroviral-naive participants, having baseline plasma HIV-1 RNA more than 100,000 copies/ml, were associated with higher rates of virologic failure and development of HIV resistance than EFV-based comparator therapy.Citation15–17 However, the switching to RPV in treatment experience studies did not show a higher virological failure.Citation12,18 Surgers et al. evaluated the efficacy and safety of switching to TDF, FTC, and RPV in 155 treatment- experienced patients by measuring ultrasensitive viral loads (US-VL) and showed that the percentage of patients with undetectable US-VL pre- and post-switch was the same.Citation19 Very few patients (1.9%) had virological failure while undergoing TDF/FTC/RPV.Citation19 This confirmed that virological suppression was maintained in treatment-experienced patients. In our study, only 1 patient experienced virologic rebound with HIV RNA of 114 copies/mL at week 24. A repeated HIV RNA on week 29 showed viral load of 192 copies/mL. Patient’s medical history was reviewed, the compliance for antiretroviral medication was good, no previous history of treatment failure, but he received influenza vaccine at week 20 and the 3rd dose of hepatitis B vaccine at week 24 (2 days prior to week 24 follow-up). This patient was closely observed to determine any association between virological failure and viral blip from immunological stimulation.
We observed grade 2 elevation of liver enzymes in only 3 (5.7%) patients. This data confirm the safety profile on liver enzymes of RPV regimen. Neuropsychiatric adverse effects were less common in subjects treated with RPV than in those treated with EFV in the THRIVE and ECHO studies.Citation15,16 A STaR (Single Tablet Regimen) study comparing the efficacy, safety, and tolerability of two STRs (RPV/FTC/TDF and EFV/FTC/TDF) also demonstrated more neuropsychiatric adverse effects when using EFV.Citation20
To the best of our knowledge, this is the first prospective study to investigate the effects of switching from EFV- to RPV-containing regimen in dyslipidemia patients, populations that are expected to benefit the most from this switch. However, there are several limitations in this study. It is a monocentric, single-arm study with a small sample size and an open-label trial, which may have an effect on patient’s bias and lifestyle modification. Further prospective randomized study with a larger population and longer duration of follow-up and more detailed evaluation of neuropsychiatric events should be performed.
Conclusions
RPV may be useful in EFV-experienced HIV patients with dyslipidemia. Our results show that switching to RPV was associated with positive changes in lipid profiles which may reduce coronary heart disease risks. This switching should be considered in these patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Faculty of Medicine, Khon Kaen University, Thailand [grant number IN58121].
References
- Günthard HF, Aberg JA, Eron JJ, et al; Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–425.10.1001/jama.2014.8722
- van Leth F, Phanuphak P, Ruxrungtham K, et al; Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–1263.10.1016/S0140-6736(04)15997-7
- Pérez-Molina JA. Safety and tolerance of efavirenz in different antiretroviral regimens: Results from a national multicenter prospective study in 1,033 HIV-infected patients. HIV Clin Trials. 2002;3:279–286.10.1310/3Q91-YT2D-BUT4-8HN6
- Squires K, Lazzarin A, Gatell JM, et al; Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004;36:1011–1019.10.1097/00126334-200408150-00003
- Drugs@FDA: FDA Approved Drug Products [Internet]. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. [Accessed August 29, 2015].
- Cohen CJ, Molina J-M, Cassetti I, et al; Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27:939–950.10.1097/QAD.0b013e32835cee6e
- Nelson MR, Elion RA, Cohen CJ, et al; Rilpivirine versus efavirenz in HIV-1–infected subjects receiving emtricitabine/tenofovir DF: Pooled 96-week data from ECHO and THRIVE studies. HIV Clin Trials. 2013;14:81–91.10.1310/hct1403-81
- Mills AM, Cohen C, DeJesus E, et al; Efficacy and safety 48 weeks after switching from efavirenz to rilpivirine using emtricitabine/tenofovir disoproxil fumarate–based single-tablet regimens. HIV Clin Trials. 2013;14:216–223.10.1310/hct1405-216
- Shalit P. Management of dyslipidemia in patients with human immunodeficiency virus. Rev Cardiovasc Med. 2014;15:S38–S46.
- Palella FJ, Fisher M, Tebas P, et al; Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS. 2014;28:335–344.10.1097/QAD.0000000000000087
- Pinnetti C, Di Giambenedetto S, Maggiolo F, et al; Simplification to co-formulated rilpivirine/emtricitabine/tenofovir in virologically suppressed patients: Data from a multicenter cohort. J Int AIDS Soc. 2014;17:19812.
- Gazaignes S, Resche-Rigon M, Yang C, et al; Efficacy and safety of rilpivirine-based regimens in treatment-experienced HIV-1 infected patients: a prospective cohort study. J Int AIDS Soc. 2014;17:19796.
- Rokx C, Verbon A, Rijnders BJA. Short communication: Lipids and cardiovascular risk after switching HIV-1 patients on nevirapine and emtricitabine/tenofovir-DF to rilpivirine/emtricitabine/tenofovir-DF. AIDS Res Human Retroviruses. 2015;31:363–367.10.1089/aid.2014.0278
- Cazanave C, Reigadas S, Mazubert C, et al; Switch to rilpivirine/emtricitabine/tenofovir single-tablet regimen of human immunodeficiency virus-1 RNA-suppressed patients, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales CO3 Aquitaine Cohort, 2012–2014. Open Forum Infect Dis. 2015;2: ofv018.
- Cohen CJ, Andrade-Villanueva J, Clotet B, et al; Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): A phase 3, randomised, non-inferiority trial. The Lancet. 2011;378:229–237.10.1016/S0140-6736(11)60983-5
- Molina J-M, Cahn P, Grinsztejn B, et al; Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): A phase 3 randomised double-blind active-controlled trial. The Lancet. 2011;378:238–246.10.1016/S0140-6736(11)60936-7
- Rimsky L, Van Eygen V, Hoogstoel A, et al; 96-week resistance analyses of rilpivirine in treatment-naive, HIV-1-infected adults from the ECHO and THRIVE Phase III trials. Antiviral Ther. 2013;18:967–977.10.3851/IMP2636
- Gantner P, Reinhart S, Partisani M, et al; Switching to emtricitabine, tenofovir and rilpivirine as single tablet regimen in virologically suppressed HIV-1-infected patients: A cohort study. HIV Med. 2015;16:132–136.10.1111/hiv.2015.16.issue-2
- Surgers L, Valin N, Viala C, et al; Evaluation of the efficacy and safety of switching to tenofovir, emtricitabine, and rilpivirine in treatment-experienced patients. J Acquir Immune Defic Syndr. 1999;2015:e10–e12.
- Cohen C, Wohl D, Arribas J, et al; Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected adults. AIDS. 2014;28:989–997.10.1097/QAD.0000000000000169