Abstract
Background: Boosted protease inhibitors (PIs), including ritonavir-boosted atazanavir (ATV/r), are a recommended option for the initial treatment of HIV-1 infection based upon clinical trial data; however, long-term real-life clinical data are limited.
Objective: We evaluated the long-term use of ATV/r as a component of antiretroviral combination therapy in the real-life setting in the REMAIN study.
Methods: This was an observational cohort study conducted at sites across Germany, Portugal, and Spain. Retrospective historical and prospective longitudinal follow-up data were extracted every six months from medical records of HIV-infected treatment-naïve patients aged ≥ 18 years initiating a first-line ATV/r-containing regimen.
Results: Eligible patients (n = 517) were followed up for a median of 3.4 years. The proportion remaining on ATV/r at 5 years was 51.5% with an estimated Kaplan-Meier median time to treatment discontinuation of 4.9 years. Principal reasons for discontinuation were adverse events (15.9%; 8.9% due to hyperbilirubinemia) and virologic failure (6.8%). The Kaplan-Meier probability of not having virologic failure (HIV-1 RNA < 50 copies/mL) was 0.79 (95% CI: 0.75, 0.83) at five years. No treatment-emergent major PI resistance occurred. ATV/r was generally well tolerated during long-term treatment with no significant changes in estimated glomerular filtration rate over five years.
Conclusions: In a real-life clinical setting over five years, treatment-naïve patients with HIV-1 infection initiating an ATV/r-based regimen showed sustained virologic suppression, an overall treatment persistence rate of 51.5%, an absence of treatment-emergent major PI resistance mutations at virologic failure, a long-term safety profile consistent with that observed in clinical trials, and no significant decline in renal function.
Introduction
Following the dramatic improvements in the survival of individuals with HIV-1 infection in the era of combined antiretroviral (ARV) therapy,Citation1 HIV-1 infection is now considered a chronic disease requiring lifelong control with ARV therapy. Thus, achieving durable virologic suppression with ARV regimens that are well tolerated is now of major importance.Citation2 However, data on long-term outcomes in patients receiving specific ARVs are limited.
Boosted protease inhibitors (PIs) possess a high genetic barrier to the development of viral resistance.Citation3 In a meta-analysis of clinical trials, initial treatment with a regimen containing a boosted PI as the third agent was associated with less resistance within and across drug classes compared with initial treatment with a non-nucleos(t)ide reverse-transcriptase inhibitor as the third agent.Citation4 These characteristics of boosted PIs have led to their inclusion as preferred agents in combination regimens for the initial treatment of HIV-1 infection in both USCitation5 and EUCitation6 treatment guidelines. Randomized controlled trials of up to four years’ duration in treatment-naïve patients with HIV-1 infection have shown that regimens containing ritonavir-boosted atazanavir (ATV/r) are an effective and well-tolerated treatment.Citation7−9 Although complementary real-life observational data evaluating ATV/r-based regimens are available,Citation10−12 these data are limited, and thus additional data with a longer follow-up duration and in different clinical settings are required.
The aim of the REMAIN observational cohort study was to evaluate long-term outcomes in ARV-naïve patients initiating ATV/r-containing regimens in a real-life clinical setting over a maximum follow-up period of five years.
Methods
Study design
The REMAIN study was a non-comparative, observational study conducted at a total of 51 sites across Germany, Portugal, and Spain (ClinicalTrials.gov identifier: NCT01236235). This study was conducted in accordance with the International Society for Pharmacoepidemiology Guidelines for Good Epidemiology Practices, the ethical principles originating from the Declaration of Helsinki, and local regulatory requirements. Local ethical committees approved the protocol, amendments, and the patient informed consent form. Written informed consent from all participants was obtained.
Patients
Consecutive patients attending the investigating center(s) with HIV-1 infection aged ≥ 18 years who were ARV treatment-naïve when an ATV/r-based regimen was initiated were selected for this study. The ATV/r-based regimen, which included at least two nucleos(t)ide reverse transcriptase inhibitors, must have been initiated between 1 February 2008 and 31 July 2010, and patients must have been seen in routine outpatient consultations between 1 January 2011 and 31 March 2012. Retrospective historical and prospective longitudinal follow-up data were extracted every six months from the medical records with follow-up continuing until 31 July 2013 (the protocol was amended in July 2012 to allow for a one-year extension to the follow-up period). Patients with more than four weeks of prior ARV exposure or who were participating in an atazanavir (ATV) clinical trial were excluded.
Study endpoints
Persistence on treatment
The primary endpoint was the proportion of patients remaining on an ATV/r-based regimen over time. Other endpoints examining treatment persistence included: (1) the probability of remaining on treatment and time to treatment discontinuation assessed using the Kaplan-Meier method; (2) baseline factors associated with treatment discontinuation, assessed using univariate and multivariate Cox proportional hazards models; and (3) the overall proportion of patients discontinuing treatment and reasons given by the investigator for treatment discontinuation.
Virologic and immunologic outcomes
Virologic and immunologic outcomes included: (1) time to virologic failure, defined as two consecutive HIV-1 RNA values of ≥ 50 copies/mL, or as one HIV-1 RNA value of ≥ 50 copies/mL followed by discontinuation, regardless of the reason, more than six months from ATV/r initiation (virologic failure was also analyzed using a ≥ 500 copies/mL cut-off); (2) the proportion of patients with virologic failure (as defined above) and its inverse, virologic response; (3) treatment-emergent new major or minor PI resistance mutations, defined using the International Antiviral Society–USA classification system,Citation13 assessed at the time of virologic failure; and (4) HIV-1 RNA changes and CD4 cell count changes over time.
Long-term safety and tolerability
The long-term safety and tolerability profile of ATV/r was assessed by the following parameters: (1) the frequency of adverse events (AEs) and AEs leading to discontinuation; (2) laboratory abnormalities graded according to the Division of AIDS system;Citation14 and (3) renal safety as measured by the frequency of renal-specific AEs (such as nephrolithiasis or renal failure) and by changes in glomerular function over time (assessed by calculating estimated glomerular filtration rate [eGFR] using the Modification of Diet in Renal Disease equationCitation15).
Statistical analysis
For the analysis of time-to-event data, the Kaplan-Meier method was considered to be the most appropriate technique. Patients with missing data or who were lost to follow-up were censored. Patients still participating in the study as of December 2012, but who did not provide their consent to continue participation in the one-year extension to the follow-up period during 2013, were considered as having discontinued ATV/r.
Baseline factors potentially associated with time to treatment discontinuation were investigated using Cox proportional hazards models. Gender, viral load at baseline (HIV-1 RNA < or ≥ 100,000 copies/mL), and co-infection with hepatitis B or C virus were entered into all models. Other factors, including country, age at initiation, disease duration, CD4 cell count, mode of HIV acquisition, and history of opportunistic infections, were preselected on the basis of a significance level of 0.2 in univariate Cox models provided excessive collinearity was not present. Pairwise interactions were tested in the final model and further selected for retention or exclusion by backward elimination.
For the statistical analysis of changes in HIV-1 RNA, CD4 cell counts, and eGFR over time, mixed-models repeated measures (MMRM) analysis was considered to be a more appropriate method for imputing missing data than the last observation carried forward (LOCF) method because MMRM generates estimates that are valid for most types of missing data, whereas LOCF does not.Citation16
The long-term safety profile of ATV/r treatment was evaluated by collecting data on reported AEs (as listed by study investigators without predefinition) and laboratory abnormalities. AE data were collected and analyzed using descriptive statistics (counts, proportions, and percentages) for all patients up to the point of ATV/r treatment discontinuation or, for those patients who remained on treatment at the end of the study, until the end of the follow-up period.
Results
Patients
Of 590 patients enrolled, 517 patients were eligible for inclusion in the study analyses. Details of patient disposition from enrollment to study analysis eligibility are summarized in Figure. Demographic and baseline characteristics are presented in Table . At baseline, median age was 40 years, the majority were men (76.0%), most patients were white (89.4%), and the route of transmission was predominantly sexual, with 16.9% acquiring HIV-1 infection from intravenous drug use. Centers for Disease Control and Prevention Class C AIDS was present in 18.8% of patients, HIV-1 RNA ≥ 100,000 copies/mL in 43.2%, CD4 count < 200 cells/mm3 in 38.2% and < 50 cells/mm3 in 10.4%, and hepatitis C virus co-infection in 18.6%. The majority of patients were receiving a fixed-dose combination of either tenofovir disoproxil fumarate/emtricitabine (TDF/FTC; 81.8%) or abacavir/lamivudine (ABC/3TC; 9.3%) as backbone ARV therapy. Patients were followed-up for a median period of 2.0 years (interquartile range [IQR]: 1.3 to 2.7) during the retrospective period and a median period of 1.5 years (IQR: 1.3 to 2.1) during the prospective period, giving a total median duration of exposure to ATV/r of 3.4 years (IQR: 1.8 to 4.3).
Figure 1 Patient disposition from enrollment to eligibility. ARV, antiretroviral; ATV/r, ritonavir-boosted atazanavir; NRTI, nucleos(t)ide reverse transcriptase inhibitors.
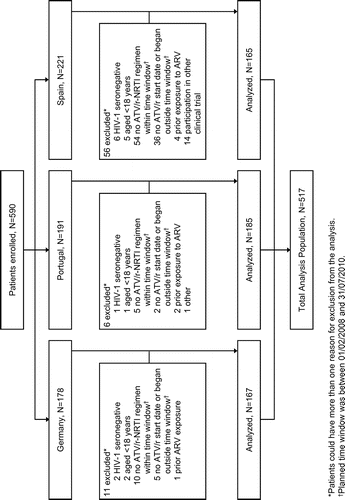
Table 1 Demographic and baseline characteristics
Persistence on treatment
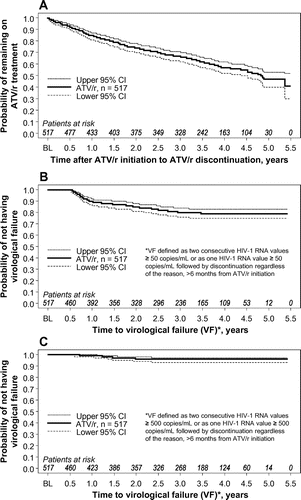
At the end of the follow-up period, after a mean follow-up duration of 3.1 (standard deviation: 1.5) years, a total of 264 patients (51.5%; 95% confidence interval [CI]: 47.0, 55.9) remained on an ATV/r-based regimen. Accounting for censored observations by survival analysis techniques (i.e. patients lost to follow-up and missing data), the probabilities of remaining on an ATV/r-based regimen were 0.85 (95% CI: 0.81, 0.87), 0.74 (95% CI: 0.70, 0.78), 0.67 (95% CI: 0.62, 0.71), 0.57 (95% CI: 0.52, 0.62), and 0.47 (95% CI: 0.40, 0.53) for the first, second, third, fourth, and fifth years, respectively (Figure A). The Kaplan-Meier estimated median time to treatment discontinuation was 4.9 years (95% CI: 4.5, upper limit non-estimable).
Figure 2 Time to discontinuation of treatment (A) and time to virologic failure using ≥ 50 copies/mL cut-off (B) and using ≥ 500 copies/mL cut-off (C). ATV/r, ritonavir-boosted atazanavir; BL, baseline; CI, confidence interval.
On univariate Cox proportional hazards analysis, country of origin and a history of opportunistic infection at baseline were associated with an increased risk of treatment discontinuation (Table ). On multivariate analysis, only country of origin remained significantly associated; specifically, patients from Germany had a reduced risk of treatment discontinuation versus those from Spain (Table ). None of the factors forced in the model (gender, baseline HIV-1 RNA level, or hepatitis B or C virus co-infection status) showed a statistically significant effect in the final model (p > 0.10).
Table 2 Univariate and multivariate Cox proportional hazards models for time to discontinuation of an ATV/r-based regimen
Overall, 223/517 patients (43.1%) had discontinued ATV/r by the end of the follow-up period for up to five years, with a lower rate in Germany than in Portugal or Spain (Table ). The most frequent reasons given by the investigator included AEs in 15.9% of patients, medical decision or regimen simplification in 9.5%, and virologic failure in 6.8% (Table ). Overall, 26/517 patients (5.0%) were lost to follow-up for up to five years and 27/517 patients (5.2%) did not provide consent to continue in the one-year extension period and were therefore considered as having discontinued ATV/r as per the protocol amendment.
Table 3 Reasons given by the investigator for discontinuation
Virologic and immunologic outcomes
Using the <50 copies/mL cut-off, the Kaplan-Meier estimated probabilities of not having virologic failure were 0.90 (95% CI: 0.86, 0.92), 0.85 (95% CI: 0.81, 0.88), 0.80 (95% CI: 0.76, 0.84), 0.79 (95% CI: 0.75, 0.83), and 0.79 (95% CI: 0.75, 0.83) for the first, second, third, fourth, and fifth years, respectively (Figure B). Using the <500 copies/mL cut-off, the Kaplan-Meier estimated probabilities of not having virologic failure were 0.99 (95% CI: 0.98, 1.00) for the first year, 0.96 (95% CI: 0.94, 0.98) for the second year, and 0.96 (95% CI: 0.93, 0.97) for the third, fourth, and fifth years (Figure C).
Eighty-five patients met the definition of virologic failure based upon the ≥50 copies/mL cut-off during the period of the study, representing a virologic response rate of 83.6%. At the time of virologic failure, 19 of these 85 patients (22.4%) had treatment-emergent minor PI resistance mutations, but no patient had treatment-emergent major PI resistance mutations.
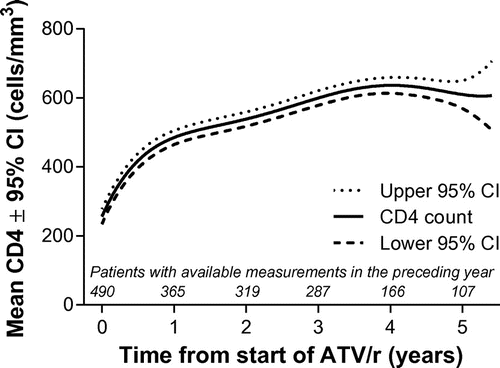
Mean HIV-1 RNA levels significantly decreased by 269,534 copies/mL (95% CI: 266,109, 272,960) over the first six months after ATV/r initiation (the associated proportion with HIV-1 RNA < 50 copies/mL at six months was 82.7%), with little change thereafter (MMRM with adjustment for baseline value, p < 0.0001). In parallel, immunologic recovery was observed; the median CD4 cell count at the last available assessment was 549 cells/mm3 (IQR: 376 to 730), representing a median increase from baseline of 331 cells/mm3 (IQR: 158 to 466). Using a statistical method robust to data missing at random, MMRM analysis of CD4 cell counts showed progressive and significant increases over time (p < 0.0001), reaching 610 cells/mm3 at five years (95% CI: 569, 651) (Figure ).
Long-term safety and tolerability
ATV/r was generally well tolerated during long-term treatment. Overall, at least one AE was reported in 48.5% of patients. Serious AEs were reported in two patients (cholecystitis and vaginal bleeding), which did not result in any change to the ATV/r-based regimen.
Treatment-emergent clinical AEs (of any grade) and laboratory AEs (of grade 3–4 severity), regardless of causality, are shown in Table . As expected from the mechanism of action of ATV, AEs of hyperbilirubinemia were reported in 29.4% and Grade 3–4 bilirubin elevations in 37.8% of patients. Gastrointestinal AEs were uncommon, occurring in ≤2.5% of patients. Grade 3–4 elevations in lipid parameters occurred in ≤6.5% of patients.
Table 4 Selected treatment-emergent clinical and laboratory AEs (regardless of causality): incidence in the overall population of eligible patients (N = 517)
Within the group of patients discontinuing because of AEs, few patients (<1.5%) discontinued for gastrointestinal or renal reasons with the most common reason being hyperbilirubinemia-related (Table ). Hyperbilirubinemia-related discontinuations are presented in detail in Table . Four patients were reported as each having two hyperbilirubinemia-related reasons for discontinuation. After excluding the four duplicated reasons, 46/517 patients (8.9%) were reported by the investigator as having discontinued for at least one hyperbilirubinemia-related reason during five years of follow-up. Among these hyperbilirubinemia-related discontinuations, investigators gave a raised blood bilirubin (defined as reporting one of three terms: ‘hyperbilirubinemia’, ‘blood bilirubin abnormal’, or ‘blood bilirubin increased’) as the reason in 21/46 patients (45.7%) and a clinical reason (defined as reporting ‘jaundice’, ‘ocular icterus’, or ‘yellow skin’) in 25/46 patients (54.3%). Not all patients had a confirmatory bilirubin measurement prior to discontinuation; for those that had, 13/517 patients (2.5%) were discontinued despite having Grade 1–2 elevations. By five years, the rate of discontinuation due to hyperbilirubinemia with a confirmed Grade 1–4 bilirubin measurement was 34/46 (6.6%) and with a confirmed Grade 3–4 bilirubin elevation was 21/46 (4.1%). Hyperbilirubinemia-related discontinuations varied by country, occurring most commonly in Germany, where ocular icterus was reported as the reason for discontinuation in seven out of the eight patients with this AE.
Table 5 Patients discontinued due to hyperbilirubinemia: reasons given by the investigator versus confirmed grading from bilirubin measurement
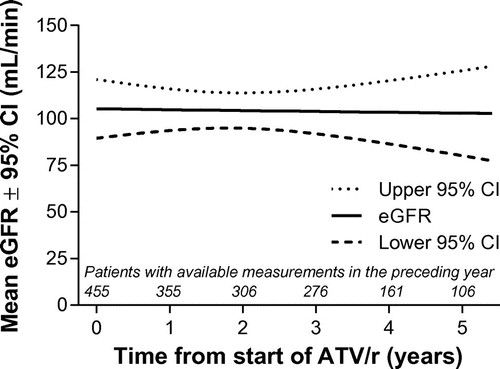
Overall, renal/urinary disorders occurred in 17 patients (3.3%). Grade 3–4 creatinine elevations were rare (<1%). The incidence of nephrolithiasis in the overall population was 0.6% per patient-year of exposure. Renal failure, which also led to treatment discontinuation in all cases, occurred in five patients (<1% of the overall population), one of whom was also receiving concomitant TDF/FTC. At the last available patient assessment, median eGFR for the overall patient population was 95.3 mL/min (IQR: 81.4 to 113.8), representing a median change from baseline of −4.7 mL/min (IQR: –21.3 to 0.0). Using a statistical method robust to data missing at random, MMRM analysis of eGFR showed no significant change over time with treatment (Figure ); mean change from baseline at five years in eGFR was −1.3 mL/min (95% CI: −30.3, 27.8). eGFR > 90 mL/min at baseline declined to < 60 and < 30 mL/min in 11 (2.6%) and one patient (<1%), respectively.
Discussion
In this large European cohort of treatment-naïve patients with HIV-1 infection commencing ATV/r-based regimens, the probability of remaining virologically suppressed (HIV-1 RNA < 50 copies/mL) for up to 5 years was high at 80%, confirming previous findings from ATV/r clinical trials.Citation7−9 No major PI resistance mutations were observed at virologic failure and no unexpected AEs were reported. Overall, the incidence of renal disorders was low and similar to that observed in clinical trials,Citation7−9,17 and no significant changes in eGFR were noted over five years of treatment with an ATV/r-based regimen.
Persistence with ATV/r-based regimens over time was good, with 51.5% of patients remaining on treatment up to five years in the current study; this compares favorably with the results of other cohort studies examining discontinuation rates with several different first-line ARV-regimensCitation10−12 and in other cohort studies in treatment-experienced patients.Citation18,19 In the Swiss HIV Cohort Study, comparing seven initial ARV treatment regimens, rates of treatment modification one year after the start of treatment were lowest for ATV/r plus TDF/FTC (24.1%) and highest for ritonavir-boosted lopinavir plus zidovudine/lamivudine (70.7%).Citation10 Similarly, in a comparative five-year cohort study of first-line combination ARV therapy, ATV/r showed greater persistence (1016 days) than efavirenz (974 days) or ritonavir-boosted lopinavir (382 days).Citation11 In a pooled analysis of 18 cohort studies conducted in Europe and North America (n = 21,801), treatment modification and interruption rates were high (51% after 2.3 years); however, patients taking ATV had lower rates of changing drug class compared with patients on efavirenz, whereas patients on lopinavir or nevirapine had higher rates of interruption.Citation12 In a retrospective European cohort study, in treatment-experienced patients (n = 1,294) who switched to an ATV/r-containing regimen, a treatment discontinuation rate of 21.2% over the first year was reported, with a probability of remaining on treatment after three years of 0.56 (95% CI: 0.52, 0.60) for patients with baseline virologic suppression (<500 copies/mL).Citation18 In a subsequent analysis of data from this study by gender, treatment discontinuation rates at one year were 40.0 and 52.1% for the male and female patients, respectively; after three years, the probability of male patients remaining on ATV/r treatment (0.58; 95% CI: 0.54, 0.61) was higher than for female patients (0.46; 95% CI: 0.40, 0.52), based on Kaplan-Meier analysis.Citation19
AEs were reported as reasons for treatment discontinuation in approximately 16% of patients in the current study, with the majority of individual events occurring in <5% of patients. Consistent with ATV inhibition of uridine 5’-diphospho-glucuronosyltransferase (UGT),Citation20 the enzyme responsible for bilirubin glucuronidation, hyperbilirubinemia was reported as a clinical or Grade 3–4 laboratory event (total bilirubin elevation > 2.5 x upper limit of normal) in 29.4% and 37.8% of patients, respectively. Discontinuation due to hyperbilirubinemia by five years occurred in 8.9% of patients overall, in 6.6% with a confirmed bilirubin measurement, and in 4.1% with Grade 3–4 bilirubin elevations. Although the rate of Grade 3–4 hyperbilirubinemia AEs in the current study (37.8%) compared favorably with the rate in a similar observational cohort study in treatment-experienced patients switched to an ATV/r-based regimen (61.0%),Citation18 the rate of discontinuation due to hyperbilirubinemia was rare in this latter study (0.9%).Citation18 However, the latter study was entirely retrospective in design, whereas the current study included a prospective component, which may have led to underreporting of the reasons for treatment discontinuation in the latter study. Despite rates of Grade 3–4 hyperbilirubinemia in the current study (37.8%) being lower than those in major randomized controlled trials (44–58%),Citation7−9,21−25 discontinuation due to hyperbilirubinemia was higher in the current study (8.9%) compared with these randomized controlled trials (0–7.8%).Citation7−9,21−25 When considering discontinuation due to clinical jaundice (includes ‘jaundice’, ‘ocular icterus’, and ‘yellow skin’ as reasons), the rate in the current study (4.8%) was similar to that reported in the AIDS Clinical Trials Group A5257 study (5.0%).Citation23 Rates of discontinuation due to hyperbilirubinemia in the current study and in A5257 were notably higher than in other cohort and clinical studies. It is unclear why this was the case for either study, although in the current study, the large proportion of patients of Hispanic origin (67.7%), for whom homozygosity for UGT1A1*28/*28 variant is overrepresented and known to increase rates of hyperbilirubinemia and discontinuation due to this cause,Citation24 may have been influential. However, the rate of discontinuation due to hyperbilirubinemia in the current study was highest in German patients, for whom ocular icterus appears to have been a prominent reason. Given this inter-study and inter-country variation, further research is required to identify differences in baseline characteristics (such as ethnicity), genetics, laboratory parameters, and local clinical practices that are associated with an increased risk of patients discontinuing ATV/r due to hyperbilirubinemia.
Given that the current multivariate Cox proportional hazards analysis indicated that the overall risk of treatment discontinuation was lowest in Germany, factors other than treatment discontinuation due to hyperbilirubinemia-related reasons need to be considered. Of note, the proportion of patients at baseline with intravenous drug use was about threefold less in Germany than in Portugal or Spain (Table ), which may have contributed to the higher rate of discontinuation in Portugal and Spain compared with Germany. Other possible reasons include regional variations in ARV treatment strategies. For example, it is common in Spain to simplify therapy in patients who achieve virologic suppression (as reflected by the highest reporting of the ‘Medical decision/Regimen simplification’ reason for discontinuation of ATV/r by Spanish investigators). Thus, higher rates of regimen simplification, rather than failure of the ATV/r regimen per se, may have contributed to the fact that the highest rates of discontinuation were observed in Spain (Table ). In addition, a higher proportion of patients in Portugal and Spain than in Germany did not provide consent to continue into the one-year extension to the follow-up period (Table ). Because the protocol amendment mandated that these patients should be considered as having discontinued ATV/r, this undoubtedly contributed to the higher rate of ATV/r discontinuation in Portugal and Spain. Had these patients provided consent, it is likely that some would have remained on treatment; therefore, the rate of ATV/r discontinuation in this study may have been overestimated.
In common with all observational studies, this study has acknowledged limitations. While the study sought to recruit patients from a wide variety of participating sites and consecutive clinic attendees were enrolled, it is not possible to state that the population studied here is representative of the general population of patients with HIV-1 infection commencing ARV therapy. Factors such as motivation to participate, reliability and completeness of historical and ongoing data collection, and ability to enroll sufficient numbers of patients within a set recruitment period were key issues contributing to the site selection process. It is possible, therefore, that patients in this study received a higher standard of clinical care leading to superior outcomes than that associated with non-participating centers. The observational and mixed retrospective–prospective study design meant that only historical data were collected for some patients, whereas for others the data collected were primarily prospective, and for both types of data collection, the length of follow-up varied. As a consequence, events may have been underreported during the retrospective phase. In common with all observational studies, bias from missing data or patients having been lost to follow-up cannot be excluded. In contrast to randomized trials where all AEs are systematically reported and assessed for causality, AEs could have been underreported. In addition, establishing whether there were causal relationships with ATV/r regimens was difficult owing to the lack of a comparator regimen in the current study.
Despite these methodological limitations, this study followed a large number of patients from different European countries over an extended period beyond that normally associated with randomized trials. In addition, the lack of narrow selection criteria, as commonly occurs in randomized trials, enabled the long-term outcomes of ATV/r-based regimens in a real-life clinical setting to be evaluated in patients who may have been ineligible for participation in randomized controlled trials.
To conclude, in a real-life clinical setting over five years, ATV/r-based regimens in treatment-naïve patients with HIV-1 infection showed sustained virologic suppression, an absence of treatment-emergent major PI resistance mutations at virologic failure, a lower overall rate of treatment discontinuation compared with other similar cohort studies examining alternative regimens, a long-term safety profile consistent with that previously observed in clinical trials, and no evidence of a significant decline in renal function for up to five years. ATV/r is an effective and well-tolerated therapeutic option for treatment-naïve patients with HIV-1 infection.
Conflicts of interest
Eugénio Teófilo has received honoraria for attendance at advisory boards and investigator grants for clinical trials from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. Nuno Rocha-Pereira has received honoraria to attend meetings and congresses both in general infectious diseases medicine and in HIV medicine from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. Birger Kuhlmann declares no conflicts of interests. Antonio Antela has received investigator grants for clinical trials, and honoraria for attendance at advisory boards or for speaker engagements from Abbvie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. Heribert Knechten has received investigator grants from Abbott and Octapharma, and has received investigator grants and personal fees from AbbVie, Biotest, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. Jesús Santos has carried out consultancy work for Bristol-Myers Squibb, Gilead Sciences, and Janssen-Cilag; he has received monetary payments for giving talks from Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, and Merck Sharp & Dohme, and has received payments for the development of educational presentations for Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, and Merck Sharp & Dohme. Maria Jesús Jiménez-Expósito is an employee of Bristol-Myers Squibb.
Adherence to ethics and reporting requirements
This study was conducted in accordance with the International Society for Pharmacoepidemiology (ISPE) Guidelines for Good Epidemiology Practices, the ethical principles originating from the Declaration of Helsinki, and local regulatory requirements.
Study registration
Registered at clinicaltrials.gov (NCT01236235).
Acknowledgements
The authors wish to thank the study investigators: Elke Lauenroth-Mai and Frank Schlote, Praxis Turmstraße, Berlin, Germany; Jörg Gölz, Praxiszentrum Kaiserdamm, Berlin, Germany; Heribert Knechten, HIV Schwerpunktpraxis Dr. Heribert Knechten, Aachen, Germany; Christiane Cordes, Praxis Dr. Cordes, Fachärztin für Allgemeinmedizin, Germany; Infektiologin (DGI), Berlin, Germany; Birger Kuhlmann, Gemeinschaftspraxis Dres. Kuhlmann/Holm/Heiken, Hannover, Germany; Albrecht Stoehr, ifi-Studien und Projekte GmbH, an der Asklepios Klinik St. Georg, Haus K, Hamburg, Germany; Axel Baumgarten, mib Dienstleistungs GmbH, Berlin, Germany; Hans-Jürgen Stellbrink, Infektionsmedizinisches Centrum Hamburg (ICH Grindel), ICH Study Center GmbH & Co. KG, Hamburg, Germany; Markus Müller, Allgemeinarztpraxis Dres. Frietsch/Ulmer/Müller, Stuttgart, Germany; Michael Rausch and Matthias Freiwald, Medizinisches Versorgungszentrum Ärztezentrum Nollendorfplatz, Berlin, Germany; Anja Meurer, Zentrum für Innere Medizin und Infektiologie, München, Germany; Stefan Mauss, Dr. med. Stefan Mauss & Kollegen Forschung GbR, Düsseldorf, Germany; Heiko Jessen, Gemeinschaftspraxis Jessen-Jessen-Stein, Berlin, Germany; Eiko Schnaitmann, Gemeinschaftspraxis Schwabstrasse, Stuttgart, Germany; Heiko Karcher, Praxis City Ost, Berlin, Germany; Franz Audebert, Gemeinschaftspraxis Alte Mälzerei, Regensburg, Germany; Winfried V. Kern, Universitätsklinikum Freiburg, Centrum Chronische Immundefizienz (CCI), Freiburg, Germany; Christoph Mayr, MVZ Ärzteforum Seestrasse, Berlin, Germany; Dieter Schuster, Mannheimer Onkologie Praxis, Mannheim, Germany; Rosario Serrão and Cátia Caldas, Hospital São João, Lisboa, Portugal; Liliane Almeida, Hospital Joaquim Urbano, Porto, Portugal; Isabel Ameida, Centro Hospitalar do Porto, EPE - Hospital Santo António, Porto, Portugal; Luisa Mocho, Hospital de São Teotónio, EPE, Viseu, Portugal; Christina Valente, Centro Hospitalar de Coimbra, Coimbra, Portugal; Fausto Roxo, Hospital Distrital de Santarém, EPE, Santarém, Portugal; Eugénio Teófilo, CHLC - Hospital dos Capuchos, Lisboa, Portugal; Isabel Germano, CHLC - Hospital São José, Lisboa, Portugal; Kamal Mansinho, CHLO - Hospital Egas Moniz, Lisboa, Portugal; Maria João Aleixo, Hospital Garcia de Orta, EPE, Pragal, Portugal; José Vera, Hospital de Cascais Dr. José de Almeida, Cascais, Portugal; Célia Oliveira, Hospital Infante Dom Pedro, Aveiro, Portugal; Carlos Santos, Hospital de Portimão, Portimão, Portugal; Dr. Rosas Vieira, Centro Hospitalar de Gaia, Vila Nova de Gaia, Portugal; Luis Tavares, Hospital do Barreiro (Nossa Senhora do Rosário), Barreiro, Portugal; Elisabeth Deig Comerma, Hospital de Granollers, Granollers, Spain; Luis Force Sanmartín, Hospital de Mataró, Mataró, Spain; Pere Domingo Pedrol, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Bonaventura Clotet Sala, Hospital Germans Trias i Pujol, Badalona, Spain; Hernando Knobel Freud, Hospital del Mar, Barcelona, Spain; Vicent Falcó Ferrer, Hospital Universitario Vall d’Hebron, Barcelona, Spain; Julián Olalla Sierra, Hospital Costa del Sol, Marbella, Spain; Pompeyo Eduardo Viciana Fernández, Hospital Virgen del Rocio, Sevilla, Spain; Juan Emilio Losa García, Fundación Hospital Alcorcon, Alcorcon, Spain; Juan Flores Cid, Hospital Arnau de Vilanova, Valencia, Spain; Enrique Ortega González, Hospital General Universitario de Valencia, Valencia, Spain; Antonio Antela, Hospital Universitario de Santiago, Santiago de Compostela, Spain; Santiago Moreno Guillén, Hospital Ramon y Cajal, Madrid, Spain; Jesús Santos González, Hospital Virgen de la Victoria, Malaga, Spain; Juan Pasquau Liaño, Hospital Virgen de las Nieves, Granada, Spain; Ignacio de los Santos Gil, Hospital La Princesa, Madrid, Spain; José Antonio Oteo Revuelta, Hospital San Pedro de La Rioja, Logrono, Spain; Miguel Cervero Jiménez, Hospital Severo Ochoa, Leganes, Spain; Carlos Barros, Hospital de Mostoles, Mostoles, Spain; Miguel Torralba González de Suso, Hospital Universitario de Guadalajara, Guadalajara, Spain; Alberto Terrón Pernía, Hospital de Jerez, Jerez de la Frontera, Spain; Desiré Gil Pérez, Hospital Universitario Miguel Servet, Zaragoza, Spain; José López Aldeguer, Hospital Universitario La Fe, Valencia, Spain.
Funding
This study was supported by Bristol-Myers Squibb, the manufacturer of atazanavir. The authors thank Caroline Perry and Julian Martins of inScience Communications, Springer Healthcare, who provided medical writing support funded by Bristol-Myers Squibb.
References
- Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137.
- Sherer RD Jr, Fath MJ, Da Silva BA, Nicolau AM, Miller NL. The importance of potency and durability in HIV patient antiretroviral therapy preferences: A telephone survey. AIDS Patient Care STDs. 2005;19:794–802.10.1089/apc.2005.19.794
- van Maarseveen N, Boucher C. Resistance to protease inhibitors. In: Geretti AM, ed. Antiretroviral resistance in clinical practice. London; 2006.
- Gupta R, Hill A, Sawyer AW, Pillay D. Emergence of drug resistance in HIV type 1–infected patients after receipt of first‐line highly active antiretroviral therapy: A systematic review of clinical trials. Clin Infect Dis. 2008;47:712–722.10.1086/592125
- Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2014. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. Accessed March 23, 2015.
- European AIDS Clinical Society. Guidelines: Version 7.1, November 2014. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Accessed March 23, 2015.
- Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655.10.1016/S0140-6736(08)61081-8
- Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53:323–332.10.1097/QAI.0b013e3181c990bf
- Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154:445–456.10.7326/0003-4819-154-7-201104050-00316
- Elzi L, Marzolini C, Furrer H, et al. Treatment modification in human immunodeficiency virus–infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arch Intern Med. 2010;170:57–65.10.1001/archinternmed.2009.432
- Maggiolo F, Leone S, Cologni G, Valenti D, Quinzan G, Suter F. Persistence on first line HAART is influenced by dosing schedule. Abstracts of the 18th International AIDS Society World AIDS conference, July 18–23, 2010, Vienna, Austria.
- Abgrall S, Ingle SM, May MT, et al. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS. 2013;27:803–813.
- Wensing AM, Calvez V, Gunthard HF, et al. 2014 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2014;22:642–650.
- DAIDS Regulatory Support Centre. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0, December, 2004; Clarification August 2009. http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf. Accessed August 13, 2014.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470.10.7326/0003-4819-130-6-199903160-00002
- National Research Council. The prevention and treatment of missing data in clinical trials. Panel on handling missing data in clinical trials. Committee on National Statistics, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press; 2010.
- Rockstroh JK, DeJesus E, Henry K, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62:483–486.10.1097/QAI.0b013e318286415c
- Jansen K, Sonnerborg A, Brockmeyer N, et al. Long-term efficacy and safety of atazanavir/ritonavir treatment in a real-life cohort of treatment-experienced patients with HIV type 1 infection. AIDS Res Hum Retroviruses. 2013;29:564–573.10.1089/aid.2012.0092
- Svedhem-Johansson V, Pugliese P, Brockmeyer NH, et al. Long-term gender-based outcomes for atazanavir/ritonavir (ATV/r)- containing regimens in treatment experienced patients with HIV. Curr HIV Res. 2013;11:333–341.10.2174/1570162X113119990037
- Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729–1739.10.1124/dmd.105.005447
- DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379:2429–2438.10.1016/S0140-6736(12)60918-0
- Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–718.10.1097/01.aids.0000216371.76689.63
- Landovitz R, Ribaudo H, Ofotokun I. Efficacy and tolerability of atazanavir, raltegravir, or darunavir with FTC, tenofovir: ACTG 5257 [Abstract 85]. 21st Conference on Retroviruses and Opportunistic Infections, March 3–6, 2014; Boston, MA, USA.
- Ribaudo HJ, Daar ES, Tierney C, et al. Impact of UGT1A1 Gilbert variant on discontinuation of ritonavir-boosted atazanavir in AIDS Clinical Trials Group Study A5202. J Infect Dis. 2013;207:420–425.10.1093/infdis/jis690
- Uy J, Hu W, Wirtz V, et al. Clinical significance of hyperbilirubinemia in the CASTLE study. J Int AIDS Soc. 2010;13:P93.10.1186/1758-2652-13-S4-P93