Abstract
Background: Scarce data exist on the efficacy and safety of the PEGylated-interferon/ribavirin/boceprevir regimen in HIV/HCV-coinfected patients who failed to respond to PEGylated-interferon/ribavirin treatment.
Objectives: To evaluate the efficacy and safety of this drug regimen and the impact of the addition of boceprevir(BOC) on atazanavir (ATV) or raltegravir (RAL) pharmacokinetic parameters in a subgroup of patients.
Methods: In this single-arm phase 2 trial, HIV-1/HCV-genotype-1-coinfected patients received PEGylated-interferonα2b (1.5 μg/kg/week)+ ribavirin (800–1400 mg/day) alone until W4 and with BOC(800 mgTID) until W48. Based on virologic response at W8, the three drugs were stopped or PEGylated-interferon/ribavirin was continued alone until W72. The primary endpoint was SVR at W24 off-therapy (SVR24). Results: 64 patients were included. SVR24 was achieved in 53% of patients (CI90%: 43–63%) and in 90% of previous relapsers. In univariate analysis, SVR24 was associated with response to previous HCV treatment, HCV-1b subtype, HCV-RNA decline, ribavirin-Ctrough at W4, and HCV-RNA at W8 but not to fibrosis score, IL28B genotype, or boceprevir-Ctrough at W8. In multivariate analysis, SVR24 remained associated with response to previous HCV treatment [non-responders versus null responders: OR = 5.0(1.3–20.0); relapsers vs. null responders: OR = 28.8(4.9–169.5)]. HCV treatment was discontinued for adverse events in 17% of patients. A 51% decrease in ATV/r-AUC0–8 h (p < 0.01) and a 57% increase in RAL-AUC0–8 h (p < 0.01) were observed, although atazanavir/r or raltegravir did not affect BOC-AUC0–8 h significantly. The ATV mean Cthrough fell from 763.8 ng/mL (CI 95%: 230.3–1297.3) without BOC to 507.7 ng/mL (CI 95%: 164–851.4) with BOC.
Conclusions: Boceprevir-based regimen demonstrated a high SVR24 rate in treatment-experienced HIV-HCV genotype-1-coinfected relapsers.
Introduction
With the use of last generation direct-acting antiviral (DAA) drugs, the majority of HCV monoinfected and HIV/HCV-coinfected patients will hopefully be cured,Citation1 and in the majority of cases, with an interferon-free regimen. However, interactions between DAA and antiretroviral drugs remain an issue for HCV/HIV-coinfected patients, and to date, access to interferon (IFN)-free regimens remains limited in many parts of the world. The objective of the ANRS HC27-BOCEPREVIH trial was to evaluate the efficacy and safety of the NS3 HCV protease inhibitor boceprevir (BOC) in combination with PEG-IFN/RBV in HIV/HCVG1-coinfected patients who failed to respond to PEG-IFN/RBV. This trial included a pharmacokinetic substudy evaluating the drug interaction between BOC and ritonavir-boosted atazanavir (ATV/r) or raltegravir (RAL) in order to further investigate previous observations in healthy volunteers regarding HIV protease inhibitors and to elucidate this issue with the integrase inhibitor RAL.Citation2
Methods
Study design
This single-arm, multicenter, open-label, phase 2 trial, enrolled HIV-1/HCV-G1-coinfected patients who had previously failed a treatment with PEG-IFN (α-2a or α-2b)/RBV (≥600 mg/day) during < 12 weeks. Other inclusion criteria included stable (>3 months) antiretroviral therapy with at least three drugs (among which abacavir, emtricitabine, lamivudine, tenofovir, ATV or ATV/r, and RAL), CD4 cell count > 200 /mm3 and > 15%, and HIV-RNA < 50 copies/mL for at least 6 months. The primary exclusion criteria were HBV coinfection, Child-Pugh score B or C, and history of decompensated cirrhosis. Virological failure to previous therapy was classified as relapse, (i.e. undetectable HCV-RNA at the end of treatment and detectable HCV-RNA thereafter), breakthrough (i.e. undetectable HCV-RNA at least once during treatment, becoming detectable thereafter under treatment), and non-response including partial response (i.e. >2 log10 HCV-RNA decline at W12 and still detectable HCV-RNA at W24) and null response (i.e. <2 log10 HCV-RNA decline at W12). The fibrosis score was documented by a liver biopsy. Inclusion of non-cirrhotic null responders was limited to 34% of patients. Cirrhotic patients with a previous null response were excluded. The study protocol (EUDRACT number 2010-023450-36) was approved by Ethical Committee CPP-Sud-Méditerranée-1 (Marseille, France) and the French Regulatory Authority ANSM (previously Afssaps, Paris, France). The protocol was registered in the ClinicalTrials.gov database under reference NCT01335529. It was conducted in compliance with the Declaration of Helsinki and ICH-GCP guidelines. All participants provided written informed consent.
Procedures
All patients received a lead-in phase with PEG-IFNα-2b/RBV during 4 weeks. BOC was added at the end of W4 (Figure ). Total treatment duration was defined according to a patient’s status regarding rapid virological response (RVR) at the end of W8 (RVR8). A complete RVR8 was defined as HCV-RNA < 15 IU/mL and a partial RVR8 as HCV-RNA between 15 and 1000 IU/mL. For patients with complete RVR8, total treatment duration was 48 weeks; SVR was evaluated at W72 (SVR24). For patients with partial RVR8, BOC was stopped at W48 but PEG-IFN/RBV was maintained for a total of 72 weeks, and SVR24 was evaluated at W96. A dosage of 800 mg BOC was prescribed (in 200 mg capsules) 3 times per day (7–9 h interval between intakes) during meals. PEG-IFN (1.5 μg/kg) was administered subcutaneously once a week. Oral RBV was given at 800–1400 mg/day (weight-based) BID during meals. Erythropoietin (EPO), granulocyte-colony-stimulating factor (G-CSF) and thrombopoietin-receptor agonists were allowed. Blood transfusions and RBV dose reduction were allowed only if EPO failed to maintain a hemoglobin concentration above 100 g/L. Futility rules for BOC were defined as an HCV-RNA > 1000 IU/mL at W8 or W12. For these patients, PEG-IFN/RBV was maintained until W72, and SVR24 was evaluated at W96. BOC, PEG-IFN, and RBV were interrupted if HCV-RNA was > 100 IU/mL at W16, if HCV-RNA was still detectable at W28 or at any time in the case of virological breakthrough.
We defined virological breakthrough as confirmed HCV-RNA detection after prior undetectability. All non-responders (failure or breakthrough) received follow-up until W72 or W96 depending on the HCV-RNA at W8. Virological relapse was defined as HCV-RNA undetectable at EOT, then detectable at the end of follow-up, without evidence of new HCV reinfection.
Virological analysis
HCV-RNA was quantified on site by real-time PCR assays using RealTime-HCV (Abbott Diagnostics) or COBAS TaqMan-HCV (Roche Diagnostics) assays (lower limits of detection (LLD) of 12 and 15 IU/mL, respectively) and carried out by the same laboratory and assay throughout the study. For virological response assessment, detectable HCV-RNA was defined as HCV-RNA > 15 IU/mL. Detectable HCV-RNA below LLD was considered positive with a value equal to LLD. Genotypic drug resistance testing was performed for all patients with virological failure, breakthrough, or relapse post-treatment. HCV-RNA was extracted from 200 μl serum with the EZ1-Virus Mini Kit v2.0 using the BioRobot EZ1 Workstation (Qiagen) following the manufacturer’s instructions. PCR amplification and direct population sequencing of the full-length HCV-NS3 protease gene were performed using in-house protocols as described previously.Citation3 Amino acid diversity was analyzed at sites associated with reduced susceptibility to BOC. HIV-RNA was measured with RealTime-HIV (Abbott Diagnostics) or COBAS TaqMan-HIV (Roche Diagnostics) assays. HIV viral load (VL) was considered detectable at > 50 copies/mL. A blip in HIV VL was defined as a positive detectable HIV VL lower than 1000 copies/mL becoming undetectable in a next measurement performed after a delay of 4 weeks.
IL28B genotype (SNP rs12979860) was determined in all patients using peripheral blood mononuclear cell DNA by Sanger population sequencing.Citation4,5
Pharmacokinetic analysis
Plasma RBV-Ctrough was determined at W4 using a high-performance liquid chromatography-diode array detector (HPLC-DAD). A pharmacokinetic (PK) substudy evaluating the drug interaction between BOC and ATV/r, or BOC and RAL was planned in a subgroup of 30 patients. Blood samples were collected at baseline for antiretroviral (ARV) and at W8 for both ARV and BOC. BOC-Ctrough was determined by a liquid-chromatography tandem-mass spectrometry method. PK parameters [Cmax, Ctrough, and the area under the curve (AUCà-!h)] were determined at steady state based on the PK profiles obtained with blood samples drawn at 0 (before ARV ± BOC intake), 1, 2, 3, 4, 6, and 8 h during an 8-h interval after intake of drugs with a standardized meal. PK analysis was performed by a non-compartmental method (PK fit Software, Montpellier, France). The reported Cmax, Tmax, and Ctrough are the values observed in individual PK profiles. Statistical analyses were performed by comparing mean PK parameters at baseline and W8 for ARV, and with historical data of BOC alone for BOC. Patients were classified according to RBV-Ctrough-W4 and BOC-Ctrough-W8 to evaluate the impact on SVR24. A 2 μg/mL threshold was chosen for RBV according to the minimum recommended Ctrough reported under PEG-IFN/RBV.Citation6 Because no efficacy threshold was established for BOC-Ctrough, the cutoff was set at the median value observed in our study (127 μg/L).
Safety analysis
Adverse events (AE) were graded by investigators according to the “ANRS-scale to grade the severity of adverse events in adults.” Non-life threatening AEs were managed by means of a dose reduction of PEG-IFN or RBV similarly to those previously described.Citation7,8 Monthly monitoring of HIV-VL during BOC exposure was initiated in March 2012 associated with ATV-Ctrough measurement at screening, at W48, or in case of HCV or HIV breakthrough, according to FDA and EMEA recommendations. An independent data and safety monitoring board regularly evaluated the safety and side effects of study regimens.
Statistical analysis
The primary efficacy endpoint was SVR24 rate measured at W72 or W96 according to treatment duration. The analysis of the main endpoint was performed with a test comparing an observed to a theoretical proportion conducted in unilateral formulation with a type I error of 5%. The rate of SVR24 below which the therapy would have no interest was fixed at 20% (null hypothesis). The rate of SVR24 above which the therapy would bring a real benefit was fixed at 35% (alternative hypothesis). To guarantee 90% power to detect such a 15% benefit and considering the statistical hypotheses, a minimum of 22 (out of 77) participants with a SVR24 were required to conclude that SVR rate is above 20%. An intent-to-treat analysis was performed for all patients who initiated treatment. Cases of missing data for the primary endpoint were considered treatment failures. Secondary efficacy endpoints included RVR8 and SVR12. Safety endpoints included treatment-emergent AEs, laboratory abnormalities, and deaths. Anemia and HCV and HIV breakthroughs were cautiously monitored. Data are presented as counts and percentages, or median and inter quartile range (IQR), as appropriate. Association of baseline characteristics with SVR24 as well as pharmacological data (RBV-Ctrough-W4, BOC-Ctrough-W8) were analyzed using Wilcoxon, χ2 or Fisher’s exact tests with a significant threshold of p < 0.05. Multivariate analysis was subsequently conducted using a logistic regression model. For this analysis, continuous variables were broken into two classes on the basis of their median values, except for pharmacological data, where the threshold was optimal (i.e. optimizing sensitivity and specificity). Statistical analyses were performed using SAS statistical software (version 9.4;Cary, NC, USA).
Results
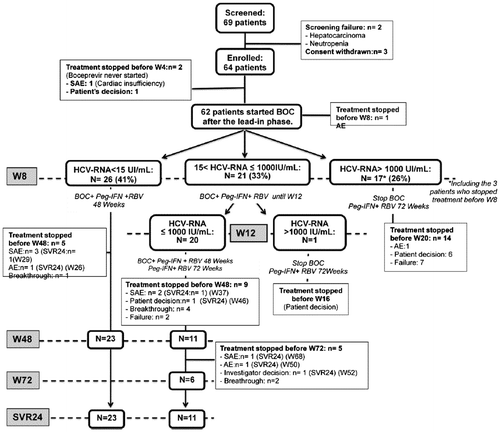
Sixty-nine patients were screened from May 2011 to April 2012, 64 patients were enrolled and started PEG-IFN/RBV, and 62 patients started BOC after the lead-in phase. The analysis was performed on these 64 patients (Figure ). The baseline characteristics of patients are presented in Table . Most were men (75%), with a median age of 49 years, most were infected with HCV-G1a (78%), and 39% had severe fibrosis or cirrhosis. The HCV VL was more than 800,000 IU/mL in 77% of cases and IL28B CT/TT genotypes were detected in 64%. Thirty-three percent of patients were previous null responders, and 31% were relapsers. Half were treated with 2 NRTI + ATV/r, 42% with 2 NRTI + RAL, and 8% with other authorized ART combinations. Among NRTIs, 92% were treated with tenofovir + emtricitabine. HIV-VL was detectable in 2 patients treated with an ATV/r-based regimen (60 and 176 copies/mL, respectively) and in 1 patient treated with an RAL-based regimen (80 copies/mL). For these three patients, HIV-VL was undetectable at screening and at W4, and remained undetectable throughout the follow-up.
Table 1 Baseline demographics and clinical characteristics
Efficacy
Sixty-two patients reached W4 (Figure ). At this time, 1 patient achieved undetectable HCV-RNA, while 28 patients (44%) had an HCV-RNA decrease ≥ 1 log10. Sixty-one patients reached W8, of whom 26 had a complete RVR8 and were assigned to continue PEG-IFN/RBV/BOC until W48. The rate of RVR8 was 27.3% among cirrhotic patients vs. 24.5% among patients without cirrhosis. A partial RVR8 was observed in 21 patients and was re-evaluated at W12, and 14 participants had to stop BOC and continue PEG-IFN/RBV until W72. All of these, 14 patients stopped the three drugs before W20 because of AE in 1 case, lack of efficacy in 7, and patient’s decision in 6. At W12, 20 patients with partial RVR8 had a partial response and were assigned to PEG-IFN/RBV/BOC until W48 and then to PEG-IFN/RBV until W72. However, 14 patients stopped the treatment before W72 because of AE in 4 cases, lack of efficacy in 2, breakthrough in 6, and patient’s decision in 2. One patient had to stop BOC and continue PEG-IFN/RBV until W72, but he decided to stop PEG-IFN/RBV before W16. No patient died after the EOT.
Overall, 37 patients (58%) discontinued treatment either before (n = 3) or after (n = 34) W8, due to AEs in 11 patients (17%), virological breakthrough in 7 (11%), lack of efficacy in 9 (14%), patient’s decision in 9 (14%; 7 before W16), and investigator’s decision in 1. Thus, 27 patients (42%) terminated the study protocol.
SVR24 was achieved in 34/64 patients (53%; 90% CI: 43%-63%; p = 0.002), including 7 patients who stopped HCV treatment prematurely following AEs (n = 5) or patient/investigator’s decision (n = 2), and was similar to SVR12. The SVR24 rate was 90% in relapsers and 79% in patients with HCV-G1b. Table shows SVR24 according to baseline characteristics, virological response at W4 and at W8, RBV-Ctrough-W4, and BOC-Ctrough-W8. Among baseline characteristics, response to previous treatment, RAL-based regimen, HCV-G1b, HCV-RNA < 6.4 log10, and HOMA > 2.5 were significantly associated with virological outcome. HCV-RNA decrease ≥ 1 log10 at W4, HCV-RNA < 15 IU/mL at W8, and RBV-Ctrough ≥ 2 μg/mL at W4 were also significantly associated with virological outcome. In multivariate analysis, response to previous treatment was the only significant variable associated with SVR24 for previous non-responders vs. null responders (OR 5.0; CI: 1.3; 20.0) and for relapsers vs. null responders (OR 28.8; CI: 4.9; 169.5).
Table 2 SVR24 rate according to baseline characteristics, week 4 and week 8 virological responses, ribavirin Ctrough at week 4 and BOC Ctrough at week 8
Resistance
The full-length HCV-NS3 protease gene was sequenced for 13 patients out of 23 who stopped treatment after HCV breakthrough, partial response, or patient’s/investigator’s decision. Eleven were infected with HCV-G1a. Clinically relevant amino acid substitutions, absent at baseline, were detected in seven patients (Table ). They included mostly R155 K and V36 M/A, and substitutions T54A and T54S were observed in 1 case each. V36A, T54A, and R155 K were concurrently detected in HCV-G1a from 1 patient.
Table 3 Resistance profile in patients with virological failure
Pharmacokinetics
The median RBV-Ctrough was 1.70 μg/mL (IQR: 1.39; 2.13) and was not significantly different (p = 0.36) between patients who achieved SVR24 and those who did not: 1.75 μg/mL (IQR: 1.39; 2.66) vs. 1.70 μg/mL (IQR: 1.45; 1.91), respectively. However, 72.2% of patients who achieved SVR24 had a RBV-Ctrough up to 2 μg/mL vs. 27.8% of patients who did not (p = 0.03). The median BOC-Ctrough was 127 μg/L (IQR: 104; 308) and was not significantly different (p = 0.40) between patients who achieved SVR24 or not: 163 μg/L (IQR: 100; 458) vs. 125 μg/L (IQR: 104; 255), respectively. Moreover, 52.4% of patients who achieved SVR24 had a BOC-Ctrough up to 127 μg/L vs. 47.6% of patients who did not achieve SVR24 (p = 0.63).
Twelve patients (10 males) fully completed the PK study, 7 on ATV/r and 5 on RAL, associated with tenofovir + emtricitabine (Table ). A decrease in ATV/r-AUC0–8 h of 51% (p < 0.01) and an increase in RAL-AUC0–8 h of 57% (p < 0.01) were observed at W8. ATV and RAL-Ctrough were, respectively, 34% and 54% lower after initiation of BOC, but these variations did not reach statistical significance and the values remained above target concentrations for both drugs. ATV/r or RAL did not significantly affect BOC-AUC0–8 h, compared with historical controls.Citation9 Variations in BOC-Ctrough and Cmax were observed when associated with ATV/r or RAL, but they did not reach statistical significance.
Table 4 Pharmacokinetic parameters of ritonavir-boosted atazanavir, raltegravir, and boceprevevir
Safety
AEs, dose modifications, and study drug discontinuation are summarized in Supplementary Tables and . The AEs most commonly observed were asthenia and flu-like symptoms. Grade 4 AEs occurred in 20 patients and included leukoneutropenia (6%), infections (6%), anemia (3%), and general disorders (3%). Although they were more frequent in patients with a severe fibrosis score, such events were observed in 23.8% and 22.2% of patients with F0/F1 and F2 fibrosis score, respectively.”Eleven (17%) patients discontinued treatment due to an AE. Four suspected unexpected serious adverse reactions (SUSAR) were reported. Two participants experienced sepsis, one had a generalized rash, and the other experienced acute pyelonephritis. All resolved. The two cases of sepsis were considered as strongly related to PEG-IFN even when they were observed under the combination of PEG-IFN/RBV and BOC. The investigator and sponsor considered those events as possibly related to study medication. No death occurred. Three patients reported anemia as a serious AE (SAE) but did not discontinue the study. Most patients with anemia received EPO (38 patients) or had a PEG-IFN or RBV dose reduction (in 2 and 13 patients, respectively), and 8 received a transfusion. G-CSF was administered to 7 patients for neutropenia. Two patients discontinued the study drug because of neutropenia (neither was infection-related) or thrombocytopenia.
During BOC exposure, 6 patients (ATV/r- and RAL-based regimen, n = 3 each) presented with one blip of HIV-VL, but no HIV breakthrough was observed. The median CD4 cell count decreased in absolute value from 728/mm3 [527–923] to 452/mm3 [301–639] at W48, but this value increased in percentage from 36% (IQR: 30–42) to 41% (IQR: 35–45). At W96, the percentage of CD4 cells returned to baseline (35%; IQR: 31–40) but remained lower in absolute value (641/mm3 (483–895).
Discussion
In this trial, the addition of BOC to PEG-IFN/RBV in HIV/HCV-coinfected patients who previously failed PEG-IFN/RBV led to an SVR24 rate of 53%, quite similar to that observed in both naive HCV-monoinfected (59 to 66%) and HIV/HCV-coinfected (63%) patients.Citation10,11 More interestingly, the SVR24 rate reached 90% in patients who had previously relapsed after PEG-IFN/RBV therapy and 61% in patients with previous partial response. These rates are higher than those observed in previously treated HCV-monoinfected patients (up to 75 and 52%, respectively).Citation10
Interestingly, among 28 patients with a decrease in HCV-RNA < 1 log10 at W4, the addition of BOC allowed for the achievement of SVR24 in 21 (75%) cases. Moreover, patients with undetectable HCV-RNA at W8 were shown to have an SVR24 rate of 88.5%, including 2 patients who stopped treatment prematurely (at W26 and W29) following AEs. Thus, patients who had an early response could benefit from shorter treatment. This hypothesis is consistent with recent reports of a response-guided shortening of BOC-based tri-therapy in HIV/HCV-coinfected patients.Citation12 However, the design of this study did not allow us to assess this hypothesis.
The limited impact of IL28B polymorphism was already described in naive coinfected patients treated with BOC.Citation10 As already reported, patients with HCV-G1 subtype 1b tended to achieve slightly higher SVR rates, as well as relapsers and previous partial responders compared to other patients.Citation13 The fact that the SVR24 was significantly higher among patients with insulin resistance confirms that the negative predictive factors previously determined for PEG-IFN/RBV therapy are no longer relevant with DAA. In contrast, some differences in SVR rates were observed among ART regimens (higher in patients treated with an RAL-based regimen = 70%), but patients were not randomized according to their cART regimen. Moreover, this result could be explained by a higher proportion of null responders among patients treated with ATV/r-based regimen (41 vs. 22%).
We report a significant impact of RBV-Ctrough-W4 on SVR24, confirming that a threshold of ≥2 μg/mL remains crucial for optimizing the virological response to an RBV-based therapy. In contrast, BOC-Ctrough-W8 did not appear significantly to affect the rate of SVR24. This could be explained by the large pharmacokinetic variability of BOC observed among our patients.
Twenty patients (33%) reported at least one SAE, and 17% discontinued treatment following an AE. Furthermore, no drug interactions occurred and the observed safety was consistent with previous reports.Citation10,11 Similar to the CUPIC study,Citation14 the rate of SAE was higher in patients with severe fibrosis. Significant hematological toxicity resulting in treatment withdrawal was observed in only 2 patients (3%), as a result of a proactive management of anemia (59% of patients). The prevalence and distribution of amino acid substitution detected in 7 patients were similar to those previously reported.Citation3
A few sets of ART regimens were allowed in this trial because data on BOC-ART interactions were not available at the beginning of the study. Nevertheless, no HIV breakthrough was observed. The results of our PK substudy are consistent with observations made in healthy volunteers. A trend toward lower ATV exposure when combined with BOC, with a significant AUC reduction, was observed. This was neither significant nor clinically relevant, as ATV-Ctrough still remained largely above the 200 ng/ml recommended threshold. However, even though ATV-Ctrough remained largely above the 200 ng/ml recommended threshold and we did not observe any HIV virological failure, our data suggest careful HIV-RNA monitoring in patients receiving combinations of BOC with ATV/r. The substantial variability in RAL-PK parameters also suggests the need for careful monitoring of tolerance, even if we did not observed related adverse events in our trial. There was also a substantial variability in RAL-PK parameters without any clinical significance. Pending more data, we suggest careful HIV-RNA monitoring in patients receiving combinations of BOC with ATV/r and, to a lesser extent, RAL.
There are several limitations to this trial. First, it is a non-randomized trial with a small sample size. Concerning the PK study, we could not include the number of planned patients. Finally, only 27 patients (42%) completed the study according to the protocol, with a high number of discontinuations due to patient’s decision.
In conclusion, the addition of BOC to PEG-IFN/RBV for HIV/HCV-coinfected patients who previously failed PEG-IFN/RBV led to an SVR24 rate of 53%, but this group included up to 90% in patients who previously relapsed after PEG-IFN/RBV therapy. Severe adverse events were reported in 20 patients and were more frequent in patients with severe fibrosis, and proactive management of anemia should be performed for patients administered this therapeutic combination. The pharmacological data justify maintaining a close monitoring of HIV-VL for patients treated with an ATV/r-based regimen, and a shorter regimen might be considered for patients with RVR8. Such a therapeutic option might be considered in countries where treatment of hepatitis C infection with the newer direct-acting antiviral drugs remains difficult because of the lack of availability, as recently emphasized.Citation15
| Abbreviations | ||
| (PEG-IFN) | = | PEGylated-interferon |
| (RBV) | = | ribavirin |
| (SVR) | = | sustained virological response |
| (HCV-G1) | = | HCV-genotype-1 |
| (DAA) | = | direct-acting antiviral drug |
| (BOC) | = | boceprevir |
| (ATV/r) | = | atazanavir combined with ritonavir |
| (ABC) | = | abacavir |
| (FTC) | = | emtricitabine |
| (3TC) | = | lamivudine |
| (RAL) | = | raltegravir |
| (TDF) | = | tenofovir |
References
- Lacombe K. Hepatitis C, from screening to treatment, a revolution. J Int AIDS Soc. 2014;17:19499.
- Hulskotte EG, Feng HP, Xuan F, et al. Pharmacokinetic interactions between the hepatitis C virus protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, darunavir, and lopinavir. Clin Infect Dis. Mar 2012;56:718–726.
- Colson P, Purgus R, Borentain P, Gérolami R. Natural presence of NS3 protease R155K hepatitis C virus variants with decreased sensitivity to protease inhibitors. J Clin Virol. 2012;53:178–180.10.1016/j.jcv.2011.10.005
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401.10.1038/nature08309
- Ito K, Higami K, Masaki N, et al. The rs8099917 polymorphism, when determined by a suitable genotyping method, is a better predictor for response to pegylated alpha interferon/ribavirin therapy in Japanese patients than other single nucleotide polymorphisms associated with interleukin-28B. J Clin Microbiol. 2011;49:1853–1860.10.1128/JCM.02139-10
- Stanke-Labesque F, Loustaud-Ratti V, Babany G, Gagnieu MC, Marquet P. Ribavirin therapeutic drug monitoring: why, when and how? Fundam Clin Pharmacol. 2010;24:401–406.
- Jacobson IM, Brown RS Jr, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: A randomized trial. Hepatology. 2007;46:971–981.
- McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593.10.1056/NEJMoa0808010
- de Kanter CT, Blonk MI, Colbers AP, Schouwenberg BJ, Burger DM. Lack of a clinically significant drug–drug interaction in healthy volunteers between the hepatitis C virus protease inhibitor boceprevir and the HIV integrase inhibitor raltegravir. Clin Infect Dis. 2013;56:300–306.10.1093/cid/cis824
- Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217.10.1056/NEJMoa1009482
- Sulkowski M, Pol S, Mallolas J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: A randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13:597–605.
- Mandorfer M, Steiner S, Schwabl P, et al. Response-guided boceprevir-based triple therapy in HIV/HCV-coinfected patients: The HIVCOBOC-RGT study. J Infect Dis. 2015;211:729–73510.1093/infdis/jiu516
- Jacobson IM, Pawlotsky JM, Afdhal NH, et al. A practical guide for the use of boceprevir and telaprevir for the treatment of hepatitis C. J Viral Hepat. 2012;19:1–26.10.1111/jvh.2012.19.issue-s2
- Hezode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French early access programme (ANRS CO20-CUPIC) – NCT01514890. J Hepatol. 2013;59:434–441.10.1016/j.jhep.2013.04.035
- Manzano-Robleda Mdel C, Ornelas-Arroyo V, Barrientos-Gutierrez T, Mendez-Sanchez N, Uribe M, Chavez-Tapia NC. Boceprevir and telaprevir for chronic genotype 1 hepatitis C virus infection. A systematic review and meta-analysis. Ann Hepatol. 2015;14:46–57.