Abstract
Background: The phase 4, METABOLIK trial demonstrated that changes in metabolic parameters with darunavir with low-dose ritonavir (DRV/r) were comparable to those observed with atazanavir with low-dose ritonavir (ATV/r). A comprehensive assessment of the effects of these agents on insulin sensitivity will provide additional, relevant clinical information.
Methods: In this substudy of METABOLIK, HIV-1–infected, antiretroviral agent–naïve male subjects aged ≥18 years with a viral load of >1,000 copies/mL were randomized to receive DRV/r 800/100 mg once daily (qd) or ATV/r 300/100 mg qd, both with a fixed dose of tenofovir disoproxil fumarate/emtricitabine 300/200 mg qd. The effects of DRV/r versus ATV/r on insulin sensitivity over 48 weeks were compared using the euglycemic hyperinsulinemic clamp, the preferred method to assess insulin sensitivity; primary end point was the effect on insulin sensitivity during the first 12 weeks.
Results: Twenty-seven subjects completed the study. In the DRV/r arm (n = 14), median glucose disposal from baseline through weeks 12 and 48 was 9.3, 11.4, and 9.9 mg/kg*min, respectively; in the ATV/r arm (n = 13), these values were 8.9, 8.6, and 9.1 mg/kg*min, respectively. Median insulin sensitivity in the DRV/r arm at baseline, week 12, and week 48 was 24.0, 25.0, and 21.5 mg/kg*min per μIU/mL × 100, respectively; these values in the ATV/r arm were 20.7, 22.0, and 22.0 mg/kg*min per μIU/mL × 100, respectively. Most subjects had ≥1 adverse event, including three serious adverse events (n = 2 [DRV/r], n = 1 [ATV/r]).
Conclusions: DRV/r and ATV/r displayed similar modest effects on insulin sensitivity using a euglycemic hyperinsulinemic clamp.
Background
Some antiretroviral (ARV) agents, notably protease inhibitors (PIs), have been associated with metabolic complications and an increased risk of cardiovascular disease in human immunodeficiency virus (HIV)-1–infected individuals.Citation1−5 The use of “older” PIs (particularly indinavir and lopinavir/ritonavir [LPV/r]) has been associated with worsening of lipid parameters,Citation6−10 increased inflammation,Citation11 insulin resistance,Citation6,Citation12−14 hyperglycemia, and diabetes mellitus.Citation12 Atazanavir (ATV) has a favorable metabolic profile in HIV-1–infected subjects, and is generally considered to be a “metabolically friendly” PI.Citation15
Darunavir with low-dose ritonavir (DRV/r) 800/100 mg once daily (qd) in combination with other ARVs is approved for the treatment of HIV-1–infected, ARV-naïve, and ARV-experienced, adult subjects who do not harbor DRV resistance-associated mutations,Citation16,17 and is one of the preferred agents in the United States Department of Health and Human Services guidelines for the treatment of ARV-naïve individuals.Citation18 The phase 4, randomized Metabolic Evaluation in Treatment-naïves Assessing the impact of two BOosted protease inhibitors on LIpids and other marKers (METABOLIK) trial evaluated the metabolic outcomes, efficacy, and safety of DRV/r-based therapy compared with ATV/r-based therapy in treatment-naïve, HIV-1–infected subjects.Citation19 Results from the METABOLIK trial revealed that changes in metabolic parameters with DRV/r were comparable to changes observed with ATV/r, with no differences in lipid parameters, fasting glucose, or insulin sensitivity (as measured by the homeostasis model assessment of insulin resistance [HOMA-IR] method).
The euglycemic hyperinsulinemic clamp (EHC) technique is considered the gold standard for the evaluation of insulin resistance.Citation20 As opposed to HOMA-IR, which is a surrogate of basal insulin sensitivity only, EHC facilitates a dynamic quantitative assessment of overall insulin sensitivity for the entire body by directly assessing glucose utilization as mediated by insulin under steady state conditions.Citation21,22 A number of small studies (n ≤30) have used this technique to evaluate insulin resistance with the use of PIs (including LPV/r, ATV, and indinavir), primarily in HIV-uninfected subjects.Citation23−26 A few small studies (n ≤27) have also evaluated insulin resistance with PIs using the EHC in HIV-1–infected subjects;Citation27−29 however, DRV/r has not been evaluated with this technique.
The present substudy of the METABOLIK trial was designed to evaluate the effects of DRV/r and ATV/r with fixed-dose tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) on insulin sensitivity using the EHC in treatment-naïve, HIV-1–infected adults over 48 weeks.
Methods
The METABOLIK substudy was a phase 4, multicenter, open-label, randomized study; details of the study design have been presented in detail previously.Citation19 In brief, eligible subjects were at least 18 years of age, naïve to ARV therapy (defined as no previous ARV therapy for >10 days), and had plasma HIV-1 RNA ≥1,000 copies/mL. Subjects were required to be infected with HIV sensitive to DRV, ATV, TDF, and FTC. Key exclusion criteria included body mass index >30 kg/m2, fasting glucose >100 mg/dL, low-density lipoprotein cholesterol >130 mg/dL, triglycerides >200 mg/dL, and presence of active AIDS defining illness (defined as category C conditions based on the Centers for Disease Control and Prevention Classification System for HIV infection),Citation13 except stable cutaneous Kaposi’s sarcoma or wasting syndrome.
The study was approved by institutional review boards at all clinical sites, and was conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. All subjects provided written informed consent.
Initially, up to 20 subjects from the main study (with complete week 48 evaluations) were to be enrolled in this substudy; however, as only 8 subjects participating in the main study were enrolled, the enrollment period and size of the METABOLIK substudy were extended to reach a target of 10 subjects per arm. Subjects were randomized in a 1:1 ratio to receive DRV/r 800/100 mg qd or ATV/r 300/100 mg qd, both with the fixed-dose combination of TDF/FTC 300/200 mg qd (Fig. ). Use of lipid-lowering agents, including over-the-counter medications, was prohibited from 28 days prior to baseline through week 12 of the trial. Atorvastatin and rosuvastatin were allowed after week 12.
Figure 1 Study design.
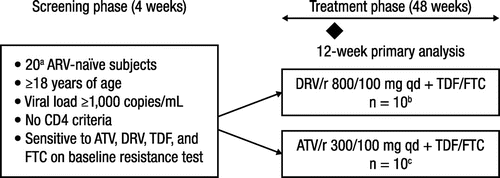
The primary end point was the effect of DRV/r qd and ATV/r qd on insulin sensitivity during the first 12 weeks of therapy using the EHC technique.Citation21,30 A secondary end point was the effect of DRV/r qd and ATV/r qd on insulin sensitivity at week 48 using the EHC technique. Safety analyses, including laboratory safety assessments (chemistry, liver function tests, hematology), vital signs and physical examination, and incidence of overall adverse events (AEs) and HIV-related events, were recorded throughout the substudy. Viral suppression at week 48 was calculated using the confirmed virologic response rate algorithm (HIV-1 RNA <50 copies/mL),Citation19 which is an adaptation of the time to loss of virologic response algorithm that classifies subjects with confirmed resuppression after virologic failure as virologic successes.Citation31
The EHC technique was performed by clamping or maintaining a predetermined blood glucose level, and the measurement was accomplished by clamping insulin at a constant rate of 40 mU/m2/min for the duration of the procedure and infusing glucose 20% at a rate titrated according to blood glucose. Prior to receiving the insulin clamp, subjects were required to abstain from vigorous exercise and to consume at least 150 g of carbohydrates for 3 days prior to the clamp procedure. They then fasted for 20 h prior to the clamp procedure. An intravenous catheter with normal saline was applied in one arm, and a retrograde draw line was inserted in the other arm. Both glucose and insulin were infused simultaneously. Blood samples to measure insulin were collected at the beginning of the procedure (t = 0) and at seven subsequent time points (t = 10 min, t = 20 min, t = 30 min, t = 3 h, t = 3 h and 10 min, t = 3 h and 20 min, t = 3 h and 30 min). Glucose was measured 10 min prior to the beginning of the procedure (t = –10 min), at the beginning of the procedure (t = 0), every 5 min from t = 30 min to t = 90 min, and every 10 min from t = 90 until completion of the procedure (t = 3 h and 30 min). Insulin infusion (100 units of insulin added to 200 cc of normal saline) was started at t = 30 min, along with dextrose 20% solution at 50 mL/h, until completion of the procedure. Blood glucose was maintained at 100 mg/dL during the procedure.
No sample size calculations were performed; however, a sample size of 20 subjects was deemed sufficient to clinically assess the substudy end points. Post hoc, it was estimated that the study was powered to detect a difference in the change in glucose disposal rate ≥3.0 mg/kg min between the two groups at the week 12 time point, based on the standard deviation of the baseline glucose disposal rate of 2.49 mg/kg*min, α = 0.05, and β = 0.20. Data were analyzed by treatment group using summary statistics. Continuous variables were summarized using descriptive statistics (n, mean, standard deviation, median, minimum, and maximum). Categorical variables are presented using frequency distributions.
Results
Study population and baseline characteristics
Twenty-nine subjects participated in the study; two subjects were not included in the analysis because they withdrew before the initiation of treatment. Of the remaining 27 subjects, 14 subjects received DRV/r-based regimens and 13 received ATV/r-based regimens. Twenty-two subjects (81.5%) completed the week 48 evaluations: 12 were in the DRV/r arm and 10 were in the ATV/r arm. Additional details on subject disposition are provided in Table .
Table 1 Subject disposition
All subjects were male, with a median age of 29 years (range: 20–47 years) in the DRV/r arm and 26 years (range: 20–48 years) in the ATV/r arm. The majority of subjects were Black or African American: 57% in the DRV/r arm and 62% in the ATV/r arm. Additional baseline characteristics are provided in Table .
Table 2 Demographics and baseline characteristics of subjects who initiated therapy
Measures of insulin sensitivity
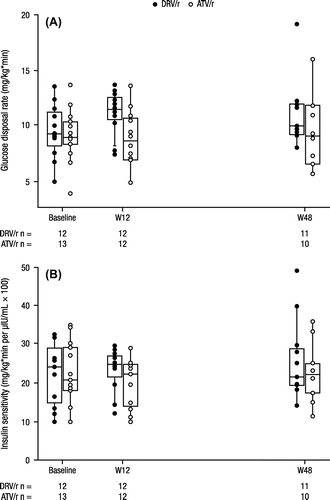
In the DRV/r arm, the median (min–max) glucose disposal rate (GDR) increased from baseline (9.3 [5–14] mg/kg*min) to week 12 (11.4 [7–14] mg/kg*min) and modestly at week 48 (9.9 [8–19] mg/kg*min); in the ATV/r arm, median GDR stayed relatively stable from baseline (8.9 [4–14] mg/kg*min) through week 12 (8.6 [5–14] mg/kg*min) and week 48 (9.1 [6–16] mg/kg*min; Fig. A). From baseline to week 12, median (range) insulin sensitivity increased modestly in both arms: from 24.0 (10–32) to 25.0 (12–175) mg/kg*min per μIU/mL × 100 in the DRV/r arm, and from 20.7 (10–35) to 22.2 (10–29) mg/kg*min per μIU/mL × 100 in the ATV/r arm (Fig. B). Median (range) insulin sensitivity declined by 48 weeks in the DRV/r arm (21.5 [14–48] mg/kg*min per μIU/mL × 100), whereas it was stable in the ATV/r arm (22.0 [11–36] mg/kg*min per μIU/mL × 100; Fig. B). The differences in the changes in glucose disposal rate and insulin sensitivity were not significantly different between the DRV/r and ATV/r arms.
Figure 2 Clamp analyses at baseline, week 12, and week 48: (A) glucose disposal rate and (B) insulin sensitivity.a
Safety evaluations
Nearly all the subjects in the DRV/r (86%) and ATV/r (92%) arms had at least one treatment-emergent AE. Two subjects in the DRV/r (14.3%) arm reported serious treatment-emergent AEs, compared with one subject in the ATV/r (7.7%) arm. These three serious AEs were a case of bronchitis/pneumonia, an incident diagnosis of diabetes, and a case of purpura; all three were determined by the site investigator to be unrelated to study treatment. The incidence of AEs, at least possibly related to study medication, was 50% in the DRV/r arm compared with 77% in the ATV/r arm, the difference related primarily to elevated bilirubin. Over the 48 weeks, there were no clinically relevant differences in safety parameters between the DRV/r and ATV/r arms other than the expected differences in bilirubin levels in the ATV/r arm.Citation18
Viral load outcomes
Viral load improved over the course of the study. Mean log10 HIV-1 RNA decreased from baseline to week 48 in both the DRV/r (change of –3.4 copies/mL) and ATV/r (change of –3.1 copies/mL) arms. At week 48, 91.7% (11/12) of DRV/r-treated subjects and 77.8% (7/9) of ATV/r-treated subjects had HIV-1 RNA <50 copies/mL (100.0% and 100.0% had <400 copies/mL in the DRV/r and ATV/r arms, respectively).
Discussion
These results demonstrate that both DRV/r and ATV/r produce similar modest changes in GDR and insulin sensitivity over 48 weeks, as measured by the EHC technique. GDR values were normal at baseline, defined previously as a value >4.7 mg/kg*min, and were stable over 48 weeks in both arms.Citation32 When accounting for insulin concentrations, insulin sensitivity improved modestly over 12 weeks and was stable over 48 weeks in both treatment arms. There were no clinically relevant differences in safety or viral suppression for either DRV/r-treated subjects or ATV/r-treated subjects.
Using EHC, certain PIs, specifically LPV/r and indinavir, have been associated with insulin resistance in healthy subjects,Citation23,24 although the effect of LPV/r was not confirmed in subsequent studies.Citation25,26 ATV has been shown to have minimal impact on insulin sensitivity in HIV-negative subjects,Citation23,25 and changing PI therapy to ATV/r improved PI-induced insulin resistance in HIV-1–infected men.Citation27 These latter results have led to the general assumption that ATV has the best metabolic profile of the available PIs. This study, the first to evaluate the use of DRV/r with the EHC technique, suggests that the effects of DRV/r on insulin/glucose metabolism are comparable to those of ATV/r. The results of this substudy are consistent with the main METABOLIK trial , which used HOMA-IR as a surrogate measure of insulin resistance.Citation19
Changes in metabolic parameters from baseline to week 48 in the DRV/r arm were comparable with those previously observed with ATV/r in ARV-naïve, HIV-1–infected subjects in the CASTLE study.Citation15 These results are also consistent with those seen in a phase 1 trial of DRV, which showed similar changes in fasting glucose parameters between DRV/r and ATV/r treatment groups, following 7 days of ritonavir and 21 days of DRV/r or ATV/r treatment.Citation33
To date, this is one of the largest EHC studies conducted in HIV-1–infected subjects initiating treatment with ARVs. Despite being one of the largest studies to use the EHC technique for the evaluation of insulin sensitivity in HIV-1–infected subjects, this study is limited by its relatively small sample size and its limited ability to detect differences between the study arms. The small sample size also prohibited analyses that were adjusted based on baseline characteristics.
Conclusions
This substudy of the METABOLIK trial demonstrated similar modest changes in glucose disposal rates and insulin sensitivity over 48 weeks using DRV/r and ATV/r, as measured by the EHC technique. These data suggest a favorable insulin sensitivity profile for both DRV/r and ATV/r.
Author description
ETO developed the protocol, served as an investigator on the protocol, oversaw data collection, assisted with data analysis, and participated in drafting and editing the manuscript. PT served as an investigator on the protocol, assisted with data collection and data analysis, and participated in drafting and editing the manuscript. BC, RR, AP, YKD, GDLR, and BPB conceived and designed the experiments, analyzed the data, and participated in drafting and editing the manuscript. All authors read and approved the final manuscript.
Clinical Trial registration: NCT00757783
Disclosure statement
ETO has served as a consultant for Gilead. PT has been a consultant for Merck and Gilead, serves on an adjudication committee for a GlaxoSmithKline vaccine study, and gave a continuing medical education talk in Spain that was sponsored by Janssen. BC was a full-time contract employee for Janssen at the time this study was conducted. RR, AP, YKD, GDLR, and BPB are employees of Janssen and stockholders of Johnson & Johnson. The METABOLIK trial was sponsored by Janssen Therapeutics.
Acknowledgements
The authors would like to thank the subjects and their families, the principal investigators (Carl Fichtenbaum, Nur Onen, Edgar T Overton, Frank Rhame, and Pablo Tebas), the METABOLIK substudy Janssen study team, and the study center staff for their participation in the substudy. Editorial support for this manuscript was provided by Courtney St. Amour, PhD, of MedErgy, and was funded by Janssen.
References
- Murphy RL, Berzins B, Zala C, et al. Change to atazanavir/ritonavir treatment improves lipids but not endothelial function in patients on stable antiretroviral therapy. AIDS. 2010;24:885–890.10.1097/QAD.0b013e3283352ed5
- Overton ET, Arathoon E, Baraldi E, Tomaka F. Effect of darunavir on lipid profile in HIV-infected patients. HIV Clin Trials. 2012;13:256–270.10.1310/hct1305-256
- Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients – association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193.10.1097/00002030-200305230-00010
- Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The data-collection on adverse effects of anti-HIV drugs (D:A:D) study. Eur J Prev Cardiol. 2015. Epub ahead of print.
- Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: The centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–955.10.1093/ije/dym231
- Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70.10.1097/00002030-199907090-00001
- Flint OP, Noor MA, Hruz PW, et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: Cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37:65–77.10.1177/0192623308327119
- Stein JH, Komarow L, Cotter BR, et al. Lipoprotein changes in HIV-infected antiretroviral-naïve individuals after starting antiretroviral therapy: ACTG study A5152s Stein: lipoprotein changes on antiretroviral therapy. J Clin Lipidol. 2008;2:464–471.10.1016/j.jacl.2008.08.442
- Gotti D, Cesana BM, Albini L, et al. Increase in standard cholesterol and large HDL particle subclasses in antiretroviral-naïve patients prescribed efavirenz compared to atazanavir/ritonavir. HIV Clin Trials. 2012;13:245–255.10.1310/hct1305-245
- Ofotokun I, Na LH, Landovitz RJ, et al. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin Infect Dis. 2015;60:1842–1851.10.1093/cid/civ193
- Zhou H, Jarujaron S, Gurley EC, et al. HIV protease inhibitors increase TNF-α and IL-6 expression in macrophages: Involvement of the RNA-binding protein HuR. Atherosclerosis. 2007;195:e134–e143.10.1016/j.atherosclerosis.2007.04.008
- Yarasheski KE, Tebas P, Sigmund C, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr. 1999;21:209–216.10.1097/00126334-199907010-00005
- Dubé MP. Disorders of glucose metabolism in patients infected with Human Immunodeficiency Virus. Clin Infect Dis. 2000;31:1467–1475.10.1086/cid.2000.31.issue-6
- Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–F173.10.1097/00002030-199815000-00001
- Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53:323–332.10.1097/QAI.0b013e3181c990bf
- PREZISTA® (darunavir) [package insert]. Titusville, NJ: Janssen Therapeutics; May 2015.
- PREZISTA® (darunavir). EPARs for authorized medicinal products for human use. December 2015.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed June 11, 2015.
- Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses. 2012;28:1184–1195.10.1089/aid.2011.0327
- Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabet Care. 2012;35:1605–1610.10.2337/dc11-2339
- Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–534.10.1046/j.1464-5491.2002.00745.x
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26.
- Noor MA, Parker RA, O’Mara E, et al. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS. 2004;18:2137–2144.10.1097/00002030-200411050-00005
- Noor MA, Seneviratne T, Aweeka FT, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: A randomized, placebo-controlled study. AIDS. 2002;16:F1–F8.10.1097/00002030-200203290-00002
- Dubé MP, Shen C, Greenwald M, Mather KJ. No impairment of endothelial function or insulin sensitivity with 4 weeks of the HIV protease inhibitors atazanavir or lopinavir‐ritonavir in healthy subjects without HIV infection: A placebo‐controlled trial. Clin Infect Dis. 2008;47:567–574.10.1086/591953
- Lee GA, Seneviratne T, Noor MA, et al. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS. 2004;18:641–649.10.1097/00002030-200403050-00008
- Busti AJ, Bedimo R, Margolis DM, Hardin DS. Improvement in insulin sensitivity and dyslipidemia in protease inhibitor-treated adult male patients after switch to atazanavir/ritonavir. J Investig Med. 2008;56:539–544.
- Randell PA, Jackson AG, Boffito M, et al. Effect of boosted fosamprenavir or lopinavir-based combinations on whole-body insulin sensitivity and lipids in treatment-naive HIV-type-1-positive men. Antivir Ther. 2010;15:1125–1132.10.3851/IMP1675
- Blümer RM, van Vonderen MG, Sutinen J, et al. Zidovudine/lamivudine contributes to insulin resistance within 3 months of starting combination antiretroviral therapy. AIDS. 2008;22:227–236.10.1097/QAD.0b013e3282f33557
- Defronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223.
- Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655.10.1016/S0140-6736(08)61081-8
- Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6:45–86.10.1210/edrv-6-1-45
- Tomaka F, Lefebvre E, Sekar V, et al. Effects of ritonavir-boosted darunavir vs. ritonavir-boosted atazanavir on lipid and glucose parameters in HIV-negative, healthy volunteers. HIV Med. 2009;10:318–327.10.1111/hiv.2009.10.issue-5
