Abstract
Objective: To compare 48-week changes in bone mineral density (BMD) and body fat distribution between patients continuing lopinavir/ritonavir and two NRTIs and those switching to lopinavir/ritonavir and lamivudine.
Methods: Substudy of a randomized, open-label, multicenter OLE study was carried out. Adult HIV-infected patients with <50 copies/mL for ≥6 months were randomized (1:1) to continue lopinavir/ritonavir and two NRTIs or switching to lopinavir/ritonavir and lamivudine. Dual-energy X-ray absorptiometry (DXA) was performed at baseline and after 48 weeks to measure bone composition and body fat distribution in both the groups.
Results: Forty-one patients (dual-therapy, n = 23; triple-therapy, n = 18) of 239, who received at least one dose of study medication, completed the study: median age, 42 years, 71% male, 73% Caucasian. At week 48, total BMD increased by 1.04% (95% CI, 0.06 to 2.01%) among patients switching to dual-therapy, whereas no significant changes occurred in patients maintaining triple-therapy. Dual-therapy and older age were independently associated with total BMD increase. Among patients discontinuing tenofovir-DF, a significant increase was seen in total BMD (1.43; 95% CI, −0.04 to 2.91) and total hip (1.33%; 95% CI, 0.44 to 2.22%). A non-statistically significant decrease in femoral and spinal BMD was observed in patients who discontinued abacavir and in those continuing triple-therapy. Regarding fat distribution, no significant changes were seen in both the treatment groups.
Discussion: BMD increased following switching to lopinavir/ritonavir plus lamivudine in HIV-infected patients on suppressive triple-therapy with lopinavir/ritonavir and two NRTIs including tenofovir-DF.
The prevalence of osteopenia and osteoporosis is higher in HIV-infected patients,Citation1 and the risk of bone fractures is increased in patients infected with HIV as compared to the general population.Citation2,3
Patients infected with HIV have frequently more traditional risk factors for low bone mineral density (BMD).Citation1 In addition, HIV infection by itself, inducing inflammationCitation4 and immune activation, favors osteoclastic bone resorption.Citation5,6 Furthermore, BMD declines 2–6% during the first months after initiation of ART regardless of the regimen,Citation7–9 and this decline appears to be greater with tenofovir DF–emtricitabine as compared to abacavir–lamivudine-containing regimens.Citation10–13 Treatment with HIV protease inhibitors (PI) might also contribute to BMD loss.Citation9,10,13,14
Although BMD decline seems to stabilize during the first 48 weeks of treatment, progressive BMD decrease might persist beyond week 48 of treatment,Citation15 at a rate of −0.8% per year at the hip level,Citation7 a loss rate similar to that observed in postmenopausal women.Citation16
On the other hand, previous data suggest a class effect of PI on abdominal fat deposit.Citation10,17,18 Visceral adipose deposit, which has been associated with an increased risk of cardiovascular disease,Citation19 might progress overtime, even in patients who have been under PI exposure for a long period of time.Citation20
The aim of this study was to compare 48-week changes in BMD and body fat distribution between patients switching to lopinavir/ritonavir and lamivudine and those continuing triple-therapy with lopinavir/ritonavir and two N(t)RTI, in the OLE study.
Patients and methods
Study design and participants
The “Only Lopinavir and Epivir” (OLE) study was a phase 3, randomized, open-label clinical trial conducted at 32 sites in Spain and France (ClinicalTrials.gov, number NCT01471821).Citation21 In the OLE study, HIV-infected adults on lopinavir/ritonavir twice-daily plus lamivudine/emtricitabine and another N(t)RTI for at least two months, with plasma HIV RNA <50 copies/mL for at least 6 months, were randomly assigned (1:1) to either switch to lamivudine 300 mg/once-daily plus lopinavir/ritonavir 400/100 mg twice-daily (dual-therapy) or to remain on the current regimen (triple-therapy).
The OLE-LIP substudy was planned prior to the initiation of the OLE study. Patients were consecutively enrolled from four sites in Barcelona, Spain. The primary endpoint was to compare changes in limb fat, trunk fat, and total fat distribution, and to compare changes in BMD and T scores in total body, lumbar spine (L1–L4), femoral neck, and total hip at study week 48, in patients randomized to switching to lopinavir/ritonavir and lamivudine and in those continuing triple-therapy (overall population).
The protocol was reviewed and approved by the Ethic Committee in all participating sites. All patients provided written informed consent.
Procedures
At baseline and at week 48 whole body, lumbar and hip DXA scans (Lunar DPXL, Madison, Wisconsin, USA) were performed to assess BMD and body fat distribution. The DXA scans were centralized in a unique center following a standardized protocol and read by an experienced radiologist unaware of the patient’s ART. The results of the DXA scans were given to the physicians in care of the patients after the OLE study was ended. Consequently, no therapeutic interventions were made, based on the results of the DXA scan, throughout the study.
Statistical analysis
Qualitative variables are reported as number of patients and percentages in each category, and compared using the chi-squared test, with the continuity correction. For quantitative variables, medians and interquartile ranges (IQRs) (25th–75th percentiles) or means and 95% confidence intervals are used as measures of central tendency and dispersion. Intragroup changes from baseline to week 48 are compared using T-test for paired data, and the Wilcoxon’s signed-rank test is used for comparison of non-normally distributed data. Comparisons between quantitative variables are performed with T-test for unpaired data, or the Mann–Whitney U-test for data with non-normal distribution.
We performed a multivariate linear regression analysis to explore the impact of treatment (dual-therapy vs. triple-therapy) on BMD outcome. Different models using global BMD, L1–L4 BMD, and femoral neck BMD as dependent variables were constructed. Models were adjusted by age, baseline BMD values, body mass index, and NRTIs backbone (ABC/3TC vs. TDF/FTC-3TC). Using a backward fitting approach, variables were left in the model if they improved the model fit and/or have a coefficient with a p value < 0.1.
To assess the effect of tenofovir-DF discontinuation on BMD changes, we also performed a stratified analysis based on the backbone treatment at baseline: tenofovir DF–emtricitabine/lamivudine vs. abacavir–lamivudine.
All statistical tests are two-tailed and are performed at a level of statistical significance of 0.05. SPSS 21.0 software for Windows (IBM Corp, Armonk, NY, USA) was used for statistical analyses.
Results
Between October 2011 and April 2013, 45 patients were included in this study. Four patients (one on dual-therapy and three in the triple-therapy arm) did not have available DXA scans data at baseline (n = 1) or at treatment week 48 (n = 3). Forty-one patients (dual-therapy, n = 23; triple-therapy, n = 18) completed the OLE–LIP study. Patients on triple-therapy were older than patients on dual-therapy: 47 vs. 37 year-old, respectively (p = <0.01). Otherwise, both study arms were well balanced (Table ).
Table 1 Demographic and baseline characteristics
Bone mineral density
At baseline, no between-group differences were observed in BMD (Table ). In addition, no statistical differences were observed in BMD in a stratified analysis based on the use of tenofovir-DF or abacavir at study entry (S1).
At week 48, total BMD increased by 1.04% (95% CI, 0.06–2.01%) in patients switching to dual-therapy, whereas no significant changes were observed in those maintaining triple-therapy (Fig. and S1).
Figure 1 Mean percentage (95% CI) change from baseline in BMD in the overall population and according to the NRTIs backbone. (A) total BMD, (B) hip BMD, (C) lumbar spine BMD.
*P value for within-group comparison; † P value for dual-therapy vs. triple-therapy comparison; DT: dual-therapy; TT: triple-therapy.
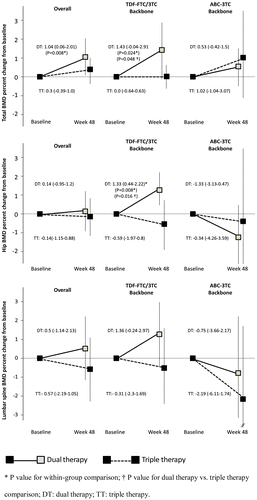
By multivariable regression analysis, dual-therapy (coefficient value, 0.403; 95% CI: 0.279–2.581; p = 0.016) and age (coefficient value, 0.406; 95% CI: 0.017–0.152; p = 0.016) were associated with an increase in total BMD. Similarly, dual-therapy (coefficient value, 2.574; 95% CI: 0.404–4.745; p = 0.021) and age (coefficient value, 0.169; 95% CI: 0.038–0.300; p = 0.013) were associated with an increase in lumbar spine BMD. No variable was associated with changes in femoral neck BMD.
Among patients on dual-therapy who discontinued tenofovir-DF, an increase was seen in total BMD (1.43; 95% CI, −0.04 to 2.91; p = 0.024), total hip (1.33%; 95% CI, 0.44–2.22%; p = 0.008), femoral neck (0.93%; 95% CI, −0.40–2.26%; p = 0.143), and lumbar spine (1.36%; 95% CI, −0.24–2.97%; p = 0.098) (Fig. and S1). A non-statistically significant decrease in BMD was observed at these locations in patients who discontinued abacavir and in those continuing triple-therapy (Fig. and S1). Furthermore, at study week 48, total hip T-score significantly increased, 0.08 (95%CI, 0.02 to 0.14), and a mild non-significant increase in T-score was observed at femoral neck and lumbar spine, among patients who discontinued tenofovir-DF (S1). Conversely, a non-significant decrease in T-score was observed at these locations in patients stopping abacavir and in those who continued triple-therapy (S1).
Fat distribution
At baseline, median total fat was similar in patients switching to dual-therapy, 16094 g (IQR, 11976 to 20359) and in those continuing triple-therapy 16789 g. In addition, limb/trunk fat ratio and fat mass ratio were comparable in both groups (Table ).
No significant differences were seen in fat distribution within or between groups after 48 weeks of treatment (S2).
Discussion
In our study, total BMD significantly increased by a mean of 1% among patients switching to dual-therapy, whereas no changes were seen in BMD among patients who continued triple-therapy, over 48 weeks of treatment. By multivariable linear regression analysis, dual-therapy was independently associated with an increase in global and lumbar spine BMD.
Data obtained from a stratified analysis, based on the baseline backbone, suggest that the improvement in BMD was driven by tenofovir-DF discontinuation. In the subgroup of patients who discontinued tenofovir-DF, total BMD and total hip BMD significantly increased (+1.4 and +1.3%, respectively) at treatment week 48. In addition, a non-statistically significant mild increase was seen in femoral neck (+0.9%) and lumbar spine BMD (+1.4%). Conversely, BMD mildly decreased at these locations in patients who discontinued abacavir and in those continuing triple-therapy, although none of these changes reached statistical significance.
In previous studies, BMD decrease was significantly higher in patients starting treatment with tenofovir-DF/emtricitabine as compared to those receiving abacavir–lamivudine.Citation10–12 In a metabolic substudy of ACTG 5202 trial, comparing emtricitabine/TDF and abacavir/lamivudine backbones as well as efavirenz and atazanavir/r as a third drug, BMD decline at week 96 was greater with emtricitabine/TDF than with abacavir/lamivudine, both at hip (−1.4%) and at lumbar spine (−2%) levels.Citation10
Furthermore, compared to N(t)RTI-sparing antiretroviral regimen with darunavir/r plus raltegravir, patients starting treatment with darunavir/r plus emtricitabine/tenofovir-DF had a significantly greater decrease in BMD at treatment week 48.Citation22 Similarly, lopinavir/r and raltegravir as a first-lineCitation23,24 or as a second-line therapy has been associated with less BMD loss than lopinavir/r regimen-containing N(t)RTIs.Citation25
The negative impact of tenofovir-DF on BMD has also been reported in treatment-suppressed patients.Citation26,27 In the STEAL study,Citation26 360 patients were randomized to switch their current NRTIs to either abacavir/lamivudine or emtricitabine/tenofovir-DF. At week 48, hip and spine T-score significantly decreased among patients treated with emtricitabine/tenofovir-DF (−0.11, at both locations), whereas T-score improved in the abacavir/lamivudine group at both locations (+0.08 and +0.05, respectively). Also, osteopenia, osteoporosis, or receipt of antiresorptive therapy were significantly more common in the emtricitabine/tenofovir-DF group, although no further changes were seen between week 48 and 96 of treatment, and the risk of bone fracture did not increased in these patients.Citation26 In other study, 64 patients on emtricitabine/tenofovir-DF/efavirenz were randomized (1:1) to continue the same regimen or to darunavir/r monotherapy (800/100 mg once-daily) for 48 weeks.Citation28 A significant increase in BMD (+2.9% at femur neck and +2.6% at lumbar spine) was seen in patients switching to darunavir/r, whereas no significant changes occurred among those who continued emtricitabine/tenofovir-DF/efavirenz. In addition, a significant increase in 25-OH vitamin D levels and a reduction in bone biomarkers were seen in patients with cin to darunavir/r as compared to those on emtricitabine/tenofovir-DF/efavirenz. However, the risk of viral rebound was higher in patients with monotherapy.Citation28
Although the pathogenesis remains to be fully characterized, tenofovir-related bone toxicity might be associated with changes in renal tubular dysfunction and disturbance of phosphate homeostasis.Citation29 Additionally, a calcilytic effect of tenofovir has been observed in vitro.Citation30 By inhibiting the activity of the calcium-sensing receptor, a fine sensor of the extracellular calcium concentration, tenofovir promotes hypersecretion of parathyroid hormone (PTH).Citation30 Nevertheless, changes in BMD might occur without alterations in PTH levels, suggesting a direct effect of tenofovir-TDF on bone metabolism.Citation31
Tenofovir alafenamide (TAF), a pro-drug of tenofovir-DF with a 90% lower plasmatic concentration, has shown a better renal and bone safety profile as compared to tenofovir-DF.Citation32 In the GS-US-292-109 study, 1463 patients receiving a TDF-based suppressive regimen were randomized (1:2) to continue the same regimen or to switch to emtricitabine/cobicistat/elvitegravir/TAF.Citation33 Median change in lumbar spine BMD was +1.79 among patients switching to emtricitabine/cobicistat/elvitegravir/TAF and –0.28% in patients continuing the TDF-based regimen (p < 0.001). Changes in total hip BMD were +1.37 and −0.26%, respectively.Citation33 In addition, we found a positive association between age and BMD outcome at week 48, both at global and lumbar spine levels. In regard to this finding, an age-dependent effect of TDF on BMD was seen in a post hoc analysis of the ACTG 5224s trial, in treatment-naive patients starting ART with TDF/emtricitabine vs. ABC/lamivudine (plus efavirenz or atazanavir/r). In this study, TDF-associated BMD decrease, both at lumbar spine and hip levels, was higher in patients younger than 30 years as compared to older patients.Citation34
Although conflicting data have been reported,Citation8 numerous studies have shown a negative impact of PI on BMD loss.Citation9,10,13,14,35,36 In the ACTG 5202Citation10, the decrease in spine BMD was significantly higher with atazanavir/r than with EFV (−3.1% vs. −1.7%; P = .035). In addition, in other study, BMD improved following the switching of PI/r to raltegravir.Citation20 In an in vitro model, exposure of human bone marrow mesenchymal stem cells (MSCs) to atazanavir or darunavir promoted MSCs senescence and impaired their capacity to differentiate into osteoblasts.Citation37 In our study, as both groups of treatment received a lopinavir/ritonavir-containing regimen, we could not evaluate the impact of this drug on BMD outcome.
In regard to fat distribution, patients included in this study have a preserved body fat composition, with a fat mass ratio under 1.5 and almost 8 kg of limb fat. In agreement with previously reported data, in patients receiving a PI-containing triple-therapy,Citation20 no significant changes were observed in fat distribution, as analyzed by DXA scans, over 48 weeks of treatment, in our study.
Some limitations are worth mentioning. The analysis was limited to 41 patients, which could influence the statistical potency for detecting fat changes in our study. However, it is worth underscoring that even with this small sample size, significant differences have been observed in BMD. Furthermore, DXA scans do not allow differentiating between subcutaneous and visceral fat, and more sensitive techniques such as CT scans or MR images were not performed. Consequently, a progressive increase in visceral abdominal fat, previously observed on patients continuing a PI/r-based regimen,Citation20 cannot be ruled out from our study.
In spite of these limitations, our study showed an improvement in BMD among patients who stopped tenofovir-DF and switched to dual-therapy with lopinavir/r and lamivudine. Although the clinical relevance of changes observed in our study over 48 weeks of follow-up is not completely understood, concerns exist in regard with the negative impact of ART on progressive BMD loss. A growing number of older persons at risk for bone fractures are living with HIV, and prevention of BMD loss has become an important component of HIV care.Citation38 Preliminary data suggest that switching from tenofovir-TD to TAF,Citation33 abacavir,Citation39 or raltegravirCitation40 might improve BMD. Alternatively, according to our data, a switch strategy of stopping tenofovir-TD and continuing dual-therapy with lopinavir/r plus lamivudine might also be beneficial in terms of BMD outcome, maintaining virological suppression.Citation21
In summary, an increase in BMD was seen in our study after switching to lopinavir/ritonavir and lamivudine in HIV-infected patients on suppressive triple-therapy with lopinavir/ritonavir and two N(t)RTIs including tenofovir-DF.
Contributors
MC is a senior consultant in the Infectious Diseases Department, Hospital Universitari Vall d’Hebrón; associate researcher at Autonomous University of Barcelona; Vall d’Hebron Research Institute, Barcelona. Spain. The author’s research interests include Clinical Trials, HCV/HIV-coinfection, HIV eradication, and Immune pathogenesis. And the author has also published books/articles: Chapters in books: 3; Indexed articles: 21
M M-R, MD, PhD, is a postdoctoral researcher, Infectious Diseases Service, Hospital Clinic, IDIBAPS, Barcelona, Spain. The author’s research interests include Antirretroviral therapy and Coinfection HIV-HCV. And the author has also published books/articles: Chapters in books: 3; Indexed articles: 21
JN, MD, in the Infectious Diseases Department, Hospital Universitari Vall d’Hebrón; Autonomous University of Barcelona, Vall d’Hebron Research Institute, Barcelona. Spain. The author’s research interests include HIV infection, HIV-HCV co-infection, antiretroviral treatment, and HIV comorbidities. And the author has also published books/articles: 0/11.
DP, is the chief of AIDS Unit. Hospital Universitari de Bellvitge, Barcelona, Spain. The author’s research interests include Infectious Diseases and HIV/AIDS. And the author has also published books/articles: 51/>100.
PD, is a professor of Medicine, Universitat de Lleida, Spain. The author’s research interests include HIV infecion, ART, co-morbidities, ART complications and Aging. And the author has also published books/articles (nº): 108/ 426.
JM, is a associate professor of Medicine, Hospital Clinic de Barcelona, Spain. The author’s research interests include HIV, HIV-HCV, Pharmacokinetics of antiretrovirals and HIV co-morbidities. And the author has also published books/articles: Books: 20 (chapters)/ Articles: 370.
MS, PhD, AIDS Unit. Hospital Universitari de Bellvitge, Barcelona, Spain. The author’s research interests include HIV indefction, cardiovascular risc, subclinical atherosclerosis and cardiovascular biomarkers. And the author has also published books/articles: 0/12.
G M-M, M.D., AIDS Unit. Hospital Santa Creu i Sant Pau , Barcelona, Spain. The author’s research interests include HIV Clinical Trials and HIV-comorbidities. And the author has also published books/articles: 0/14.
AC, MD, Infectious Diseases Department, Hospital Universitari Vall d’Hebrón; Autonomous University of Barcelona; Vall d’Hebron Research Institute, Barcelona. Spain. The author’s research interests include HIV co-morbidities, Pharmacokinetics, and STD. And the author has also published books/articles: 5/45.
JG, is a professor of Medicine, University of Barcelona. The author’s research interests include HIV antiretroviral treatment and HIV vaccine. And the author has also published books/articles: >50/>400.
ER, is a senior consultant, Infectious Diseases Department, Hospital Universitari Vall d’Hebrón. The author’s research interests include HIV Clinical Trials, Clinical Pharmacology and Complication of HIV and antiretroviral therapy. And the author has also published books/articles: 45/>200.
Financial support/disclosures
AbbVie provided funding for the OLE trial (OLE-LIP is a substudy of the OLE trial); [RD12/0017/0003]. Acción Estratégica en Salud. Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica 2008–2011. Instituto de Salud Carlos III. Fondos FEDER.
Conflict of interest
M Crespo has received consultancy fees from Gilead, ViiV, MSD; research grants from Gilead, ViiV, MSD, and Janssen; and payment for lectures including service on speakers bureaus from Gilead, ViiV, BMS, Abbot, and JanssenCilag
Publication ethics
The protocol was reviewed and approved by the Ethic Committee in all participating sites. All patients provided written informed consent. All authors have approved the manuscript, which describes original work that is not under consideration by any other journal.
OLE-LIP study team
H. Clínic: J. Mallolas, J. Gatell, M. Martínez, J. Pich, J.A. Arnaiz.
H. Universitari Vall d’Hebrón: M. Crespo, E. Ribera, Adria Curran, Jordi Navarro, Ariadna Torrella.
H. Universitari de Bellvitge: D. Podzamczer, J.M. Tiraboschi, M. Saumoy.
H. de la Santa Creu i Sant Pau: P. Domingo, G. Mateo, M. Gutiérrez, J. Muñoz-
References
- McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: A practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946.10.1086/652982
- Young B, Dao CN, Buchacz K, et al. Increased rates of bone fracture among HIV-infected persons in the HIV outpatient study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52:1061–1068.10.1093/cid/ciq242
- Prieto-Alhambra D, Güerri-Fernández R, De Vries F, et al. HIV infection and its association with an excess risk of clinical fractures. J Acquir Immune Defic Syndr. 2014;66:90–95.10.1097/QAI.0000000000000112
- Hileman CO, Labbato DE, Storer NJ, et al. Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS. 2014;28:1759–1767.10.1097/QAD.0000000000000320
- Titanji K, Vunnava A, Sheth AN, et al. Dysregulated B cell expression of RANKL and OPG correlates with loss of bone mineral density in HIV infection. PLoS Pathog. 2014;10:e1004497.10.1371/journal.ppat.1004497
- Gazzola L, Bellistri GM, Tincati C, et al. Association between peripheral T-Lymphocyte activation and impaired bone mineral density in HIV-infected patients. J Transl Med. 2013;11:51.10.1186/1479-5876-11-51
- Grund B, Peng G, Gibert CL, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23:1519–1529.10.1097/QAD.0b013e32832c1792
- Brown TT, McComsey GA, King MS, et al. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561.10.1097/QAI.0b013e3181adce44
- Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;27:817–824.10.1097/QAD.0b013e328328f789
- McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801.10.1093/infdis/jir188
- Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir‐lamivudine versus tenofovir‐emtricitabine in HIV‐infected adults: 48‐week results from the ASSERT study. Clin Infect Dis. 2010;51:963–972.10.1086/652982
- Moyle GJ, Stellbrink HJ, Compston J, et al. 96-week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antiviral Ther. 2013;18:905–913.10.3851/IMP2667
- Grant PM, Kitch D, McComsey GA, et al. Low baseline CD4 + count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis. 2013;57:1483–1488.10.1093/cid/cit538
- Negredo E, Domingo P, Ferrer E, et al. Peak bone mass in young HIV-infected patients compared with healthy controls. J Acquir Immune Defic Syndr. 2014;65:207–212.10.1097/01.qai.0000435598.20104.d6
- Huang JS, Hughes MD, Riddler SA, et al. Bone mineral density effects of randomized regimen and nucleoside reverse transcriptase inhibitor selection from ACTG A5142. HIV Clin Trials. 2013;14:224–234.10.1310/hct1405-224
- Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–490.10.1007/s001980050093
- Moyle GJ, Hardy H, Farajallah A, et al. Changes in bone mineral density after 96 weeks of treatment with atazanavir/ritonavir or lopinavir/ritonavir plus tenofovir DF/emtricitabine in treatment-naive patients with HIV-1 infection. J Acquir Immune Defic Syndr. 2015;68:40–45.10.1097/QAI.0000000000000383
- Martinez E, Gonzalez-Cordon A, Ferrer E, et al. Differential body composition effects of protease inhibitors recommended for initial treatment of HIV infection: A randomized clinical trial. Clin Infect Dis. 2015;60:811–820.10.1093/cid/ciu898
- Lake JE, Wohl D, Scherzer R, et al. Regional fat deposition and cardiovascular risk in HIV infection: The FRAM study. AIDS Care. 2011;23:929–938.10.1080/09540121.2010.543885
- Curran A, Martinez E, Saumoy M, et al. Body composition changes after switching from protease inhibitors to raltegravir. AIDS. 2012;26:475–481.10.1097/QAD.0b013e32834f3507
- Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): A randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:785–792.
- Bernardino JI, Mocroft A, Mallon PW, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: A substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2015;2:e464–e473.10.1016/S2352-3018(15)00181-2
- Bedimo RJ, Drechsler H, Jain M, et al. The RADAR study: Week 48 safety and efficacy of raltegravir combined with boosted darunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. impact on bone health. PLoS One. 2014;9:e106221.10.1371/journal.pone.0106221
- Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2013;29:256–265.
- Martin A, Moore C, Mallon PW, et al. Bone mineral density in HIV participants randomized to raltegravir and lopinavir/ritonavir compared with standard second line therapy. AIDS. 2013;27:2403–2411.10.1097/01.aids.0000432534.47217.b4
- Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir‐emtricitabine or abacavir‐lamivudine: A randomized, 96‐week trial. Clin Infect Dis. 2009;49:1591–1601.10.1086/599193
- Rasmussen TA, Jensen D, Tolstrup M, et al. Comparison of bone and renal effects in hiv-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS ONE. 2012;7:e32445.10.1371/journal.pone.0032445
- Hamzah L, Tiraboschi JM, Iveson H, et al. Effects on vitamin D, bone and the kidney of switching from fixed-dose tenofovir disoproxil fumarate/emtricitabine/efavirenz to darunavir/ritonavir monotherapy: A randomized, controlled trial (MIDAS). [Epub ahead of print Oct 13, 2015]. Antivir Ther. PMID: 26460504.
- Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. 2009;23:689–696.10.1097/QAD.0b013e3283262a64
- Menzaghi BQT, Bonfanti P, Molteni C, et al. Bone metabolism and tenofovir: Evidence of direct effect on calcium-sensing receptor. In: 22nd Conference on Retroviruses and Opportunistic Infections (CROI); 2015, February 23–26; Seattle P-765.
- Cotter AG, Vrouenraets SM, Brady JJ, et al. Impact of switching from zidovudine to tenofovir disoproxil fumarate on bone mineral density and markers of bone metabolism in virologically suppressed HIV-1 infected patients; a substudy of the PREPARE study. J Clin Endocrinol Metab. 2013;98:1659–1666.10.1210/jc.2012-3686
- Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615.10.1016/S0140-6736(15)60616-X
- Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: A randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16:43–52.10.1016/S1473-3099(15)00348-5
- Grant PM, Kitch D, McComsey GA, et al. Differential skeletal impact of tenofovir disoproxil fumarate in young versus old HIV-infected adults. HIV Clin Trials. 2015;16:66–71.10.1179/1528433614Z.0000000010
- Kinai E, Nishijima T, Mizushima D, et al. Long-term use of protease inhibitors is associated with bone mineral density loss. AIDS Res Hum Retroviruses. 2014;30:553–559.10.1089/aid.2013.0252
- Bonjoch A, Figueras M, Estany C, et al. High prevalence of and progression to low bone mineral density in HIV-infected patients: A longitudinal cohort study. AIDS. 2010;24:2827–2833.10.1097/QAD.0b013e328340a28d
- Hernandez-Vallejo SJ, Beaupere C, Larghero J, et al. HIV protease inhibitors induce senescence and alter osteoblastic potential of human bone marrow mesenchymal stem cells: Beneficial effect of pravastatin. Aging Cell. 2013;12:955–965.10.1111/acel.2013.12.issue-6
- Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. 2015;60:1242–1251.10.1093/cid/civ010
- Negredo E, Diez-Perez A, Bonjoch A, et al. Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: Changes in bone turnover markers and circulating sclerostin levels. J antimicrob chemother. 2015;70:2104–2107.
- Bloch M, Tong WW, Hoy J, et al. Switch from tenofovir to raltegravir increases low bone mineral density and decreases markers of bone turnover over 48 weeks. HIV Med. 2014;15:373–380.10.1111/hiv.2014.15.issue-6
