Abstract
Objectives: Despite substantial improvements in HIV outcomes with combination antiretroviral therapy (cART), morbidity and mortality remain above population norms. The gut mucosal immune system is not completely restored by cART, and the resultant microbial translocation may contribute to chronic inflammation, inadequate CD4 T-cell recovery, and increased rates of serious non-AIDS events. Since the microbial environment surrounding a CD4 T cell may influence its development and function, we hypothesize that probiotics provided during cART might reduce inflammation and improve gut immune health in HIV-positive treatment-naïve individuals (PROOV IT I) and individuals with suboptimal CD4 recovery on cART (PROOV IT II).
Methods: These prospective, double-blinded, randomized, placebo-controlled, multicenter pilot studies will assess the impact of the probiotic Visbiome at 900 billion bacteria daily. Forty HIV positive cART-naïve men will be randomized in the PROOV IT I study, coincident with antiretroviral initiation, and be followed for 24 weeks. In PROOV IT II, 36 men on cART, but with a CD4 T-cell count below 350 cells/mm3 will be followed for 48 weeks. The primary outcome for both studies is the comparison of blood CD8 T-cell immune activation. Secondary analyses will include comparison of blood inflammatory biomarkers, microbial translocation, blood and gut immunology and HIV levels, the bacterial community composition, diet, intestinal permeability, and the safety, adherence and tolerability of the study product.
Discussion: These studies will evaluate the ability of probiotics as a safe and tolerable therapeutic intervention to reduce systemic immune activation and to accelerate gut immune restoration in people living with HIV.
Background
Current combination antiretroviral therapy (cART) has transformed the clinical care and the lived experience of HIV infection.Citation1 However, increased rates of serious non-AIDS events (SNAEs) such as cardiovascular disease (CVD), bone, renal, and neurodegenerative disease occur in cART-treated individuals, and may be caused in part by persistent immune activation/inflammation.Citation2, 4 An important contributor to this inflammation may be the “leaking” of luminal gut microbes across the bowel wall and into the bloodstream, a process referred to as microbial translocation.Citation5, 6 Circulating microbial products such as lipopolysaccharide (LPS) are potent triggers of the innate immune system, and thus prolonged microbial translocation during cART may be a major contributor to chronic inflammation.Citation5 Therapeutic interventions to enhance gut immune defenses may therefore reduce chronic inflammation, restore CD4 T cells, and decrease rates of SNAEs in HIV-infected individuals.
HIV infection may lead to microbial translocation and inflammation through a few mechanisms.Citation7, 8 First, HIV directly damages the integrity of the gut epithelial lining,Citation7, 9 allowing microbes that normally reside in the gut lumen to more readily cross into the lamina propria.Citation10 Second, HIV preferentially replicates within the gut mucosal tissues, which are rich in highly susceptible CD4+ T cells, resulting in a rapid mucosal CD4 depletion and T-cell dysregulation.Citation11, 12 In particular, tissue-reparative Th22 cells and microbe-responsive Th17 cells are preferentially depleted,Citation8, 13, 14 which may compromise the integrity of the epithelial barrier and the clearance of invading microbes.Citation15–18 Third, there is a dysbiotic shift of the gut microbiome and translocation of the pathogenic microbes across the gut wall during HIV infection and cART that may induce local and systemic inflammation.Citation19–21 These events lead to increased levels of microbial products such as lipopolysaccharide (LPS) in the bloodstream, and to downstream host immune activation/inflammation.Citation10, 22 Despite rapid viral suppression and more gradual CD4 T-cell reconstitution in the blood after effective cART initiation, to restore the number and function of gut CD4 T-cell subsets may require several years of suppressive cART.Citation23, 24 This slow reconstitution of protective mucosal cells and HIV-associated dysbiosis may allow for persistent microbial translocation, increased immune activation and chronic inflammation.Citation8, 13, 25
Up to 25% of cART-treated individuals demonstrate suboptimal blood CD4 recovery despite effective viral suppression; this “immunologic non-responder” (INR) phenotype is associated with increased immune activationCitation26–28 and with higher rates of AIDS and non-AIDS related conditions,Citation29, 30 and death.Citation31 Poor gut integrity, increased microbial translocation, and reduced CD4 T-cell trafficking to the gutCitation32, 33 could be a source of ongoing inflammation in INR individuals. Since current standard cART does not timely restore the full depth and breadth of the gut immune system, a treatment regimen that is deemed successful should ideally aim to normalize the gut immune cells soon after cART initiation.
Complementary and alternative therapies are used by approximately half of people living with HIV.Citation34, 35 However, there is a paucity of clinical trials in this field, and people living with HIV/AIDS in Ontario have identified evaluating the efficacy of complementary therapies as a research priority.Citation36 The World Health Organization defines probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”.Citation37 Since the development of immune cells such as Th17 and Th22 cells can be driven by exposure to specific bacteria,Citation38, 39 such a product may be able to alter mucosal immunity in HIV-infected people, particularly since HIV infection is associated with gut microbial dysbiosis.Citation19, 20, 40 Therefore, we hypothesize that probiotic supplementation may enhance the recovery of gut Th17 and Th22 number and function in people living with HIV, and subsequently reduce microbial translocation and immune activation.
Visbiome (Exegi Pharma LLC.) is the new generic replica of VSL#3® (the brand VSL#3 belongs to VSL Pharmaceuticals Inc.), and is a Health Canada approved natural health product with an excellent safety profile.Citation41 Visbiome is composed of the specific combination of eight different freeze-dried bacteria (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, and Streptococcus thermophiles), as the active principle which is currently known according to the published literature as the probiotic mix VSL#3. The term “VSL#3” has become the usual nomenclature to identify the specific mixture of probiotic bacteria as a common name both by consumers and by the scientific community in all peer-reviewed publications and guidelines. Visbiome contains the same strains in the same concentrations and proportions as the original VSL#3 brand probiotic blend, which has been utilized in all the clinical and experimental studies reported here. In SIV-infected non-human primates, the addition of the probiotics VSL#3 and Culturelle or VSL#3 and IL-21 to standard cART enhanced the reconstitution of the number and function of mucosal CD4+ T cells, including Th17 cells, and enhanced clinical outcome.Citation6, 42 Sixteen weeks of synbiotic (probiotic and prebiotic combination) supplementation in the absence of ART improved CD4 counts, but a recent systematic review of a range HIV probiotic clinical trials found mixed results regarding CD4 count.Citation43 Several probiotic studies of cART-treated individuals have been conducted with varying results. One study demonstrated that probiotic Saccharomyces boulardii supplementation (60 million bacteria daily) for 12 weeks reduced levels of IL-6,Citation44 another study showed that multistrain probiotic use for 8 weeks reduced D-dimer levels,Citation45 while probiotic Bacillus coagulans (2 billion bacteria daily) consumption for 3 months did not increase blood CD4 T-cell counts or reduce levels of SNAE biomarkers IL-6 and D-dimer.Citation46 The Probio-HIV study assessed the impact of a similar probiotic blend to Visbiome for 48 weeks in an open label single arm study and concluded that probiotics may improve gut immune health and reduce inflammation; however, a placebo comparison arm was not included nor were mucosal tissues analyzed.Citation47 Therefore, carefully designed RCTs to evaluate the effect of high-potency probiotics on gut immune health and on systemic inflammation in HIV are warranted. Given the delayed improvement in gut health that occurs after the initiation of standard cART, we designed the two PROOV IT pilot trials called “Probiotic Visbiome for Inflammation and Translocation” or PROOV IT to examine the ability of Visbiome at a daily dose of 900 billion bacteria to accelerate the reduction of systemic immune activation in HIV-infected treatment-naïve individuals initiating cART (PROOV IT I) and in the context of INR participants (PROOV IT II).
Methods/Design
Study Design
The PROOV IT pilot trials (protocol date: September 2015, protocol identifier CTNPT 022A and 022B) are two phase-two prospective, double-blinded, randomized, placebo-controlled, and multicenter studies (Toronto, Canada) designed to evaluate the impact of the probiotic Visbiome (2 sachets daily at 450 billion bacteria per sachet) on blood immune activation in HIV-infected individuals. The PROOV IT I study will enroll 40 male antiretroviral-naïve participants who will be randomized 1:1 into the probiotic vs. placebo arms coincident with ART initiation for 24 weeks; all participants will then have the option of taking Visbiome for an additional 24 weeks in an open-label manner. The PROOV IT II study will enroll 36 INR participants who will be randomized in a 2:1 ratio into the probiotic vs. placebo study arms, respectively, for 48 weeks. Participants in the placebo arm will subsequently be offered a 48-week supply of the probiotic at no cost. Both studies have been registered with ClinicalTrials.gov: NCT02441244 (PROOV IT I) and NCT02441231 (PROOV IT II).
Ethics Approval
The study protocols, informed consent forms and all required documentation were approved by Health Canada’s Natural Health Product Directorate (#179546 and #179613), the Research Ethics Committee of the University Health Network (#14-8023 and #14-8030) and the University of Toronto (#31262 and #31261) for PROOV IT I and II, respectively. All study participants will sign a written consent form prior to enrolment. These studies will be conducted in accordance with the International Conference on Harmonization Good Clinical Practice (ICH GCP) regulations and guidelines and the current version of the Declaration of Helsinki.
Participant Eligibility
Study participants will be recruited from two sites in Toronto, Canada: a primary care setting (the Maple Leaf Medical Clinic) and a tertiary care HIV referral center (the Immunodeficiency Clinic of University Health Network). The study inclusion and exclusion criteria for the studies are outlined in Table . Eligibility for PROOV IT I will include participants in whom the treating physician makes a clinical decision to initiate a cART regimen that consists of recommended or alternate antiretroviral agents as outlined in the most current Department of Health and Human Services (DHHS) guidelines.Citation48 Likewise, PROOV IT II participants may be on any cART regimen.
Table 1 Inclusion and exclusion criteria for PROOV IT I and II
Treatment Groups
In both clinical trials, the participants in the intervention group will receive oral probiotic Visbiome at a Health Canada approved dose of 900 billion bacteria daily (two sachets daily) and participants in the placebo arm will receive identically packaged placebo sachets (two sachets daily) that are indistinguishable in appearance from the active product. The placebo product will contain maltose and silicon dioxide as inactive agent and both active product and placebo will be manufactured and labeled by the same company (Exegi Pharma LLC). All study products will be stored in 4°C.
Randomization and Allocation Concealment
A simple randomization scheme using a secure website will be used to generate a randomization list by Exegi Pharma LLC. using block sizes of 4 and 3 for PROOV IT I (1:1 randomization) and II (2:1 randomization), respectively. A randomization list including the treatment allocation for each study and for each participant will be prepared in individually sealed envelopes, and will be stored in a locked, secured box by the Central Statistician. All participants and study personnel (including investigators, research coordinators and data analysts) will be blinded with respect to treatment allocation for the duration of the study. Access to the allocation code will be restricted to a study statistician or a research pharmacist who will not perform the final study analyses.
Study Duration and Visits
PROOV IT I is a 24 week, randomized double-blinded study that includes the option of extending the study duration for an additional 24 weeks in an open-label manner such that all participants have the opportunity to access probiotics (Figure (a)). Study visits will occur at weeks 0 (baseline), 2, 4, 8, 16, and 24 for the double-blinded portion of the study, and at weeks 26, 28, 32, 40, and 48 for the open-label portion of the study. Each visit will include a physical exam, a blood draw, and a study questionnaire; the baseline, week 24 and week 48 visits will also include a sigmoid biopsy, an intestinal permeability urine test, anal and penile swabs, and a diet questionnaire.
Figure 1. Study design and study visit schedule for the (a) PROOV IT I double-blind and open-label portion, and (b) PROOV IT II studies.
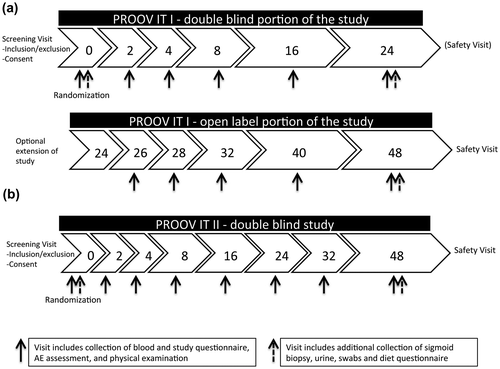
PROOV IT II is a 48-week, randomized double-blinded study. For PROOV IT II, study visits will occur at weeks 0 (baseline), 2, 4, 8, 16, 24, 32, and 48 (Figure (b)) and will include a physical exam, a blood draw and a study questionnaire; the sigmoid biopsy, urine test, anal and penile swabs, and diet questionnaire will occur at the baseline and week 48 visits.
Given the relative invasiveness and increased time requirements of the sigmoid biopsy procedure and the intestinal permeability urine test, both of these procedures will be optional in both studies. For each participant, a post-study safety visit will be scheduled four weeks after the last study visit.
Study Endpoints
The primary endpoint of both trials is a comparison of the blood immune activation as measured by the frequency of HLA-DR and CD38 co-expression on CD8+ T cells and will be evaluated at 24 weeks for PROOV IT I and at 48 weeks for PROOV IT II. The secondary and exploratory endpoints are:
Secondary endpoints
| • | plasma levels of inflammation and coagulation markers (including IL-6 and D-dimer) | ||||
| • | plasma markers of microbial translocation and monocyte activation (including LPS and sCD14) | ||||
| • | CD8 T-cell activation in the sigmoid mucosa | ||||
| • | frequency and absolute number of CD4 T cells in blood and sigmoid mucosa | ||||
| • | number and function of blood and sigmoid immune subsets (including Th17 and Th22 cells) | ||||
| • | small intestinal permeability | ||||
| • | blood viral load and gut mucosal HIV provirus levels | ||||
| • | bacterial community diversity and composition, determined by16S rRNA gene sequencing of anal swabs and sigmoid biopsies | ||||
| • | feasibility, safety, tolerability, adherence, and acceptability of study product and procedures | ||||
Exploratory endpoints
| • | bacterial community diversity and composition, determined by16S rRNA gene sequencing of penile swabs | ||||
| • | metabolomic measurements from blood plasma (including levels of vitamin D, glucose and insulin, and lipid profiling) | ||||
Laboratory Measurements
Blood Immune Activation
Peripheral blood will be collected into Acid Citrate Dextrose (ACD) solution A vacutainers (BD biosciences) and freshly isolated peripheral blood mononuclear cells (PBMCs) will be obtained by Ficoll-Hypaque density gradient centrifugation. The PBMCs will be stained with fluorochrome-labeled monoclonal antibodies specific for CD3, CD4, CD8, CD45, HLA-DR, or CD38; the cells will also be stained with a live/dead dye (Invitrogen). Data will be acquired using the BD LSR Fortessa X20 flow cytometer and data analysis will be performed on live, singlet lymphocytes based on cell size, granularity, and CD45 expression using the latest version of Flow Jo analytical software (TreeStar, Ashland, OR). Flow cytometric gating will be determined by all fluorescence minus one controls or by isotype controls. Unused PBMCs from each study visit will be stored for future use in ancillary studies.
Assay for CD4 T-Cell Subset Numbers and Functions in Gut
CD4 T-cell subset numbers will be assessed in freshly isolated PBMCs and in gut mononuclear cells at baseline and at weeks 24 (for PROOV IT I) and 48 (for both studies), as previously described.Citation8, 13 Briefly, about 20 sigmoid biopsies will be collected, 25–30 cm from the anal verge, weighed, and will undergo two rounds of Collagenase Type II digestions for 30 min each at 37 °C on a shaking, heated block. Isolated gut cells will be filtered and enumerated. Blood and sigmoid mononuclear cells will be stimulated with phorbol 12-myristate acetate (PMA, 1 ng/ml) and ionomycin (1 μΜ), or with media for 6 h at 37 °C, with the final 5 h with Brefeldin A (1 μM). Cells will be washed with 1% fetal bovine serum (FBS) in phosphate-buffered saline (PBS), permeabilized, stained for 30 min with fluorochrome-labeled monoclonal antibodies and with a viability dye (Invitrogen), and fixed in 2% paraformaldehyde. The monoclonal antibodies will be specific for CD45, CD3, CD4, CD8, IL-10, IL-17a, IL-22, IFNγ, and TNFα. Th17 cells will be defined as CD4+ IL-17a+ T cells, Th1 cells will be defined as CD4+ IFNγ+ T cells, and Th22 cells will be defined as CD4+ IL-22+ IL-17a- IFNγ- T cells.Citation8, 49 The functional profile of Th17 cells will be assessed by their capacity to co-produce TNFα, IFNγ, IL-10, and IL-22 using Boolean gating on FlowJo (Treestar) and using data analysis on the SPICE software (NIH/NIAID).Citation13 Flow cytometric gating will be determined using media control and/or all fluorescence minus one controls; values for functional cell subsets will be background-corrected where applicable.
Absolute Cell Numbers
The approximate number of gut cells will be calculated by multiplying the percentage of live lymphocytes, as determined by flow cytometry, by the number of gut mononuclear cells per gram of tissue, as previously described.Citation50 Mononuclear cells from pre-weighed tissues prior to Collagenase digestion will be counted, and the total number of cells per gram of tissue will be determined. This value will be multiplied by the frequency of cells in the live lymphocyte gate and multiplied by subsequent gates based on flow cytometric analysis. The number of blood cells will be calculated by multiplying the percentage subsequent gates in the live lymphocyte gate to the number of lymphocytes per μl of blood determined by the Complete Blood Count (CBC).
Assessment of Soluble Markers
Blood markers of microbial translocation, inflammation, and coagulation will be assessed in frozen blood plasma; these markers have been associated with mortality and SNAEs.Citation10, 51 Limulus amoebocyte lysate assay will be used to quantify LPS (Lonza), a Gram-negative bacterial cell wall component, and the LPS co-receptor soluble CD14 (sCD14) will be measured by a solid phase sandwich ELISA (R&D Systems), which are markers of microbial translocation and monocyte activation. Levels of IL-6 (Meso Scale Discovery), an inflammatory cytokine, and D-dimer (Sekisui Diagnostics), a coagulation marker, along with other inflammatory and coagulation biomarkers will be assayed by standard ELISAs. All assays will be performed in duplicate according to the manufacturers’ instructions.
In Vivo Intestinal Permeability Test
Paracellular permeability of the small intestine will be measured non-invasively through the oral administration of small, nontoxic, non-charged, water-soluble probes (lactulose and mannitol) that are not metabolized. Urine lactulose and mannitol levels will then be measured using high-performance liquid chromatography, and the ratio of lactulose to mannitol will be used to determine the degree of small intestinal permeability.Citation52 This intestinal permeability assay will be performed at baseline and week 24 (PROOV IT I) and/or 48 (PROOV IT I and II).
Diet Questionnaire
Diet is an important modulator of the gut microbiome and of the immune system.Citation53 All study participants will complete the validated online Canadian Diet History Questionnaire II (C-DHQII, National Cancer Institute) at baseline and at weeks 24 (for PROOV IT I) and/or 48 (PROOV IT I and II). The C-DHQII’s past month food frequency questionnaire will provide nutrient information regarding the nutritional intake of study participants including major nutrient food groups (including micronutrients and energy, carbohydrate constituents, minerals, vitamins, fats, and others).
Microbiome Analysis
The bacterial microbiome of sigmoid biopsies and self-collected anal and penile swabs will be assessed through 16s rRNA gene sequencing, as previously described.Citation54 Anal and penile swabs will be collected into 1 mL of certified human DNA free PBS (Mo Bio, USA), and sigmoid biopsies will be flash frozen and stored at -80 °C. Profiling of mucosal bacterial communities will be performed by unbiased 16S rRNA gene sequencing. In brief, DNA will be extracted from swabs and sigmoid biopsies with the PowerSoil DNA isolation kit (MoBio) and barcoded V4 amplicons will be sequenced on the Illumina platform. Taxonomic and phylogenetic diversity will be assessed using the Quantitative Insights Into Microbial Ecology (QIIME) suite of microbiome analysis tools.Citation3
Gut Mucosal HIV Provirus Levels
CD8+ T cells will be depleted from gut mononuclear cells by a column-based cell separation technique (StemCell Technologies) and proviral DNA levels will be measured as previously described.Citation55 Briefly, genomic DNA will be isolated from CD8-depleted gut mononuclear cells using the Puregene DNA isolation kit. Real-time PCR will be used to amplify proviral DNA and the copy number of HIV DNA per million CD8-depleted T cells will be reported.
Metabolomic Profiling
Metabolomic profiling will be performed on blood plasma samples collected at baseline and at weeks 24 (for PROOV IT I) and/or 48 (PROOV IT I and II). Metabolomic measurements may include parameters such as lipidomics (fatty acids, TAG, DAGs, phospholipids, etc.), nutrients (general metabolite analysis) and metabolic footprinting and fingerprinting.
Storage of Biological Samples
Biological specimens will be stored at the University of Toronto for analysis in the PROOV IT trials and for future use in ancillary studies.
Safety and the Data Safety Monitoring Board
The Data Safety Monitoring Committee (DSMC) of the CIHR Canadian HIV Trials Network (CTN) will be responsible for safety monitoring of these trials; the DSMC will also conduct safety analyses of serious adverse events at weeks 24 and 48 for PROOV IT I and II, respectively. The DSMC, which consists of clinicians, biostatisticians, and a community member, will convene ad hoc when necessary to discuss any safety issues that arise; no interim analysis is planned. Participants may be prematurely discontinued from the study if there is a treatment related toxicity, if they require prohibited concomitant medications, or if there are clinical indications believed to be life-threatening or harmful by the attending physician, regardless of their inclusion in the potential toxicities listed in the protocol.
All adverse events, and their possible or probable association with HIV disease or study treatment, will be recorded. Adverse events will be graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (DAID AE grading table). Adverse events will be considered serious according to the standard ICH GCP definition (resulted in hospitalization, disability, death, etc.). SAEs will be reported to the project manager within 24 h of the study site becoming aware of the event.
Since there have been rare reported cases of bacteremia related to probiotic use, participants will be informed of signs of bacteremia (fever) and will have a physical exam every study visit. Participants will remain under surveillance for SAEs until the follow-up safety visit or until the Investigator agrees that the event is satisfactorily resolved, becomes chronic, or that no further follow-up is required. For all collected AEs/SAEs, the clinician who examines and evaluates the participant will determine the likely cause of the adverse event based on temporal relationship and on his/her clinical judgment.
Adherence Assessments
Adherence will be assessed at each visit by sachet count and by the number of self-reported missed sachets as reported on Case Report Forms. Participants will also be asked randomly to provide an anal swab for adherence assessment by microbiome analysis.
Sample Size Justification
The sample sizes of 40 and 36 participants for the PROOV IT I and II pilot studies respectively were determined primarily by feasibility, and with the goal of collecting data for the design of larger clinical trials. Our preliminary studies of cART-naïve men showed that blood immune activation, defined as CD38 and HLA-DR co-expression by blood CD8+ T cells, fell from 24.3 to 10.7% (standard deviation (SD) 7.38%) after one year of therapy (unpublished data), although this was still higher than the level seen in HIV-uninfected individuals (3.7%). With 40 participants in PROOV IT I, we will be able to detect a difference of 6.71% (0.91 SD) in blood immune activation at week 24 between the two study arms, with a significance level of 0.05 and 80% power.
A separate INR preliminary study using a different flow cytometer and antibody panel showed higher blood immune activation level in INR participants (mean, 2.59%; SD, 1.86%) compared to HIV-uninfected controls (0.70%; unpublished). With the assumption that blood immune activation in INRs is stable over a 48-week period, a total of 36 participants in PROOV IT II with 2:1 randomization will allow us to detect a difference of 1.90% (1.02 SD) in blood immune activation between the two groups at week 48 with a significance level of 0.05 and 80% power. The sample size calculations were not adjusted for missed visits, assay failures, or missed sample collection. Larger clinical trials will be required to detect smaller differences in immune activation, which may also be of clinical interest.
Unblinding Procedures
Upon completion of the analysis, the study team and participants will be unblinded to the randomization code. In the event of an emergency during the study when the knowledge of the treatment is essential for further management of the participant, investigators, and the Medical Monitor will discuss and determine if the code should be broken; all efforts will be made to maintain the blind during the study. For example, if a contraindication to study product develops (e.g. suspected hypersensitivity), the study product should simply be stopped rather than breaking the blind. Randomization codes will be stored securely with restricted access in individually sealed envelopes at the sites and in a list form by the code holder.
Statistical Methods
Analysis will be performed on all participants who were randomized into the study arms (intention to treat analysis) and for participants who completed the trial (adherence > 80% of participants randomized to intervention by sachet count; per-protocol analysis). We will attempt to minimize the amount of missing data by continuing to follow participants who discontinue the study treatment. Primary and secondary endpoints in the two study arms will be compared using analysis of covariance in which baseline values will be treated as a covariate (excluding microbiome and diet information). For endpoints measured at more than two time points, we will examine trends over time between treatment groups using generalized linear mixed models to account for correlation among repeat observations within individuals.
We will address two main aims with analysis of the mucosa-associated microbiota: (1) to determine the effect of probiotic administration on the composition of the sigmoid, anal and penile microbiota, as well as the temporal durability of any such effect, and (2) to identify correlations between bacterial community composition, mucosal proviral burden, mucosal, and systemic inflammation and systemic biomarkers of coagulation. We will assess taxonomic and phylogenetic alterations in the paired sigmoid tissue and anal and penile swab DNA samples from each participant. Diversity and community composition will be computed using QIIME.Citation3 Within-sample (alpha) diversity will be estimated by the Shannon Diversity Index (SDI, taxonomic diversity), which is a composite measure of community evenness and richness, as well as phylogenetic diversity. Between-sample (beta) diversity, i.e. the similarity or dissimilarity of community structure between samples, will be estimated by Bray-Curtis dissimilarity (for taxonomic similarity) and weighted and unweighted UniFrac distances (phylogenetic similarity, Lozupone PMID: 16332807).
Subgroup Analyses
Analyses will be conducted to compare the differences in the primary outcome by adherence and by the study site for both studies (Toronto General Hospital vs. Maple Leaf Medical Clinic). These analyses will determine the impact of adherence and account for site-specific factors that may alter the study endpoint.
Discussion
Chronic inflammation despite suppressive cART is a major burden for the long-term care of HIV-infected individuals. It is an important contributor to the increased rates of comorbidities and mortality seen in this population, and may relate to alterations in gut immunology and increased bacterial translocation that can take a decade or longer to resolve on effective therapy.Citation4 Novel therapeutic interventions to accelerate the restoration of gut immunology and barrier function may reduce microbial translocation and inflammation. The presence and composition of the gut bacterial flora is central to mucosal CD4 T-cell development and function.Citation38, 39 Since a pathogenic gut dysbiosis is observed during HIV infectionCitation19, 20 probiotics may provide an adjunctive therapy to accelerate the process of mucosal immune restoration. We hypothesize that probiotic Visbiome supplementation (900 billion bacteria daily) in addition to effective cART may enhance gut immune health and reduce systemic immune activation. Two RCTs have been designed to address this research question: PROOV IT I will enroll HIV-infected cART-naïve individuals initiating cART for the first time, while PROOV IT II will enroll cART-experienced participants with undetectable viral load (<50 copies/ml), but with suboptimal CD4 recovery.
Several probiotic products with regulatory approval are available in North America, but there has generally been little rigorous scientific investigation of their specific benefits, particularly in the context of HIV. Therefore, Visbiome was selected for these proof-of-concept HIV clinical studies for several reasons. VSL#3, the identical former product of Visbiome demonstrated in a non-human primate SIV model to improve gut immune function.Citation6, 42 It is the most concentrated probiotic product available on the market (450 billion bacteria per sachet), with a blend of eight different bacterial strains. Given the approved dosage of Visbiome in Canada and in the USA of up to four sachets daily (1800 billion bacteria per day), this results in a probiotic dose that is several-fold higher than most alternatives. Furthermore, Visbiome has an excellent safety profile, even when 3600 billion bacteria were taken daily for a year by individuals with pouchitis and ulcerative colitis, with no major serious adverse events.Citation41, 56 Finally, independent quality testing of Visbiome by our group confirmed that only contents listed on the label were included in the sachet, ensuring high quality control.
Probiotic-related bacteremia is a rare complication of probiotic use that is a potential health concern for HIV-infected individuals. However, the theoretical risk of developing bacteremia from probiotics containing lactobacilli is less than 1 per million usersCitation57; furthermore, cases of sepsis due to probiotic use have mainly occurred in people who were significantly immunosuppressed, and have not been reported in healthy persons.Citation58 The proposed clinical trials will either enroll individuals who are in good general health with a high CD4+ T-cell count (PROOV IT I), or those already on stable cART (PROOV IT II), and will only provide a moderate daily dose of Visbiome. Although the acceptable daily dose of Visbiome in Canada is up to 1600 billion bacteria daily, 900 billion bacteria was selected due to the minimal adverse events seen at this dose.Citation41 In the case of clinical conditions requiring an antibiotic prescription, participants will stop taking the study product temporarily, and thereafter may continue the study if they choose to. While it is reassuring that prior studies prescribing the use of VSL#3 involving gut inflammatory disease have not reported probiotic-associated bacteremia.Citation41
The PROOV IT pilot studies have been carefully designed to minimize biases and to maximize feasibility; however, limitations within the study protocol should be noted. The sample sizes for both studies are relatively small, therefore, data gathered from these pilot studies will be utilized to inform future RCTs, and will be carefully interpreted. The placebo control and double-blinded design minimize selection and ascertainment biases. Due to the pilot nature of these studies, potential gender -based immunological differences Citation59, only men will be considered for these studies. The exact cART regimen administered to participants will be left to the discretion of the individual and their primary care physician, ensuring that probiotic impact is assessed across a diverse set of cART treatments.
Data from these pilot studies will be used to design future full-scale clinical trials in treatment naïve and treatment experienced patients. In particular, data on the variability in the primary outcome will be used to determine the required sample sizes of the future trials and the rate of recruitment and proportion of patients approached who agree to participate will be used to plan the number of centers and time required to recruit the necessary study participants. Assessment of secondary endpoints (including microbiome analysis, gut immune parameters, and intestinal permeability) may be adjusted according to experience obtained from measurement of these items during the pilot study. A follow-up trial will not be pursued if there is evidence of harm from the probiotic or if the acceptability of the intervention is low. Lack of a statistically significant difference in the primary outcome will not be a reason not to pursue a subsequent larger trial.
There are several limitations to our study design. The PROOV IT studies are lengthy in duration, require long study visits, and include invasive procedures; therefore, recruitment and retention may be challenging. To enhance feasibility, we have designed the study so that lengthy and/or invasive procedures, such as the intestinal permeability urine test and sigmoidoscopy, are optional. In addition, all participants will eventually have the option to receive the probiotic product; the latter in PROOV IT I may determine if long-term use of probiotics (48 vs. 24 weeks) within an individual may provide additional benefit. To encourage compliance and retention in the study, most study visits will coincide with regular clinic visits with their primary care physician, and participants will be compensated for their time and effort. The timing of primary and secondary analyses for PROOV IT I (24 weeks) and PROOV IT II (48 weeks) were based on our hypothesis that more rapid immune changes will be observed in participants starting cART (PROOV IT I) compared to cART pre-treated INR individuals (PROOV IT II) with slow CD4 recovery in blood. The latter group typically has lower nadir CD4 counts, start cART later, has greater immune activation, and more fibrosis in lymphoid tissues.Citation61, 62 Given the likeliness of greater prior immune damage in INRs, we hypothesize that it may take longer for probiotics to have an impact this population. Finally, the immune endpoints measured in these studies are only surrogate markers for subsequent adverse clinical outcomes, rather than actual clinical endpoints. The primary endpoint of blood CD8 T-cell immune activation was selected because this is associated with death, inflammation, microbial translocation, and increased progression to AIDS.Citation63, 64 More recent studies in ART-experienced populations have also identified soluble blood biomarkers that strongly predict mortality and SNAs, including IL-6, D-dimer, and sCD14, and so these are included as secondary endpoints. Other secondary endpoints will provide mechanistic information. For example, we hypothesize that probiotics may enhance epithelial integrity and reduce gut leakiness, and so the in vivo intestinal permeability test will provide important information. Sigmoid biopsies will permit the research team to directly assess the impact of probiotic supplementation on gut immunology and the gut microbiome.
In summary, the use of complementary medicines is very common among HIV-infected individuals, and the HIV community in Ontario has identified the establishment of efficacy as a key research priority. There are compelling data from in vitro and non-human primate models to suggest that probiotic supplementation as an adjunct to cART may accelerate the restoration of gut immunity and barrier function, while reducing the systemic immune activation caused by bacterial translocation. Therefore, the PROOV IT trials are designed to assess whether Visbiome, a potent probiotic product, can improve surrogate markers of HIV morbidity and mortality among cART-treated individuals in two important clinical contexts. The research team understands their responsibility to the prioritization stakeholders and will execute a public knowledge translation and exchange plan of key study findings.
| = | List of Abbreviations | |
| ACD | = | Acid Citrate Dextran |
| AEs | = | adverse events |
| cART | = | combination antiretroviral therapy |
| CBC | = | complete blood count |
| CTN | = | CIHR Canadian HIV Trials Network |
| CVD | = | cardiovascular disease |
| DMSB | = | data monitoring and safety board |
| FBS | = | fetal bovine serum |
| INR | = | immune non-responders |
| LPS | = | lipopolysaccharides |
| PBMCs | = | peripheral blood mononuclear cells |
| PBS | = | phosphate-buffered saline |
| RCTs | = | randomized controlled trials |
| SAEs | = | serious adverse events |
| SNAEs | = | serious non-AIDS events |
Disclaimer statements
Authors’ Contributions
CJK and RK conceived the original idea for the trials. CJK, CK, JR, RR, RR, RR, RK, and SLW contributed to the trial design. CJK wrote the original version of the manuscript and all authors critically reviewed and approved the final version.
Conflicts of interest
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the CIHR Canadian HIV Trials Network (CTNPT 022), Canadian Institutes of Health Research and Ontario HIV Treatment Network [ROG G869].
Acknowledgments
We thank the CIHR Canadian HIV Trials Network (CTN)’s Wendy Zubyk, Dana Nohynek, Gina Graziani, Kay Kuang, and Jim Pankovich for study management, regulatory guidance, manuscript review, and/or data management services. We would like to acknowledge the Exegi Pharma LLC. (study product, randomization, and labeling services). Dr. Connie J. Kim is supported by a CTN Postdoctoral Fellowship Award.
References
- van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F, study Anoc. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527–1535.10.1097/QAD.0b013e32833a3946
- Capeau J. Premature aging and premature age-related comorbidities in HIV-infected patients: Facts and hypotheses. Clin Infect Dis. 2011;53:1127–1129.10.1093/cid/cir628
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336.
- Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123.
- Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection AIDS. 2013;27:1199–1208.10.1097/QAD.0b013e32835ecb8b
- Klatt NR, Canary LA, Sun X, et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest. 2013;123:903–907.
- Estes JD, Harris LD, Klatt NR, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052.10.1371/journal.ppat.1001052
- Kim CJ, Nazli A, Rojas OL, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–680.10.1038/mi.2012.72
- Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852.10.1371/journal.ppat.1000852
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371.
- Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759.10.1084/jem.20040874
- Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431.10.1126/science.280.5362.427
- Kim CJ, McKinnon LR, Kovacs C, et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol. 2013;191:2164–2173.10.4049/jimmunol.1300829
- Chege D, Sheth PM, Kain T, et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS. 2011;25:741–749.10.1097/QAD.0b013e328344cefb
- Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735.10.1056/NEJMoa0810719
- Symons A, Budelsky AL, Towne JE. Are Th17 cells in the gut pathogenic or protective? Mucosal Immunol. 2012;5:4–6.10.1038/mi.2011.51
- Kisand K, Bøe Wolff AS, Podkrajšek KT, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308.10.1084/jem.20091669
- Cavani A, Pennino D, Eyerich K. Th17 and Th22 in skin allergy. Chem Immunol Allergy. 2012;96:39–44.10.1159/000331870
- Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193–191.
- Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–994.
- Klase Z, Ortiz A, Deleage C, et al. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 2015;8:1009–1020.10.1038/mi.2014.128
- Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790.10.1093/infdis/jiq118
- Mehandru S, Poles MA, Tenner-Racz K, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484.10.1371/journal.pmed.0030484
- Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247.10.1128/JVI.00120-06
- Fernandes SM, Pires AR, Ferreira C, et al. Enteric mucosa integrity in the presence of a preserved innate interleukin 22 compartment in HIV type 1-treated individuals. J Infect Dis. 2014;210:630–640.10.1093/infdis/jiu126
- Marchetti G, Gori A, Casabianca A, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727–1736.10.1097/01.aids.0000242819.72839.db
- Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–1915.10.1097/00002030-200309050-00009
- Lederman MM, Calabrese L., Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226.10.1093/infdis/jir507
- Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–447.10.1086/652978
- Zoufaly A, Cozzi-Lepri A, Reekie J, et al. Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe. PLoS One. 2014;9:e87160.10.1371/journal.pone.0087160
- Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014;58:1312–1321.10.1093/cid/ciu038
- Tincati C, Merlini E, Braidotti P, et al. Impaired gut junctional complexes feature late-treated individuals with suboptimal CD4+ T-cell recovery upon virologically-suppressive cART. AIDS. 2016;30:991–1003.
- Klatt NR, Funderburg NT, Brenchley JM, et al. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13.10.1016/j.tim.2012.09.001
- Bica I, Tang AM, Skinner S, et al. Use of complementary and alternative therapies by patients with human immunodeficiency virus disease in the era of highly active antiretroviral therapy. J Altern Complement Med. 2003;9:65–76.10.1089/107555303321222955
- Josephs JS, Fleishman JA, Gaist P, Gebo KA, Network HIVR. Use of complementary and alternative medicines among a multistate, multisite cohort of people living with HIV/AIDS. HIV Med. 2007;8:300–305.10.1111/hiv.2007.8.issue-5
- Ontario HIV Treatment Network (OHTN). Priority populations. 2014; Accessed June, 2015. http://www.ohtn.on.ca/otw-portfolio/priority-populations/
- Morelli L, Capurso L. FAO/WHO guidelines on probiotics. J Clin Gastroenterol. 2012;46:S1–S2.10.1097/MCG.0b013e318269fdd5
- Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518.10.1038/nature10957
- Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498.10.1016/j.cell.2009.09.033
- Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829.10.1371/journal.ppat.1003829
- Chapman TM, Plosker GL, Figgitt DP. VSL#3 probiotic mixture: A review of its use in chronic inflammatory bowel diseases. Drugs. 2006;66:1371–1387.10.2165/00003495-200666100-00006
- Ortiz AM, Klase ZA, DiNapoli SR, et al. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunology. 2016;9:458–467.
- Miller H, Ferris R, Phelps BR. The effect of probiotics on CD4 counts among people living with HIV: A systematic review. Benef Microbes. 2016;1–8.10.3920/BM2015.0163
- Villar-García J, Hernández JJ, Güerri-Fernández R, et al. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients. J Acquir Immune Defic Syndr. 2015;68:256–263.10.1097/QAI.0000000000000468
- Stiksrud B, Nowak P, Nwosu FC, et al. Reduced levels of D-dimer and changes in gut microbiota composition after probiotic intervention in HIV-infected individuals on stable ART. J Acquir Immune Defic Syndr. 2015;70:329–337.10.1097/QAI.0000000000000784
- Yang OO, Kelesidis T, Cordova R, Khanlou H. Immunomodulation of antiretroviral drug-suppressed chronic HIV-1 infection in an oral probiotic double-blind placebo-controlled trial. AIDS Res Hum Retroviruses. 2014;30:988–995.10.1089/aid.2014.0181
- d’Ettorre G, Ceccarelli G, Giustini N, et al. Probiotics reduce inflammation in antiretroviral treated, HIV-infected individuals: Results of the “Probio-HIV” clinical trial. PLoS One. 2015;10:137200.
- Services DoHaH. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. . Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. 2015; Accessed June 18, 2015.
- Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585.
- Ciccone EJ, Read SW, Mannon PJ, et al. Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal Immunol. 2010;3:172–181.10.1038/mi.2009.129
- Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203.10.1371/journal.pmed.0050203
- Teshima CW, Meddings JB. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep. 2008;10:443–449.10.1007/s11894-008-0083-y
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336.10.1038/nature10213
- Liu CM, Osborne BJ, Hungate BA, et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathog. 2014;10:e1004262.10.1371/journal.ppat.1004262
- Sheth PM, Chege D, Shin LY, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. 2008;1:382–388.10.1038/mi.2008.23
- Adams MR. Safety of industrial lactic acid bacteria. J Biotechnol. 1999;68:171–178.10.1016/S0168-1656(98)00198-9
- Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36:775–780.10.1086/cid.2003.36.issue-6
- Boyle RJ, Tang ML. The role of probiotics in the management of allergic disease. Clin Exp Allergy. 2006;36:568–576.10.1111/cea.2006.36.issue-5
- Sankaran-Walters S, Macal M, Grishina I, et al. Sex differences matter in the gut: Effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013;4:10.10.1186/2042-6410-4-10
- Remis RS, Liu J. HIV/AIDS in Ontario: Preliminary Report, 2011. Toronto: University of Toronto; 2013.
- Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus–infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543.10.1086/jid.2003.187.issue-10
- Schacker TW, Reilly C, Beilman GJ, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19:2169–2171.10.1097/01.aids.0000194801.51422.03
- Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and Death in the multicenter AIDS cohort study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92.10.1097/00042560-199710010-00003
- Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101.10.1111/imr.12079
