Abstract
Background: Coenzyme Q10 (CoQ10) deficiency has been associated with statin-induced myopathy, and supplementation with CoQ10 may reduce inflammation markers. The effects of statins on CoQ10 and its anti-inflammatory properties have not been investigated in HIV-positive patients.
Objective: The objectives of this study were to examine the effect of rosuvastatin on CoQ10 and CoQ10/LDL ratio over 24-week SATURN-HIV trial, explore the associations between CoQ10 levels and markers of vascular disease, inflammation, and immune activation, and assess whether changes in CoQ10 affected the anti-inflammatory effects of statin therapy or were associated with myalgia symptoms.
Methods: This was a secondary analysis of the SATURN-HIV trial, a 96-week randomized clinical trial of 10 mg daily rosuvastatin vs. placebo in HIV-infected patients on antiretroviral therapy. We assessed the statin treatment effect on CoQ10 levels and CoQ10/LDL ratios and whether changes in these markers were related to myalgias. Relationships between CoQ10, subclinical vascular disease, and biomarkers of inflammation and immune activation were explored using Spearman correlations and multivariable regression models.
Results: Overall, 147 patients were included. Median age was 46 years; 78% were male and 68% African American. At baseline, CoQ10 levels and CoQ10/LDL ratio were modestly correlated with markers of HIV disease, immune activation, and carotid distensibility. After 24 weeks of statin therapy, CoQ10 levels decreased (p = 0.002 for between group difference) and CoQ10/LDL ratio increased (p = 0.036). In the statin treatment arm, we did not find evidence of a relationship between changes in CoQ10 or CoQ10/LDL ration and changes in markers of inflammation or immune activation. There was a borderline statistically significant association between changes in CoQ10 and myalgia symptoms [OR 4.0 per 0.1 mg/L decrease in CoQ10, p = 0.07].
Conclusion: Twenty-four weeks of 10 mg daily rosuvastatin decreases CoQ10 concentration and increases CoQ10/LDL ratio in HIV-infected patients on antiretroviral therapy.
Introduction
Statins—3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors—are widely used to lower serum cholesterol and reduce cardiovascular mortality in both primary and secondary prevention.Citation1,2 It is also well established that statins lower plasma coenzyme Q10 (CoQ10) levels.Citation3−Citation10 CoQ10 is a naturally occurring quinolone that is an integral part of the electron transport chain and oxidative phosphorylation in the mitochondria.Citation11 CoQ10, in its reduced form, acts as an antioxidant providing protection for cell membranes.Citation12 Because of evidence that CoQ10 deficiency can cause peripheral myopathy,Citation10,13−Citation14 there has been a long-standing concern that statin-induced myopathy may be mediated in part by CoQ10 deficiency. Although pre-statin CoQ10 levels predict the risk of myalgias, clinical trials of oral CoQ10 supplementation have had mixed results.Citation10,14−Citation18
Interestingly, studies have also demonstrated that CoQ10 lowers inflammatory markers when orally supplemented, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), oxidized low-density lipoprotein (LDL), and high-sensitivity C-reactive protein (hs-CRP).Citation19−Citation22 A CoQ10- mediated increase in inflammation may explain the lack of statin benefit in patient with heart failure,Citation23 though the observational studies linking CoQ10 levels to heart failure outcomes are conflicting.Citation24−Citation25 There is also limited evidence that CoQ10 supplementation may improve endothelial function in healthy volunteers.Citation26
Chronic HIV infection is characterized by residual inflammation, immune activation, profound endothelial dysfunction, and high cardiovascular risk despite effective antiretroviral therapy (ART),Citation27 yet little is known about the relationships between CoQ10, inflammation, and vascular disease in HIV. Small, single-center studies have been published describing conflicting results about the effect of CoQ10 in HIV-infected individuals.Citation28−Citation30 One study conducted before effective ART showed no statistically significant difference in CoQ10 levels between HIV-infected patients and uninfected controls.Citation28 More recently, there is evidence that CoQ10 supplementation may ameliorate the neurotoxicity and endothelial dysfunction associated with the use of mitochondrial toxic ART.Citation31−Citation32 No study has examined CoQ10 changes in response to statin therapy in chronic HIV infection.
To this end, the primary objective of this study was to examine the effect of rosuvastatin on CoQ10 and CoQ10/LDL ratio over 24 weeks in the Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV (SATURN-HIV) trial.Citation33 Second, we explored associations between CoQ10 levels and markers of vascular disease, inflammation, and immune activation at baseline. Third, we assessed whether changes in CoQ10 affected the anti-inflammatory effects of statin therapy or were associated with myalgia symptoms.
Methods
Study design
This study was a secondary analysis of the recently completed SATURN-HIV trial. SATURN-HIV was a randomized, double-blind placebo-controlled trial designed to measure the effect of rosuvastatin on markers of cardiovascular risk and skeletal bone health in patients with well-treated HIV infection. All subjects were ≥ 18 years of age, on stable ART for ≥3 months, with HIV-1 RNA level ≤1000 copies/ml. Additional entry criteria included LDL cholesterol <130 mg/dL and evidence of heightened inflammation and/or T-cell activation (high-sensitivity C-reactive protein (hsCRP) >2.0 mg/dL and/or CD8 + CD38 + HLA-DR + T-cells ≥ 19%). Full inclusion/exclusion criteria can be found at clinicaltrials.gov (NCT01218802). Randomization was conducted by the primary investigational pharmacist at 1:1 to active rosuvastatin 10 mg daily vs. matching placebo. Randomization was stratified by protease inhibitor use and by the presence or absence of coronary artery calcification and osteopenia at baseline. The study was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH), and all subjects signed a written consent before enrollment.
Demographics, medical history, and clinical variables were obtained at the baseline visit. HIV-1 RNA and CD4 + T-cell count were obtained as part of routine clinical care. Venous blood was drawn after a 12-h fast at baseline and 24 weeks. Lipoproteins, insulin, glucose, and creatinine were measured at the University Hospitals clinical laboratories. Insulin resistance was calculated from fasting glucose and insulin using the homeostatic model assessment of insulin resistance (HOMA-IR). Peripheral blood mononuclear cells (PBMCs) were separated by centrifugation with Ficoll-Hypaque and were cryopreserved until analyzed by flow cytometry in batch. Frozen plasma samples were stored at −80 °C and analyzed in batch.
CoQ10, inflammation, and immune activation
CoQ10 concentrations were measured from frozen plasma using high-performance liquid chromatography (Quest Diagnostics; Madison, NJ, USA). HsCRP was measured by particle enhanced immunonephelometric assay on a BNII nephelometer (Siemens; Munich, Germany). Other soluble biomarkers of inflammation [IL-6 and TNF-α receptors I and II (sTNFR-I and II)] and monocyte activation [soluble CD14 and CD163] were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota). Interassay coefficients of variation ranged from 0.4 to 18%.
Monocytes and T-cells were phenotyped by flow cytometry as previously described.Citation28 Three monocyte subsets: (1) CD14 + CD16+, (2) CD14dimCD16+, and (3) CD14 + CD16- were each quantified as a percentage of the overall monocyte population. T-cell activation was quantified as the percentage of CD4 + or CD8 + cells that expressed both CD38 and HLA-DR. PD1 expression on CD4 + and CD8 + cells was measured as a marker of T-cell exhaustion.
Ultrasound measurement of subclinical vascular disease
Common carotid artery intima-media thickness (CCA-IMT) and brachial artery endothelial function (flow-mediated dilation [FMD] and hyperemic velocity time integral [VTI]) were measured by ultrasound using semiautomated edge detection software (Medical Imaging Applications LLC, Coralville, Iowa) as previously described.Citation34 Carotid distensibility was measured with semiautomated edge detection software from 10-beat ultrasound cine loops. The diameter of the distal 1 cm of the right carotid artery was measured in systole (Ds) and diastole (Dd). Blood pressure was obtained at the time of carotid ultrasound to determine the pulse pressure (PP). Carotid distensibility was calculated using the same formula [(2*(Ds − Dd) / Dd) / PP] used in the Women’s Interagency Health Study and the Multicenter AIDS Cohort Study and is reported in units of 10−6 × N−1 m2.Citation35−Citation36
Statistics
This was a secondary analysis of a clinical trial using data from the baseline and week 24 study visits. The analyses of treatment effect were performed using intent-to-treat principles based on randomized treatment assignments. Baseline characteristics of the study participants were described using median and interquartile ranges for continuous variables of frequency and percent for categorical variables.
Comparisons of baseline characteristics by group were made using unpaired t-tests, Wilcoxon rank-sum tests, or Fisher exact tests as appropriate. Zero- to 24-week changes in CoQ10 and CoQ10/LDL among those assigned to statin versus placebo were compared using t-tests with assumption of unequal variances. The relationship between changes in CoQ10 levels and odds of developing myalgia symptoms was assessed with logistic regression. Separately for baseline CoQ10 levels and CoQ10/LDL ratio, we used Spearman correlation coefficients to test associations with traditional cardiovascular risk factors, HIV disease characteristics, markers of inflammation and immune activation, and ultrasound markers of subclinical vascular disease. We constructed scatter plots and used linear regression to explore the associations of 24-week changes in CoQ10 level and CoQ10/LDL ratio with 24-week changes in inflammation and immune activation markers. Two biomarkers of interest (IL-6 and hs-CRP) were strongly associated with CoQ10 changes in univariate analyses, but appeared to be driven primarily by a small number of outliers. For comparison, we therefore repeated the regression models after excluding these outliers.
All statistical tests were two-sided and considered significant at a level of p < 0.05. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina).
Results
Of the 202 subjects screened for the SATURN-HIV study between March 2011 and August 2012, 147 were enrolled: 72 were randomized to the rosuvastatin (10 mg) arm and 75 to the placebo arm. The characteristics of the 55 patients who screened out and the 11 (5 statin; 6 placebo) patients who were lost to follow-up in the first 24 weeks of the study have been described previously.Citation37−Citation38
Baseline characteristics of study participants are described in Table . There were no baseline differences between the treatment and placebo arm (p < 0.05). Briefly, the overall median age was 46 (IQR 40–53) years, 78% of patients were male, and 68% were African-American. Participants had low-risk lipid profiles and calculated 10-year Framingham scores.
Table 1 Baseline characteristics of study participants by treatment group
CoQ10 and markers of CVD risk and inflammation
Table shows the correlations of CoQ10 concentrations and CoQ10/LDL ratio with traditional CVD risk factors, HIV-specific factors, biomarkers of inflammation and immune activation, and subclinical vascular disease. As expected, CoQ10 concentrations were modestly positively correlated with LDL cholesterol levels (r = 0.208, p = 0.012) and there was a stronger, negative correlation between CoQ10/LDL ratio and LDL levels (r = −0.441, p < 0.0001). Interestingly, both CoQ10 concentration and CoQ10/LDL ratio were negatively correlated with Caucasian race but were not correlated with age, gender, or smoking status.
Table 2 Correlations of baseline CoQ10 and CoQ10/LDL with traditional risk factors, HIV parameters, markers of inflammation and immune activation, and subclinical vascular disease
CoQ10 and CoQ10/LDL were positively correlated with HIV disease duration and undetectable HIV-1 viremia, and negatively correlated with CD4 + T-cell count. Despite modest positive correlations with activated T-cells and proinflammatory monocyte phenotypes [T-cell activation (CD38 + HLA-DR + on CD4 + and CD8+), T-cell exhaustion (PD1 + CD38 + HLA-DR+), and “patrolling” monocytes (CD14dimCD16+)], there were no baseline correlations with soluble markers of systemic inflammation or immune activation. Finally, carotid distensibility was negatively correlated with both CoQ10 concentrations and CoQ10/LDL ratio, but no correlation was seen with carotid IMT or brachial FMD.
CoQ10 changes on statin
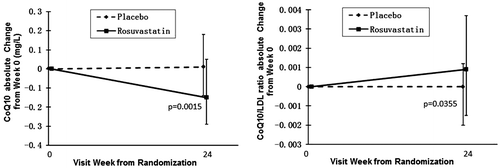
At baseline, median (IQR) CoQ10 concentration was borderline statistically higher in the placebo group [0.77(0.60–1.04) vs. 0.89(0.67–1.12) mg/L, statin vs. placebo; p = 0.06] but CoQ10/LDL ratio was similar [0.008(0.006–0.012) vs. 0.009(0.007–0.012); p = 0.27]. Twenty-four-week changes in CoQ10 and CoQ10/LDL ratio are displayed in Figures (A) and (B), with the treatment effect analysis displayed below the graphs. As hypothesized, absolute CoQ10 levels declined after 24 weeks of statin therapy. Because of a relatively larger reduction in LDL cholesterol (−28% vs.+3.8%; statin vs. placebo, p < 0.01), the CoQ10/LDL ratio increased slightly. Overall, there was a statistically significant correlation between changes in CoQ10 and changes in LDL (r = 0.341, p < 0.001) which was borderline significant when the statin treatment arm was analyzed separately (r = 0.228, p = 0.06).
CoQ10 and changes in biomarkers of inflammation
Among those assigned to statin therapy, 0- to 24-week changes in IL-6 and hs-CRP were associated with CoQ10/LDL changes in univariate linear regression models (p < 0.001); other biomarkers shown in Table were not associated (all p > 0.05). In further analyses, however, these associations were driven primarily by a small number of outliers (n = 2 with IL-6 change >–20 pg/mL and n = 4 with hs-CRP change >−20 μg/mL). When outliers were excluded, there was no evidence of an association (p = 0.86 for IL-6 and p = 0.79 for hs-CRP).
CoQ10 and muscle symptoms
Four participants in the statin arm (5.6%) experienced low-grade myalgia symptoms (Grade 1 or 2) within the first 24 weeks of the study. Only one of these four developed clinically significant myalgias (Grade 3) with mild elevation of creatinine kinase at week 5 requiring cessation of study drug. His symptoms quickly resolved and he continued to be followed on study but off study drug. Baseline CoQ10 concentration was not associated with higher odds of developing myalgia symptoms during the study (p = 0.42); however, there was a borderline statistically significant inverse association between changes in CoQ10 and myalgia symptoms [OR 4.0 per 0.1 mg/L decrease in CoQ10, p = 0.07]. Twenty-four-week changes in CoQ10 were unrelated to changes in creatinine kinase or aspartate transaminase (AST) concentrations (p > 0.4).
Discussion
In this study, we present the first evidence that statin therapy is associated with reductions in plasma CoQ10 and increases in CoQ10/LDL ratio in the HIV-infected population. Despite modest correlations between CoQ10 and biomarkers of inflammation and immune activation, we did not find any evidence of an association between changes in CoQ10 on statin therapy and changes in inflammation markers. These findings may be relevant for the management of cardiovascular disease in patients with HIV infection or other chronic inflammatory conditions.
We used both CoQ10 and CoQ10/LDL ratio in this study. Approximately 60% of CoQ10 is transported via the LDL molecule; therefore, large reductions in LDL may significantly affect the concentrations of free CoQ10 in the blood.Citation39 This can be corrected using the CoQ10/LDL ratio. Studies are conflicting regarding the effect of statins on CoQ10/LDL ratio, though some have demonstrated an increase in the ratio.Citation3,6−Citation7, 39
The changes in CoQ10 and CoQ10/LDL in our study are consistent with those seen in studies of HIV-negative individuals. A study in heart failure patients using the same dose of rosuvastatin showed a 27–51% change at 12 weeks in CoQ10.Citation44 A smaller study of the general population using rosuvastatin 3 mg demonstrated only a 2% decrease of CoQ10 at 20 weeks.Citation45 Our study conducted over a 24-week period demonstrated a 24% change. Although this is the first study on HIV patients, our study did not have an HIV-uninfected control group, and thus, we cannot definitively say whether HIV infection is associated with any more or less change in CoQ10 compared to the general population.
The decrease in CoQ10 is mediated by the inhibition of the conversion of HMG-CoA to mevalonic acid in the sterol biosynthesis pathway. This sterol precursor is shared by both cholesterol and ubiquinone. As reported previously, the 25% reduction in LDL cholesterol over 24 weeks in SATURN-HIV was somewhat lower than the ~45% reduction that would be expected for rosuvastatin 10 mg in the general population.Citation38,40 Despite this modest reduction in LDL, the CoQ10/LDL ratio still increased in our study.
To our knowledge, no prior study has evaluated the effect of statin therapy on CoQ10 levels in the context of any chronic inflammatory disorder. Yet, prior studies have suggested that both statins and CoQ10 supplementation may reduce inflammation. The anti-inflammatory properties of statins have been extensively studied and have been reviewed previously.Citation41 The anti-inflammatory effects of CoQ10 are relatively less well-studied,Citation19−Citation22,42−Citation43 and even fewer clinical studies have been performed. In one clinical study, Lee et al. demonstrated that high dose CoQ10 supplementation appears to raise CoQ10 and reduce plasma IL-6 concentrations in subjects with coronary artery disease; however, there was no correlation between changes in CoQ10 and changes in IL-6 in their study.Citation19 Similarly, in subjects with multiple sclerosis, CoQ10 supplementation reduced plasma IL-6 and TNF-α.Citation22
In the SATURN-HIV trial, 10 mg of daily rosuvastatin led to early and sustained reductions in several biomarkers of immune activation such as soluble CD14 (a marker of monocyte activation), proportion of non-classical “patrolling” monocytes expressing tissue factor, and cell-surface markers of T-cell activation and exhaustion; yet the effect on circulating inflammatory cytokines and acute phase reactants was less consistent.Citation37,38 We hypothesized that adverse effects on CoQ10 or the CoQ10/LDL ratio may underlie the blunted statin effect on these markers of inflammation, relative to cellular markers of immune activation. Although we did observe an inverse relationship between changes in CoQ10/LDL and changes in IL-6 and hs-CRP in initial analyses, this was driven primarily by a small number of outliers. When these outliers were excluded, there was no evidence of any relationship. It is unknown whether co-supplementation with oral CoQ10 in an HIV-infected population might augment the anti-inflammatory effect of statin therapy; however, our results suggest that the effect is likely to be very small.
The incidence of myalgias over 24 weeks in the statin group was 5.6%. This rate of myalgia symptoms is comparable to other rosuvastatin trials such as JUPITER or CORONA.Citation23,44 There was no relationship between baseline CoQ10 and odds of developing muscle symptoms, although there was a borderline statistically significant association with changes in CoQ10 level over 24 weeks. Because of a small number of myalgia events, our study had limited power to detect a significant relationship.
CoQ10 levels and CoQ10/LDL were both inversely associated with carotid distensibility at baseline, but there were no other significant relationships with measures of subclinical vascular disease. The clinical significance of the relationship with carotid stiffness is unclear but should be investigated in future studies.
A major strength of our study is the randomized clinical trial design and extensive phenotyping of study participants in terms of inflammation, immune activation, and subclinical vascular disease. Although SATURN-HIV is the largest placebo-controlled trial of statin therapy conducted in treated HIV infection to date, we may have lacked power to detect clinically significant relationships between CoQ10 and myalgia symptoms. The majority of our population had suppressed HIV-1 viremia on ART and was predominately African American and male, which may affect the generalizability of our results.
In conclusion, 24 weeks of rosuvastatin 10 mg daily reduces CoQ10 concentrations but modestly raises CoQ10/LDL ratio in a population of HIV-infected subjects on ART. In this study, changes in CoQ10 and CoQ10/LDL ratio on statin are not associated with changes in inflammation or immune activation. The relationships between CoQ10, statins, and inflammation could be further explored in larger clinical studies of patients with treated HIV infection or other chronic inflammatory conditions.
Abbreviations
CoQ10 – Coenzyme Q10, HIV – Human immunodeficiency virus, LDL – Low-density lipoprotein, TNF-α – Tumor necrosis factor-α, IL-6 – Interleukin-6, hs-CRP – High-sensitivity C-reactive protein, ART – Antiretroviral therapy, CCA-IMT – Common carotid artery intima-media thickness, FMD – flow-mediated dilation, VTI – Hyperemic velocity time integral.
Funding
This work was supported by the National Institutes of Health [grant number R01 NR012642], [grant number K23 HL123341]; Center for AIDS Research, CWRU [grant number P30 AI36219]; Case Western Reserve University, CTSC [grant number UL1 TR000439].
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
JT.M is a PGY-2 Internal Medicine Resident. CT.L is an assistant professor of Medicine. AM is a Registered Dietitian. YJ has completed his Ph.D. in Biostatistics and Epidemiology. SM.D is a professor of Community Dentistry (secondary appointment).Profesor Plenario (adjunct appointment) Departamento de Investigacion, Universidad de Belgrano. Buenos Aires, Argentina. GA.M, an endowed Chair is a professor of Pediatrics and Medicine.
References
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110:227–239.10.1161/01.CIR.0000133317.49796.0E
- Stone NJ, Robinson JG, Lichtenstein AH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the ACC/AHA task force on practice guidelines. Circulation. 2014;129:S1–S45.10.1161/01.cir.0000437738.63853.7a
- Mabuchi H, Higashikata T, Kawashiri M, et al. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Athero Thromb. 2005;12:111–119.10.5551/jat.12.111
- Rundek T, Naini A, Sacco R, Coates K, DiMauro S. Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Arch Neurol. 2004;61:889–892.10.1001/archneur.61.6.889
- Suzuki T, Nozawa T, Sobajima M, et al. Atorvastatin-induced changes in plasma coenzyme Q10 and brain natriuretic peptide in patients with coronary artery disease. Int Heart J. 2008;49(4):423–433.10.1536/ihj.49.423
- Berthold H, Naini A, Di Mauro S, et al. Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma. Drug Saf. 2006;29(8):703–712.10.2165/00002018-200629080-00007
- Bleske BE, Willis RA, Anthony M, et al. The effect of pravastatin and atorvastatin on coenzyme Q10. Am Heart J. 2001;142(2):e2–e7.
- Mortensen SA, Leth A, Agner E, Rohde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med. 1997;18:s137–s144.10.1016/S0098-2997(97)00014-9
- Littarru GP, Langsjoen P. Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion. 2007;7:s168–s174.10.1016/j.mito.2007.03.002
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–1412.10.1016/j.amjcard.2006.12.063
- Crane FL. Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion. 2007;7:s2–s7.10.1016/j.mito.2007.02.011
- Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7:s41–s50.10.1016/j.mito.2007.02.006
- Marcoff L, Thompson PD. The role of coenzyme Q10 in statin associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–2237.10.1016/j.jacc.2007.02.049
- Lamperti C, Naini AB, Lucchini V. Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol. 2005;62:1709–1712.10.1001/archneur.62.11.1709
- Keith M, Mazer CD, Mikhail P, Jeejeebhoy F, Briet F, Errett L. Coenzyme Q10 in patients undergoing CABG: effect of statins and nutritional supplementation. Nutr Metab Cardiovasc Dis. 2008;18(2):105–111.10.1016/j.numecd.2006.09.011
- Young JM, Florkowski CM, Molyneux SL, et al. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol. 2007;100:1400–1403.10.1016/j.amjcard.2007.06.030
- Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526–529.10.1016/j.amjcard.2012.04.026
- Mabuchi H, Nohara A, Kobayashi J, Kawashiri M, Katsuda S, Inazu A. Effects of CoQ10 supplementation on plasma lipoprotein lipid, CoQ10 and liver and muscle enzyme levels in hypercholesterolemic patients treated with atorvastatin: a randomized double-blind study. Atherosclerosis. 2007;195(2):e182–e189.10.1016/j.atherosclerosis.2007.06.010
- Lee B, Huang Y, Chen S, Lin P. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition. 2012;28:767–772.10.1016/j.nut.2011.11.008
- Gökbel H, Gergerlioğlu HS, Okudan N, Gül İbrahim, Büyükbaş S, Belviranlı M. Effects of coenzyme Q10 supplementation on plasma adiponectin, interleukin-6, and tumor necrosis factor- α levels in men. J Med Food. 2010;13(1):216–218.10.1089/jmf.2008.0310
- Bessler H, Bergman M, Blumberger N, Djaldetti M, Salman H. Coenzyme Q10 decreases TNF-α and IL-2 secretion by human peripheral blood mononuclear cells. J Nutr Sci Vitaminol. 2010;56:77–81.
- Sanoobar M, Eghtesadi S, Azimi A, et al. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutr Neurosci. 2015;18(4):169–176.10.1179/1476830513Y.0000000106
- Rogers JK, Jhund PS, Perez AC, et al. Effect of rosuvastatin on repeat heart failure: the CORONA trial. JACC Heart Fail. 2014;2(3):289–297.10.1016/j.jchf.2013.12.007
- Molyneux SL, Florkowski CM, George PM, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52(18):1435–1441.10.1016/j.jacc.2008.07.044
- Mortensen SA, Vadhanavikit S, Folkers K. Deficiency of coenzyme Q10 in myocardial failure. Drugs Under Exp Clin Res. 1984;7:497–502.
- Gao L, Mao Q, Cao J, Wang Y, Zhou X. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:311–316.10.1016/j.atherosclerosis.2011.10.027
- Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 counts: does it reduce the risk of cardiovascular disease. Curr Opin HIV AIDS. 2014;9(1):54–62.10.1097/COH.0000000000000015
- Folkers K, Langsjoen P, Nara Y, et al. Biochemical deficiencies of coenzyme Q10 in HIV-infection and exploratory treatment. Biochem and Biophys Res Comm. 1988;153(2):888–896.10.1016/S0006-291X(88)81179-3
- Folkers K, Hanioka T, Xia LJ, McRee JT, Langsjoen P. Coenzyme Q10 increases T4/T8 ratios of lymphocytes in ordinary subjects and relevance to patients having the aids related complex. Biochem Biophys Res Commun. 1991;176(2):786–791.10.1016/S0006-291X(05)80254-2
- Folkers K, Morita M, Mcree J. The activities of coenzyme Q10 and vitamin B6 for immune responses. Biochem Biophys Res Commun. 1993;193(1):88–92.10.1006/bbrc.1993.1593
- Cherry CL, Mobarok M, Wesselingh SL, et al. Ubisol-Aqua™: coenzyme Q10 prevents antiretroviral toxic neuropathy in an in vitro model. Current HIV Res. 2010;8:232–239.10.2174/157016210791111106
- Xue SY, Hebert VY, Hayes DM, Robinson CN, Glover M, Dugas TR. Nucleoside reverse transcriptase inhibitors induce a mitophagy-associated endothelial cytotoxicity that is reversed by coenzyme Q10 cotreatment. Toxicol. Sci. 2013;134(2):323–334.10.1093/toxsci/kft105
- Longenecker CT, Hileman CO, Funderburg NT, McComsey GA. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: the SATURN-HIV trial. Clin Infect Dis. 2014;59(8):1148–1156.10.1093/cid/ciu523
- Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(4):396.10.1097/QAI.0000000000000478
- Kaplan RC, Sinclair E, Landay AL, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213.10.1016/j.atherosclerosis.2011.03.011
- Seaberg EC, Benning L, Sharrett AR, et al. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke. 2010;41:2163–2170.10.1161/STROKEAHA.110.583856
- Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209(8):1156–1164.10.1093/infdis/jiu012
- Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58(4):588–595.10.1093/cid/cit748
- Liu CS, Lii CK, Chang LL, et al. Atorvastatin increases blood ratios of vitamin E/low-density lipoprotein cholesterol and coenzyme Q10/low-density lipoprotein cholesterol in hypercholesterolemic patients. Nutr Res. 2010;30:118–124.10.1016/j.nutres.2010.01.007
- Adams SP, Sekhon SS, Wright JM. Lipid-lowering efficacy of rosuvastatin. Cochrane Database Syst Rev. 2014;11: epub. doi: 10.1002/14651858.CD010254.pub2
- Tousoulis D, Psarros C, Demosthenous M, Patel R, Antoniades C, Stefanadis C. Innate and adaptive inflammation as a therapeutic target in vascular disease. J Am Coll Cardiol. 2014;63(23):2491–2502.10.1016/j.jacc.2014.01.054
- Lee J, Hong YS, Jeong JH, et al. Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines. PLoS ONE. 2013;8(7):e69362–69369.10.1371/journal.pone.0069362
- El Morsy EM, Kamel R, Ahmed MA. Attenuating effects of coenzyme Q10 and amlodipine in ulcerative colitis model in rats. Immunopharmacol immunotoxicol. 2015;37(3):244–251, epub.
- McMurray JJ, Dunselman P, Wedel H, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure. J Am Coll Cardiol. 2010;56(15):1196–1204.10.1016/j.jacc.2010.02.075
- Toyama K, Sugiyama S, Oka H, et al. Rosuvastatin combined with regular exercise preserves coenzyme Q10 levels associated with a significant increase in high-density lipoprotein cholesterol in patients with coronary artery disease. Atherosclerosis. 2011;217:158–164.10.1016/j.atherosclerosis.2011.02.050