Abstract
Patients with prior virologic failure (VF) are at an increased risk of subsequent failure, emergence of resistance, and death. This analysis identifies outcomes and correlates of VF in a high-risk population.
Methods: A5251 was designed to evaluate an enhanced adherence counseling intervention delivered by nurses from a central call site on virologic suppression. Due to slow enrollment, the study was closed prematurely and revised study endpoints were evaluated (week 24 VF (HIV-1 RNA ≥200 copies/ml) and non-perfect adherence (<100% self-reported using both the ACTG adherence questionnaire and visual analog scale (VAS)).
Results: Fifty-nine participants were enrolled, 43 (73%) black non-Hispanic and 23 (39%) women. Median prior antiretroviral regimen changes were three and the co-morbidity in this population was higher than typical for HIV clinical trials. At week 24 (n = 41), 24 (59%) failed to reach virologic suppression (HIV-1 RNA <200 copies/ml) and 25 (63%) reported non-perfect adherence. Higher depression (CES-D10) and adverse illness perceptions (IPQ-B) were associated with week 24 non-adherence. Early clinical assessments (week 12 HIV-RNA ≥200 copies/mL and non-perfect adherence) as well as higher depression and adverse illness perceptions were associated with week 24 VF.
Discussion: In this high-risk population, the proportion of participants with suboptimal adherence and VF was unacceptably high. Interventions to address this treatment gap are clearly needed. Depression and a higher illness perception score, failure to achieve virologic suppression by week 12, and less than perfect adherence could be used to target individuals for early interventions in treatment-experienced, high-risk individuals at high risk for VF.
Combination antiretroviral therapy (ART) has dramatically decreased HIV-related morbidity and mortality.Citation1, 2 HIV treatment is also an effective strategy for prevention.Citation3–5 However, a substantial proportion of people living with HIV (PLWH) have detectable HIV virus due to virologic failure (VF).Citation6 CDC estimates show that of Americans with HIV, 86% are diagnosed, 40% in HIV care, 37% prescribed ART, and 30% virally suppressed.Citation7,8 These estimates suggest approximately 20% of those prescribed ART are not virologically suppressed and pose an ongoing risk of transmitting HIV.Citation5,8 To achieve an “AIDS Free Generation,” it is essential to develop effective interventions for PLWH who are currently failing ART.Citation9–11
Many strategies have been employed to improve HIV medication adherence.Citation12 However, a recent review concluded that few interventions have improved both adherence and clinical outcomes.Citation13 Additionally, persons of color and individuals with co-morbidities and psychologic/behavioral factors are associated with higher rates of VF and are underrepresented in trials.Citation14, 15 Few studies enroll high-risk participants with recent VF, partly because they are known to have higher risk of subsequent failure.Citation16
In AIDS Clinical Trials Group (ACTG) A5251, we sought to enroll a diverse sample of PLWH with recent VF to test the efficacy of a phone-based adherence counseling intervention. This study followed earlier adherence intervention studies.Citation17–19 A5251 closed prematurely due to slow enrollment and we were unable to evaluate the intervention’s efficacy. In this paper, we report the challenges encountered enrolling a high-risk population for non-adherence and recurrent VF and present results of post hoc analyses to identify characteristics associated with VF.
Methods
Study design
A5251 original
A5251 was a multi-site randomized controlled trial to evaluate virologic efficacy of an enhanced adherence counseling intervention compared to standard of care (SOC) over 48 weeks. The targeted sample size was 296 participants (1:1 randomization). The intervention was delivered by phone from nurses at one call center. Calls incorporated motivational interviewing,Citation20 individualized to participants’ illness perceptions, and focused on helping participants develop knowledge and skills to overcome barriers.Citation21–24 Participants in both arms received individualized SOC adherence education/counseling by a physician, nurse, and/or pharmacist. The primary endpoint was virologic suppression at week 48. Data were collected at baseline and every 12–16 weeks after randomization to week 96. Inclusion criteria were HIV positives, age ≥18 years, English or Spanish speaking, non-adherent to antiretrovirals in the prior year, VF on ART (≥1,000 copies/mL within 16 weeks of study enrollment), co-enrolled in an ACTG study, and starting a new regimen with ≥2 active antiretrovirals at study entry. The ACTG study’s co-enrollment criterion was amended to allow enrollment of any patient followed at participating domestic ACTG sites. Additionally, participants could restart a regimen with ≥2 active antiretrovirals documented by HIV resistance testing rather than switching to a new regimen. The protocol was approved by the institutional review boards at participating ACTG sites; participants provided written informed consent.
A5251 post hoc
Premature study closure, both enrollment and follow-up, resulted in insufficient power to evaluate the planned primary endpoint. We refocused the study aims to: (1) describe the enrolled high-risk population; and (2) identify characteristics associated with VF. Revised endpoints were: (1) week 24 VF (HIV-1 RNA ≥200 copies/ml); and (2) week 24 non-perfect adherence (<100% self-reported visual analog scale [VAS]).
Study assessments
CD4 cell count, plasma HIV-1 RNA, and self-reported adherence were assessed at baseline, weeks 12, 24, and 48. Baseline questionnaires included: antiretroviral history, adherence, depression score (CES-D10),Citation25 self-efficacy score,Citation26 and the Brief Illness Perception Questionnaire (IPQ-B),Citation27 a nine-item scale assessing cognitive and emotional representations of illness: a higher score indicating a more threatening view of HIV. At baseline and weeks 12 and 24, participants completed the (a) ACTG adherence self-report questionnaire,Citation26 and (b) a short adherence questionnaire composed of two questions: four-day adherence recall and a VAS that asked participants to indicate on a scale of 0–100% the percentage of antiretrovirals taken over the last 30 days.Citation28 Prior to study closure, sites were asked to complete a questionnaire on enrollment barriers.
Analysis
The post hoc analyses combined all participants, irrespective of randomization, and all study follow-up that occurred prior to premature study closure. Univariable and multivariable exact logistic regression models for the revised endpoints assessed associations. Covariables with p < 0.2 in univariable models were included in multivariable models, unless considered co-linear, in which case, they were removed from multivariable models one at a time. Baseline covariables included age, sex, race/ethnicity, restarting a antiretroviral regimen vs. starting a new one, being on vs. off antiretrovirals, number of prior regimens, CES-D10, IPQ-B, self-efficacy, smoking history (none vs. ever), and substance use in the last year. Week 12 covariables included VAS adherence (perfect vs. not), ACTG four-day adherence (perfect vs. not), and HIV-1 RNA (< vs. ≥200 copies/ml). Participants with missing data were excluded.
Results
Study enrollment
Fifty-nine participants enrolled between October 2010 and October 2012. Thirty (51%) were randomized to adherence counseling and 29 to SOC. Follow-up ended in January 2013. Eighty-five percent (29 of 34) of participating sites completed a questionnaire on enrollment barriers. Most sites (64%) reported not enrolling some participants due to active drug use, mental health problems, or being deemed unreliable. Some failed to keep appointments for screening and/or baseline visits. Eight of the 29 responding sites (28%) cited reluctance to enroll participants viewed as high risk for incomplete follow-up because of the ACTG evaluation process that penalizes sites for participants who miss follow-up visits or are lost to follow-up.
Baseline characteristics
Forty-three (73%) participants were black non-Hispanic and 23 (39%) were women (Table ). Median baseline age was 47 (IQR 37-53) years, CD4 count 180 (57-333) cells/mm3, and HIV-1 RNA 4.0 (3.2–4.9) log10copies/mL. Median prior antiretroviral regimen changes (≥2 drugs and/or a change in drug class) were three (range 0, >10), and 22% reported ≥5. Psychosocial co-morbidities were common: 28 (47%) had a history of depression, 10 (17%) of anxiety, 18 (31%) of substance use, and 40 (68%) of smoking cigarettes, with 32 (54%) current smokers. HIV drug resistance was common: 38 (64%) had reverse transcriptase and 35 (59%) protease mutations. Integrase resistance mutations were infrequent, 2 (3%).
Table 1 Baseline characteristics of the study participants
Antiretroviral regimens (re)started at study entry comprised two to six medications, ≥2 nucleoside analogs (91%), non-nucleoside (28%), protease inhibitor (76%), integrase inhibitor (38%), and/or entry inhibitor (12%). At entry, 38 (64%) participants were taking their prescribed cART, and of these, 17 (45%) reported missed dose(s) in the last four days. All but seven participants reported missing doses in the last three months. Overall, only 13 (22%) reported always taking their antiretrovirals on time, with an additional 13 (22%) doing so “most of the time.” Median adherence to antiretrovirals over 30 days prior to entry using the VAS was 76%.
Follow-up
At study closure, 55 participants had been followed through week 12, and 46 through week 24. Four were on study for less than 12 weeks: 2 due to study closure and 2 due to deaths. Nine others were on study for less than 24 weeks: 8 due to study closure and only 1 participant was lost to follow-up. Median weeks from study entry to last study were 33.3 weeks (IQR: 19.9–45.4). Forty-three (78%) had HIV-1 RNA data at week 12 and 41 (89%) at week 24 (Fig. ).
Figure 1 Enrollment and follow-up of study participants.
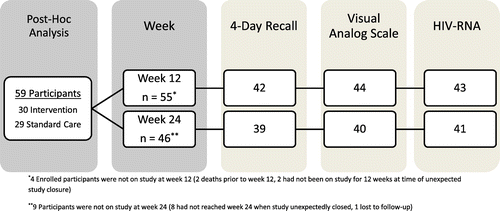
Of 30 randomized to the adherence counseling intervention, 20 (67%) were successfully contacted by the call center, 9 (30%) were not contactable, despite multiple attempts, and study-related technical difficulties prevented contacting one. Nurses reported some participants had run out of phone minutes or changed phone numbers. During the short follow-up, 25 participants had a total of 73 acute illnesses including 11% bacterial or mycobacterial infections, 10% candidal infections, and 12% gastrointestinal diseases; 12 hospitalizations, and 3 deaths. Two deaths were HIV-related (disseminated Histoplasmosis or septic shock), neither attributed to the intervention, and for one participant, the cause of death was unknown.
On study adherence
Forty-two (76%) completed the four-day adherence recall at week 12 and 39 (85%) at week 24. Forty-four (80%) completed the VAS adherence at week 12 and 40 (87%) at week 24. At week 12, nine reported missed dose(s) in the last four days, and by VAS, median adherence was 95% (IQR: 80%, 100%) with 24 reporting non-perfect adherence. Sixteen reported missed dose(s) in the last four days and by VAS, week 24 median adherence was 95% (IQR: 65%, 100%), with 25 (63%) reporting non-perfect adherence (<100%).
Week 48 viral suppression
Nine of the 14 (64%) participants with week 48 data had HIV-1 RNAs <200 copies/mL. As stated above, the study was underpowered for the planned primary endpoint at week 48 and there was no statistical difference between randomized arms (Fisher’s exact p > 0.99).
Characteristics associated with non-suppression at Week 24
Twenty-four (59%) of the 41 participants with week 24 HIV-1 RNAs were ≥200 copies/mL. In univariable models, several covariables were significantly associated with week 24 non-suppression: week 12 HIV-1 RNA ≥200 copies/mL (OR 25.7 [95%CI 3.4, 357.7]; p < 0.001), week 12 non-perfect VAS adherence (OR 7.4 [1.3, 57.6]; p = 0.018), and higher IPQ-B score, indicating a more threatening view of HIV (OR 1.09 [1.02, 1.17] per unit; p = 0.008) (Table ). Associations with week 12 ACTG four-day recall (p = 0.20) and CES-D10 (p = 0.21) were weaker and not included in the multivariable model. In the multivariable model, only week 12 HIV RNA ≥200 copies/mL remained a significant predictor of week 24 viral non-suppression.
Table 2 Variables associated with week 24 detectable HIV virus and non-perfect adherence by visual analog scale (exact logistic regression)
Characteristics associated with non-perfect adherence by VAS at Week 24
Twenty-five (63%) of the 40 participants reported week 24 non-perfect VAS. Covariables associated with non-perfect VAS were higher CES-D10 score (OR 1.31 [1.11, 1.62] per unit; p < 0.001), higher IPQ-B score (OR 1.15 [1.06, 1.28] per unit; p < 0.001), and week 12 non-perfect VAS (OR 25.5; p < 0.001) (Table ). All participants reporting week 12 non-perfect VAS adherence also reported week 24 non-perfect VAS adherence. The association between week 12 non-perfect four-day adherence and week 24 VAS adherence was not significant (p = 0.15). In the multivariable model that excluded week 12 measures, CES-D10 (OR 1.24 [1.03, 1.54]; p = 0.022) and IPQ-B (OR 1.11 [1.02, 1.24]; p = 0.013) remained independently associated with week 24 non-perfect VAS adherence.
Discussion
Adherence to antiretroviral medication is key to improved outcomes and minimizing HIV transmission, but a variety of barriers impede adherence in high-risk populations. This study was designed to improve our understanding of how to better support PLWH with poor adherence and recent VF.
Women, populations of color, those with history of depression or anxiety, and substance use were well represented. Enrollment, however, was slower than expected and the study closed prematurely to recruitment and follow-up. In retrospect, this might have been anticipated, given the target population and ACTG standard procedures. Although there was wide interest from ACTG sites across the United States, many sites excluded eligible patients from enrollment because of concerns about potential missed visits and increased loss-to-follow-up of high-risk participants. ACTG sites are evaluated on maintaining patients on study and on study data completeness; dropout rates adversely affect ACTG site scores. Enrolling subjects that drop out might jeopardize sites’ future participation as a site within the ACTG, as site scores are used to select clinical sites. This was especially important as sites were concerned about the downsizing in the number of sites that was expected to occur shortly after the study closed. Despite concerns about dropout, retention of enrolled participants was excellent; only 1 person was lost to follow-up prior to study closure.
Participants were heavily ART experienced, most had failed more than one regimen and a quarter over five. They reported numerous co-morbidities, more than typically seen in HIV trials. Participants were also sicker, and experienced more acute illnesses and hospitalizations than expected. In contrast to our prior work, the central call center was unable to reach 30% of those randomized to the intervention arm. We believe this reflects the challenges of scaling up the intervention to a large multi-site study in an era where most participants only have cell phones and many do not maintain the same cell phone number over time.Citation17–19 Even after enrollment into a clinical trial targeting high-risk individuals, ACTG sites’ efforts failed to achieve acceptable levels of adherence or virologic outcomes for most of this high-risk population: nearly 60% had week 24 HIV-1 RNAs ≥200 copies/mL. Many covariables associated with non-adherence and VF in previous studies (e.g. age, sex, race/ethnicity, restarting the same regimen vs. starting a new regimen, and substance use) did not have significant associations in this underpowered study.Citation16 However, several variables did reach significance including a higher baseline illness perception score, which was associated with non-perfect adherence by VAS and poorer HIV suppression at week 24. Higher depressive symptoms were also significantly associated with week 24 non-perfect VAS adherence. Early assessments, non-perfect VAS adherence, and failure to suppress HIV-1 RNA to <200 copies/mL at week 12 were strongly associated with week 24 VF. Interestingly, the VAS, a simple single assessment, was more strongly associated with poor outcomes than the four-day adherence recall, which has been widely used within and outside the ACTG for over 25 years.Citation26 However, our numbers were small and these results should be interpreted cautiously until validated in large studies.
Conclusions
Novel interventions for individuals with prior VF due to suboptimal adherence are clearly needed. This study shows that enrolling these ART-experienced participants at high-risk of non-adherence and VF is challenging, but once enrolled, participants can be retained. Findings indicate that higher baseline illness perception and depression score, <100% adherence by VAS, and/or failure to achieve virologic suppression by week 12 might identify participants at greatest risk of VF soon after starting a new ART regimen. This study highlights the importance of obtaining information on HIV illness perception and depression in addition to antiretroviral adherence measures to allow for early targeted interventions, rather than waiting for the traditional week 24 assessment. In addition, the single-item VAS adherence measure outperformed the longer ACTG four-day recall questionnaire. The high rate of suboptimal adherence and VF underscore the need for further research in this population to improve the state of engagement in the HIV care continuum cascade and work toward achieving an AIDS-free generation.
Disclaimer statements
Contributors GKR was involved in conceiving and designing the study, obtaining funding and ethics approval, analyzing the data, writing the article in whole, revising the article. SEC was involved in conceiving and designing the study, obtaining funding and ethics approval, analyzing the data, writing the article in part, revising the article. LJH was involved in analyzing the data, writing the article in part, revising the article. LS was involved in designing the study, analyzing the data, revising the article. LM and DR was involved in designing the study, collecting and analyzing the data, revising the article. MD was involved in designing the study, analyzing the data, revising the article. TF was involved in collecting and analyzing the data, revising the article. SL was involved in analyzing the data, writing the article in part, revising the article. AWW was involved in designing the study, analyzing the data, revising the article. SAS was involved in designing the study, analyzing the data, revising the article. NRR was involved in conceiving and designing the study, obtaining funding and ethics approval, analyzing the data, writing the article in part, revising the article.
Funding AIDS Clinical Trial Group and National Institute of Nursing Research.
Conflicts of interests No relevant conflicts.
Acknowledgments
The authors are grateful for the patients’ commitment and participation in this study and the New Start Call Center (Kirsis Ham NP, Valery Hughes NP, Christina Megill PA and Todd Stroberg). The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Office of the Global AIDS Coordinator, the National Institutes of Health, or the U.S. Department of State. The following individuals assisted in conducting A5251: Beverly E. Sha, MD and Tondria Green, RN, BSN, ACRN – Rush University Medical Center (Site 2702) Grant AI069471; Miriam Chicurel-Bayard RN BSN and Erin Elizabeth Hoffman BS – Chapel Hill CRS (Site 3201) ACTG CTU Grant UM1 AI069423, CTSA: 1UL1TR001111 CFAR: P30 AI50410; Shobha Swaminathan, MD and Christie Lyn Costanza, MPH – Rutgers New Jersey Medical School CRC (Site 31786) Grant 2UM1AI069419; Kerry Upton, RN and Karen Savage, RN – Alabama Therapeutics CRS (Site 31788) Grant UM1AI069452; Kim Whitely and Traci Davis – MetroHealth CRS (Site 2503) Grant 1U01AI069501-01; Susan E. Cohn, MD, MPH, Nina Lambert, BSN – Northwestern University CRS (Site 2701) Grant AI 069471; Elizabeth Fletcher MS APN-C and Yolanda Smith BA – Cooper University Hospital (Site 31476) Grant UM1 AI069503; Amy Sbrolla RN BSN and Teri Flynn ANP-BC – Massachusetts General Hospital (Site 101); Albert Wu, MD AI069465 Johns Hopkins University. Grant 2UM1AI069412-08; Kirsis Ham NP, Valery Hughes NP, Christina Megill PA-C and Todd Stroberg RN – Weill Cornell Chelsea CRS (Site 7804) Grant UM1AI069419 and CTSC UL1TR000457; Roger Bedimo MD and Michelle Mba MPH – Trinity Health & Wellness Center (Site 31443) Grant UM1AI069471; Linda Meixner RN and Edward Seefried RN – UCSD (Site 701) Grant AI069432; Brenda Jackson, RN and Becky Basham, Regulatory – Vanderbilt Therapeutics Clinical Research (Site 3652) Grant 2UM1AI069439-08 and Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH; and UM1 AI068634 ACTG Statistical and Data Management Center.
References
- Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infec. Diseas. 2006;194(1):11–19.10.1086/jid.2006.194.issue-1
- Palella FJ, Jr., Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2006;43(1):27–34.10.1097/01.qai.0000233310.90484.16
- Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England J Med. 2000;342(13):921–929.10.1056/NEJM200003303421303
- Dieffenbach CW. Preventing HIV transmission through antiretroviral treatment-mediated virologic suppression. Curr Opin HIV AIDS. 2012;7(2):106–110.10.1097/COH.0b013e32834f3f13
- Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Internal Med. 2015;175(4):588–596.10.1001/jamainternmed.2014.8180
- Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin. Infec. Diseas. 2011;52(6):793–800.10.1093/cid/ciq243
- Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: From cascade to continuum to control. Clin. Infec. Diseas. 2013;57(8):1164–1171.10.1093/cid/cit420
- Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV–United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–17.
- Hull MW, Montaner JS. HIV treatment as prevention: The key to an AIDS-free generation. J Food Drug Anal. 2013;21(4):S95–S101.10.1016/j.jfda.2013.09.043
- Needle R, Fu J, Beyrer C, et al. PEPFAR’s evolving HIV prevention approaches for key populations—people who inject drugs, men who have sex with men, and sex workers. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S145–S151.10.1097/QAI.0b013e31825f315e
- The Office of the Global AIDS Coordinator. PEPFAR BluePrint: Creating an AIDS-free Generation. 2012. http://www.pepfar.gov/documents/organization/201386.pdf.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-infected Adults and Adolescents. Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- Mbuagbaw L, Sivaramalingam B, Navarro T, et al. Interventions for enhancing adherence to antiretroviral therapy (ART): A systematic review of high quality studies. AIDS Patient Care STDs. 2015;29(5):248–266.10.1089/apc.2014.0308
- Blashill AJ, Bedoya CA, Mayer KH, et al. Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS Behav. 2015;19(6):981–986.10.1007/s10461-014-0925-6
- Castillo-Mancilla JR, Cohn SE, Krishnan S, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials. 2014;15(1):14–26.10.1310/hct1501-14
- Robbins GK, Johnson KL, Chang Y, et al. Predicting virologic failure in an HIV clinic. Clin Infect Dis. 2010;50(5):779–86.
- Collier AC, Ribaudo H, Mukherjee AL, et al. A randomized study of serial telephone call support to increase adherence and thereby improve virologic outcome in persons initiating antiretroviral therapy. J Infec. Diseas. 2005;192(8):1398–1406.10.1086/jid.2005.192.issue-8
- Reynolds NR, Testa MA, Su M, et al. Telephone support to improve antiretroviral medication adherence. J Acquir Immune Defic Syndr. 2008;47(1):62–68.10.1097/QAI.0b013e3181582d54
- Robbins GK, Testa MA, Su M, et al. Site nurse–initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy, ACTG 5031: Substudy of ACTG 384. HIV Clin Trials. 2013;14(5):235–253.10.1310/hct1405-235
- Miller WR, Rollnick S. Motivational Interviewing. 2nd ed. New York, NY: The Guilford Press; 2002.
- Leventhal H, Meyer D, Nerenz D. The common sense representation of illness danger. Contrib Med Psychol. 1980;2:7–30.
- Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: Using common sense to understand treatment adherence and affect cognition interactions. Cognitive Ther Res. 1992;16(2):143–163.10.1007/BF01173486
- Reynolds NR. The problem of antiretroviral adherence: A self-regulatory model for intervention. AIDS Care. 2003;15(1):117–124.10.1080/0954012021000039815
- Weinman J, Petrie KJ, Moss-Morris R, Horne R. The illness perception questionnaire: A new method for assessing the cognitive representation of illness. Psychol Health. 1996;11(3):431–445.10.1080/08870449608400270
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.10.1177/014662167700100306
- Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2000;12(3):255–266.10.1080/09540120050042891
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637.10.1016/j.jpsychores.2005.10.020
- Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–277.10.1097/00002030-200201250-00017
