Abstract
Background: Tuberculosis (TB) remains a main cause of morbidity and mortality among individuals infected with HIV. We investigated the incidence of TB among a cohort of HIV infected patients attending a setting with low TB burden where screening for latent TB infection is not routinely carried out.
Methods: an observational cohort study on HIV-infected adults attending the HIV clinic at Queen Elizabeth Hospital Birmingham, UK between 1 January 2011 and 30 September 2015. Patients with culture-proven TB after HIV diagnosis, or those treated for clinical diagnosis of the infection, were classified as having “active TB”.
Results: 1824 patients were included in the study (5347 patient years of follow up), of whom 21 patients developed TB (16 microbiology confirmed). Of the 666 new HIV diagnoses, six patients developed TB within one month, giving a TB prevalence at the time of HIV diagnosis of 0.9%. The total TB incidence for the remaining 1818 patients was 2.81 cases per 1000 patient years (95% CI: 1.63–4.53). TB incidence was significantly more common among patients with CD4 ≤ 200 cells/mm3 compared to those with CD4 > 500 cells/mm3 (28.2 vs. 1.22 per 1000 patient years, p < 0.001), and in patients with VL ≥ 40 copies/mL compared to <40 copies/mL (8.30 vs. 1.42, p < 0.001).
Conclusion: In settings with low TB prevalence, early start of combined antiretroviral therapy and intensified TB case finding protocols may significantly reduce the incidence of TB.
Background
Patients with HIV-associated immunosuppression have 10 times the risk of reactivation of latent Tuberculosis (TB) infection (LTBI) compared to non-HIV infected individuals.Citation1 By prevention of reactivation of LTBI, isoniazid prevention therapy (IPT) reduces the incidence of TB and is recommended among HIV-infected patients.Citation2 This has been supported by data from settings with high rates of TB.Citation3 Earlier studies from resource rich settings with low TB burden supported IPT among HIV-infected individuals.Citation4 British HIV Association (BHIVA) recommends IPT for HIV-infected patients at risk of LTBI when confirmed by an interferon gamma releasing assay (IGRA).Citation5
Intensified TB case finding has also been part of the WHO recommendations for prevention of infection.Citation6 The advent of novel high performance TB assays that can reliably detect active infection provide the opportunity for implementation of the policy in most clinic settings.Citation7
Combination of antiretroviral therapy (cART) has also been shown to prevent active TB infection among HIV-infected patients.Citation8–12
In settings with low TB burden, improved diagnosis and treatment of active TB infection with cART may reduce the incidence of TB in HIV-infected individuals to the extent that IPT may not be required. In one such setting, we aimed to investigate the incidence of TB.
Methods
This was an observational study on a cohort of HIV-infected patients attending Queen Elizabeth Hospital Birmingham, UK followed up between 1 January 2011 and 30 September 2015 (inclusive).
In our center, we do not screen HIV-infected patients for LTBI, nor do we offer IPT. Instead, we follow the protocols for intensified TB case finding for prevention of TB.Citation6 Patients with clinical presentations suspicious of TB are routinely screened for active infection, irrespective of their country of birth, CD4+ T cell or plasma viral load (VL) counts.
Patients with evidence of possible pulmonary TB are followed up with a bronchoscopy and mycobacterial culture of materials from broncho-alveolar lavage. Those with fever are screened with at least three samples of blood and early morning urine for culture of mycobacterial infection. Patients with persistent lymphadenopathy undergo biopsy for histopathology examination. Tissue samples are cultured for mycobacterial infection. All cases of positive cultures of mycobacterial infection are confirmed in the West Midlands’ mycobacterial reference laboratory.
In this study, patients with active TB had positive growth of Mycobacterium tuberculosis (MTB) in culture media, evidence of MTB on GeneXpert assay or positive acid fast bacilli on microscopy examination of tissue and sputum specimens. HIV-infected patients with clinical diagnosis of TB who completed the standard duration of treatment were also classified as having active TB.
HIV-infected patients with CD4+ T-cells of less than 350 cells/mm3 were offered cART. VL and CD4+ T-cells were measured after four weeks. These were measured every 16 weeks after VL count measured less than 40 copies/mL.
Study end points and data collection
The primary end point of the study was the incidence of TB among a cohort of HIV-infected patients attending a large tertiary center in the UK where cART and intensified TB case finding without IPT is routinely offered. Our secondary end point was to investigate the number of patients at risk of LTBI who needed IPT in order to prevent one case of active TB. We used BHIVA guidelines to identify those at risk of LTBI.
All patients who attended the HIV clinic during the study period were included in the analysis. For each patient, the dates of all clinic appointments within the study period were collected. The period between the first and last appointments was treated as the total follow up for each patient. Values of age, duration of ARV, CD4+ T-cells count, and VL were recorded for each appointment. Where CD4+ T-cells and VL measurements were not available at an appointment, the last values were carried forward. Where the data were not available at the first appointment, the first available measures were carried backward.
At each appointment, the CD4+ T-cells measurement, duration of cART, and country of origin were used to identify whether patients were at high risk according to BHIVA guidelines (Table ). Patients with missing data for any of the criteria used in the risk score calculation were excluded from the risk criteria analysis.
Table 1 Risk criteria for latent TB according to British HIV association guidelines
The time between consecutive appointments for a patient was then calculated. VL, CD4+ T-cell, age, and duration of CD4+ T-cells were assumed to remain constant for the duration between appointments. Patients could move between low and high risk groups over the course of follow up, as their age, CD4+ T-cells count, VL, and duration of cART varied.
Where patients had multiple TB diagnoses, only the first was considered in the analysis. The factors relating to the patient (e.g. CD4+ T-cells, risk category) at the time of TB diagnosis were then recorded and used in the calculation of TB incidence by patient subgroup.
Statistical analysis
Because TB incidence was not stable over time, the data were analyzed using two different approaches. The first set of analyses considered the short-term TB prevalence in patients newly diagnosed with HIV. Patients who developed TB within a month of HIV diagnosis were compared to those that did not using t-tests for normally distributed variables, Mann–Whitney tests for skewed variables, and Fisher’s exact test for nominal variables, with IBM SPSS 22 (IBM Corp. Armonk, NY).
In our calculation, we considered TB cases diagnosed within 30 days of HIV diagnosis for measurement of prevalence of TB in our cohort. This time span reflects the number of days for identifying and reporting isolates of mycobacterial infection by the regional reference TB laboratory. We considered TB cases after 30 days of HIV diagnosis for calculation of incidence of TB.
The second analysis included the whole cohort of HIV patients, but excluded those diagnosed concurrently with HIV and TB. The long-term incidence of TB was then calculated within this group, by dividing the number of TB cases by the total number of patient years (PYs) of follow up.
Long-term TB incidence was then calculated within subgroups of patients, based on a range of factors. Some of these factors (e.g. CD4) varied over time, hence a patient could potentially be in multiple subgroups during their period of follow up. In order to reflect this, the number of patient years between subsequent measurements of the factor were calculated, and added to the total for the respective subgroup. For example, take a patient who had an initial CD4 level of 150 cells/mm3, which increased to 300 cells/mm3 at one year, before their follow-up ended at two years. This patient would then contribute one patient year to the ≤200 cells/mm3 group and one patient year to the 201–350 cells/mm3 group. If the patient developed TB between one and two years, then one case of TB would be added to the CD4 = 201–350 cells/mm3 subgroup. The same process was then applied to all patients, and to all of the factors being considered, to calculate the total number of TB cases and patient years in each subgroup, which were then used to calculate the TB incidence. Comparisons of TB incidence across factors were then made using the mid-P exact test from the OpenEpi person-time calculator.Citation13
A number needed to treat (NNT) was then calculated for the patients in the high risk group according to the BHIVA guidelines. This made the assumption that all patients identified as high risk would be treated with isoniazid prophylaxis, which would have 60% effectiveness for IPT.Citation8 As such, the NNT to prevent one case of TB would be 1/(0.4X), where X is the yearly incidence of TB in the high risk group.
Results
Patient demographics
Between 1 January 2011 and 30 September 2015, 1824 patients attended 94,563 appointments, contributing to 5347 PY of follow-up (Table ). Patients had a mean age of 39.3 ± 10.3 at their first appointment in the period, and 65% were male.
Table 2 Demographics
During the period, 21 patients developed TB, of whom 16 cases were confirmed by positive MTB culture (Table ). One patient died within two weeks of the diagnosis of HIV and TB.
Table 3 Summary of cases of active TB. Unless stated otherwise, sputum samples were negative for AFB
Within the cohort as a whole, 666 (36.5%) were newly diagnosed with HIV. At the point of HIV diagnosis, the median CD4 count was 452 (IQR: 263 – 634) cells/mm3, and 54.0% of these patients were classified as “high risk” mostly due to the first criteria (from Sub Saharan Africa and on cART for less than two years). TB cases were recorded in eight patients, of which seven were confirmed by microbiology.
Short-term TB prevalence
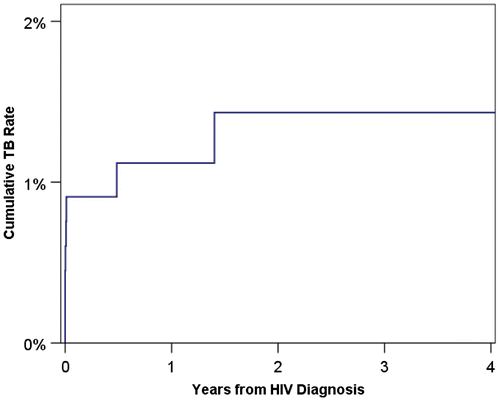
Of the eight TB cases recorded in new HIV diagnoses, six (75%) occurred within one month of HIV diagnosis (Fig. ), giving a TB prevalence of 0.9%. All of these patients were of black ethnicity, resulting in a significant difference in ethnicity distributions between those patients with and without TB at one month (p = 0.011). Patients with TB had a significantly lower CD4 at the time of HIV diagnosis, with a median of 120 cells/mm3, compared to 459 cells/mm3 in patients TB-free at one month (p = 0.001). TB prevalence was 1.7% in the high risk patient group, compared to 0.0% in the low risk group (p = 0.034). Comparisons of other factors are reported in Table .
Table 4 Short term TB prevalence
Long-term TB incidence
The six patients with TB recorded within a month of HIV diagnosis were removed from the full cohort, leaving 1818 patients and 15 cases of TB (10 microbiology confirmed). These patients contributed to 5336 PY, giving a total TB incidence of 2.81 per 1000 PY (95% CI: 1.63 – 4.53). Eight patients (53%) were lost to follow up for HIV care for at least one year prior to diagnosis of TB (Table ). Comparison of TB incidence across a range of factors is reported in Table .
Table 5 Long term TB incidence
Patients originating from medium risk countries had significantly higher incidence than those from low risk countries (7.32 vs. 1.18 per 1000 PY, p = 0.043), although high risk countries were not found to have a significantly higher TB incidence (3.86 vs. 1.18 per 1000 PY, p = 0.068). The three patients from medium incidence countries that developed TB originated from Latvia (Table ).
TB incidence did not differ significantly by patient age (p = 0.233) or gender (p = 0.440). Incidence was raised in black, relative to white patients, although not significantly so (3.97 vs. 1.40 per 1000 PY, p = 0.098). TB incidence did not differ significantly by cART, with a small reduction from 4.10 cases per 1000 PY where the duration was less than six months, to 2.29 per 1000 PY for cART durations greater than two years (p = 0.354).
The best predictors of TB incidence were disease related factors of VL and CD4 measurements. Patients with VL ≥ 40 copies/mL had a TB incidence of 8.30 per 1000 PY, compared to 1.42 in patients with a VL of less than 40 copies/mL (p < 0.001). TB incidence in patients with CD4 ≤ 200 cells/mm3 was 23 times greater than those with CD4 > 500 cells/mm3 (28.2 vs. 1.22 per 1000 patient years, p < 0.001).
Despite the effect of CD4 count, patients classified as high risk by BHIVA guidelines were not found to have a significantly higher TB incidence than low risk patients (6.31 vs. 2.23 per 1000 patient years, p = 0.079).
Assuming that every high-risk patient were to be treated with IPT and every patient was to complete the six month treatment course, the number needed to be treated to prevent one case of TB per year would be 264, with a 60% effectiveness for IPT.Citation8
Discussion
In our study, the overall incidence of TB among a cohort of HIV-infected patients was relatively low at 0.28 per 100 PY. This was considerably less than that of 4.69 cases per 100 PY reported for over 17,000 HIV-infected individuals enrolled in 12 cohorts in Europe and North America between 1996 and 2003.Citation4 Lower incidence of active TB has been reported among HIV-infected adults in resource rich settings; 0.22 cases per 100 PY in a Swiss HIV cohort study.Citation9
The incidence of TB among HIV-infected individuals in high TB burden settings has been reported at significantly higher rates; between 2.6 and 11.25 per 100 PY.Citation10–12
We identified most cases of active TB at the time of HIV diagnosis where IPT would not have been useful. The highest incidence of active TB has been reported in the first 12 months after start of cART.Citation10,11 A prospective study of 1544 HIV-infected patients in South Africa reported TB incidence of 12.43 cases per 100 PY in the first year of HIV diagnosis that reduced to 4.92 cases per 100 PY after five years.Citation12
We believe that intensified TB case finding with early start of cART significantly reduced the incidence of the infection in our cohort. In the recently published TEMPRANO study on 2056 patients, cART reduced the rate of TB by 50%.Citation8 Other studies have similarly reported reduced incidence of TB with cART.Citation10–12
We found that low CD4+ T cell and detectable plasma VL counts were the best predictors of active TB in long-term follow-up. This was also the case among individuals from high TB burden areas as reported in other studies.Citation9,11,12,14
Data on the role of reactivation of LTBI in causing cases of active TB among HIV-infected patients remain inconsistent. A US study on 22,857 TB cases reported 80% were related to reactivation of LTBI.Citation15 A systemic review of seven studies carried out in different African countries with over 2000 HIV-infected participants, however, concluded that cases of active TB in HIV endemic settings were more likely to develop after recent MTB transmission.Citation16 A significant number of our patients with HIV were from sub-Saharan African countries where recent transmission would have accounted for active TB infection.
The benefits of treatment of LTBI in prevention of active TB in HIV patients have been reported in earlier studies. A systemic review on 8578 HIV-infected patients concluded that IPT reduced the rate of active TB by 32% among patients not on cART.Citation3 More recent studies have reported up to a 60% reduction of active TB with IPT among patients taking cART.Citation8
We would have to treat 264 patients to prevent one case of active TB in our cohort. In a systemic review of earlier studies, 50 HIV-infected individuals had to be treated with isoniazid to prevent one case of active TB in high TB burden settings.Citation3 In our calculation, we assumed all patients on IPT would complete the treatment course. IPT completion rate has been universally reported to be sub-optimal. In a large study on 15,035 patients with LTBI, 45% completed IPT.Citation17 IPT completion rate has been reported between 60 and 78% among HIV-infected adults in cohort and retrospective studies.Citation18,19 In a prospective randomized study, 41% of 53 HIV-infected adults completed a six month course of IPT.Citation20
IPT can cause hepatotoxicity leading to its discontinuation among 2–22% of patients.Citation18–20
Concerns that failure of IPT may lead to development of isoniazid resistant TB infection have not been supported in large studies.Citation21
Intensified TB case finding relies on screening patients for active infection. This has been shown to be beneficial in areas with high prevalence of TB where between 7 and 31% of HIV-infected patients have been reported to have active TB infection before start of cART.Citation14
WHO has proposed symptoms that should be investigated for active TB. A systemic review on over 8000 HIV-infected individuals in high TB burden settings concluded that absence of the symptoms was associated with a low probability of active TB.Citation22 A more recent study in a high TB burden setting, however, reported a poor sensitivity of WHO symptoms in identification of 126 patients with active TB.Citation23 We believe that presence of WHO’s proposed symptoms should be followed up by extensive investigation for active TB. We rely on microbiological techniques for successful culture of mycobacterial infections for intensified TB case finding that may not be readily available in resource constrained settings.
The novel TB assays have improved the diagnosis of active infection particularly in resource constrained settings.Citation7,14 Screening for active TB infection with these assays should reduce the incidence of infection in those settings.
In our study, we identified high incidence of active TB among patients from Latvia. This is consistent with the available data that show high TB burden in some Eastern European countries.Citation24 We propose that HIV centers should implement intensified TB case finding protocols for patients from those countries.
BHIVA’s risk classification for patients at risk of LTBI is an effective tool for identifying newly diagnosed HIV-infected patients at risk of active TB. The classification had low yield for active TB in long-term follow-up.Citation5
Our study had a number of limitations. This was a cohort study and we could not adjust for all possible bias. Also, we do not know how many patients lost to follow up during the study period developed active TB. Our analysis was based on 5336 PY of follow up that should partly account for this limitation. In calculating the number of patients at risk of LTBI, we assumed all at-risk patients to have LTBI because IGRA has not been used in our center. Our method therefore suffered from type 1 error. Our data should be used with caution in low TB burden HIV care settings not adhering to intensified TB case finding protocols and early start of cART.
Early diagnosis of active TB infection should remain the cornerstone of management of HIV-infected patients. Following the publication of the results of INSIGHT START study,Citation25 many national guidelines now recommend start of cART with any CD4 count. We propose that the national guidelines in areas with low TB burden should consider the favourable impact of cART on incidence of active TB and recommend protocols for intensified TB case finding among HIV-infected adults.
Conflict of interest
No potential conflict of interest was reported by the authors.
References
- Horsburgh CR, Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350(20):2060–2067.10.1056/NEJMsa031667
- WHO, UNAIDS. Policy Statement on Preventive Therapy against Tuberculosis in People Living with HIV. Geneva: WHO; 1998.
- Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171.
- Girardi E, Sabin CA, d’Arminio MA, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41:1772–1782.
- British HIV Association. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med. 2011;12:517–524.
- World Health Organization. Guidelines for Intensified Tuberculosis Case-finding and Isoniazid Preventive Therapy for People Living with HIV in Resource Constrained Settings. Geneva: Department of HIV/AIDS, Stop TB Department, World Health Organization; 2010.
- Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015.10.1056/NEJMoa0907847
- TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822.10.1056/NEJMoa1507198.
- Elzi L, Schlegel M, Weber R, et al. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin Infect Dis. 2007;44:94–102.10.1086/cid.2007.44.issue-1
- Yirdaw KD, Jerene D, Gashu Z, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoSOne. 2014;9(8):e104557. 10.1371/journal.pone.0104557.
- Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AIM, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban hiv clinic in sub-saharan Africa. PLoSOne. 2010;5(5):e10527.10.1371/journal.pone.0010527
- Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoSOne. 2012;7(3):e34156.10.1371/journal.pone.0034156
- Dean AG, Sullivan KM, Soe MM. OpenEi: Open source epidemiologic statistics for public health, version 3.03a. www.OpenEpi.com, updated 04/05/2015, Accessed November 11, 2015.
- Balcha TT, Sturegård E, Winqvist N, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of xpert MTB/RIF compared with smear microscopy and liquid culture. PLoSOne. 2014;9(1):e85478.10.1371/journal.pone.0085478
- Shea KM, Kammerer JS, Winston CA, Navin TR, Horsburgh CR, Jr. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am J Epidemiol. 2014;179(2):216–225.10.1093/aje/kwt246
- Houben RM, Crampin AC, Ndhlovu R, et al. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. Int J Tuberc Lung Dis. 2011;15(1):24–31.
- Li J, Munsiff SS, Tarantino T, Dorsinville M. Adherence to treatment of latent tuberculosis infection in a clinical population in New York City. Int J Infect Dis. 2010;14(4):e292–e297. 10.1016/j.ijid.2009.05.007.
- van Griensven J, Choun K, Chim B, Thai S, Lorent N, Lynen L. Implementation of isoniazid preventive therapy in an HIV clinic in Cambodia: high rates of discontinuation when combined with antiretroviral therapy. Trop Med Int Health. 2015;20(12):1823–1831. 10.1111/tmi.12609.
- Oni T, Tsekela R, Kwaza B, et al. A recent HIV diagnosis is associated with non-completion of isoniazid preventive therapy in an HIV-infected cohort in Cape Town. PLoSOne. 2012;7(12):e52489. 10.1371/journal.pone.0052489.
- Matteelli A, Casalini C, Raviglione MC, et al. Supervised preventive therapy for latent tuberculosis infection in illegal immigrants in Italy. Am J Respir Crit Care Med. 2000;162(5):1653–1655.10.1164/ajrccm.162.5.9912062
- van Halsema CL, Fielding KL, Chihota VN, Russell EC, Lewis JJ, Churchyard GJ, et al. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS. 2010;24(7):1051–1055.10.1097/QAD.0b013e32833849df
- Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391.10.1371/journal.pmed.1000391
- Rangaka MX, Wilkinson RJ, Glynn JR, et al. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis. 2012;55(12):1698–1706. 10.1093/cid/cis775.
- Walls T, Shingadia D. The epidemiology of tuberculosis in Europe. Arch Dis Child. 2007;92:726–729.10.1136/adc.2006.102889
- The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807.