Abstract
Background and Objective: Diabetes mellitus (DM) is common in human immunodeficiency virus (HIV)-infected patients. However, the relationship between dysglycemia, lipid metabolism, and immune activation in HIV patients is poorly understood.
Methods: We retrospectively analyzed the clinical data of 180 HIV patients, including 153 patients undergoing highly active antiretroviral therapy (HAART) and 27 HAART-naive patients. DM was defined as fasting serum glucose levels ≥126 mg/dl, and impaired fasting glucose (IFG) was defined as serum glucose levels of 101–125 mg/dl at two different time points. Lipid metabolic indexes were measured. CD4+, CD8+, and CD8+ HLA-DR+ T cells were determined by flow cytometry.
Results: IFM and DM percentages were higher in the HAART group than in the HAART-naive group (59.5% vs. 48.1% and 21.6% vs. 7.4%, respectively; p < 0.01). Additionally, DM percentage was high in patients receiving HAART containing protease inhibitors. Serum levels of triglycerides and very low-density lipoprotein cholesterol were higher in IFG and DM HAART patients than in euglycemic HAART patients (p < 0.05). Serum triglyceride levels were higher in HAART-naive DM patients than in other patients (p < 0.05). CD8+ and CD8+ HLA-DR+ cell counts were higher in IFG and DM HAART patients than in euglycemic HAART patients (p < 0.05). Ordinal logistic regression analysis suggested that TRIG, VLDL, CD8, and HAART were predictors of glucose metabolic disorders.
Conclusion: HIV patients with hyperglycemia have severe dyslipidemia and immune activation, and HAART is an important impact factor of glucose and lipid metabolic disorders.
Introduction
Since the introduction of highly active antiretroviral therapy (HAART), there has been a remarkable reduction in human immunodeficiency virus (HIV)-induced mortality rates and in the incidence of acquired immune deficiency syndrome (AIDS)-related diseases. However, the prevalence rate of non-AIDS-related diseases, e.g. cardiovascular disease (CVD), endocrine disorders, and cancer, has increased in HIV patients.Citation1 It has been reported that more than 10% of HIV patients die from CVD.Citation2 Moreover, the risk of diabetes mellitus (DM), atherosclerosis, and CVD is higher in HIV patients than in healthy subjects.Citation3–6 In HIV/HAART-associated lipodystrophy syndrome (HALS), patients present fat maldistribution with dyslipidemia, insulin resistance, and other metabolic complications.Citation7 Adipokine, leptin, and adiponectin levels decrease in patients with HALS and lipoatrophy or lipodystrophy.Citation7,8
Glucose metabolic disorders, including DM, are common in HIV patients. Brown et al.Citation9 reported that the DM prevalence rate in HIV patients is 14%. HAART increases the risk of metabolic disorders, including insulin resistance, DM, dyslipidemia, lipoatrophy, and CVD, decreases serum levels of high-density lipoprotein cholesterol (HDL-c), and contributes to hypertriglyceridemia, lactic acidosis, and hypercoagulability.Citation10,11 The prevalence rate of impaired fasting glucose (IFG) and DM is higher in patients undergoing HAART than in controls.Citation12,13 Zhang et al.Citation13 reported that the incidence of DM for HIV-infected Chinese individuals is 2.62/100 person-years, which is considerably higher than that for the general Chinese population (0.43 to 1.55/100 person-years). DM patients have lipid metabolic disorders.Citation14 However, there is little information on lipid metabolism in HIV patients with glucose metabolic disorders. In addition, HIV patients are susceptible to persistent immune overactivity, which is related to the occurrence of non-AIDS-related complications such as atherosclerosis and metabolic disorders.Citation15 It is unclear whether glucose and lipid metabolic disorders in HIV patients are related to immune activation. In this study, we measured the serum glucose and lipid levels of 180 HIV patients and analyzed immune activation. We found that HIV patients with hyperglycemia have severe dyslipidemia and immune activation and that HAART is an important impact factor of glucose and lipid metabolic disorders.
Subjects and methods
Subjects
We retrospectively analyzed clinical data of HIV patients registered at St. Josef Hospital, Ruhr-University Bochum, from June to September of 2014. HIV infection was diagnosed on the basis of positive results from serological and HIV RNA detection assays. The inclusion criteria were (1) confirmed HIV infection, (2) patients ≥18 y of age, and (3) presence of follow-up records including fasting glucose at two different time points. Exclusion criteria were (1) the presence of any medical condition in which exercise was contraindicated and (2) pregnancy. The clinical data of 180 HIV patients were analyzed: 153 underwent HAART and 27 were HAART-naive. Patients with serum glucose levels within the normal range (74–100 mg/dl) were diagnosed as euglycemic. Overt DM was defined as serum glucose levels ≥ 126 mg/dl, while IFG was defined by serum glucose levels of 101–125 mg/dl at two different time points.Citation13 In our study, all DM patients had Type 2 DM.
Laboratory tests
CD4+, CD8+, and activated CD8 + HLA-DR + T cells in peripheral blood were measured by flow cytometry (FACSCantoII, Becton Dickinson, Franklin Lakes, NJ, USA). Plasma HIV load (HIV RNA) was determined using a standardized reverse transcriptase-polymerase chain reaction assay (Cobas Amplicor HIV-1 Monitor Test, version 1.5, Ultra sensitive specimen preparation, Roche Diagnostic Systems Inc., Branchburg, NJ, USA). The lower limit of detection in plasma was 50 HIV-1 RNA copies/ml. Serum lipid levels, including serum total cholesterol (CHOL), triglyceride (TRIG), HDL-c, low-density lipoprotein cholesterol (LDL-c), and very low-density lipoprotein cholesterol (VLDL-c), were measured with an automatic biochemical analyzer (AU5800, Beckman Coulter, Brea, CA, USA).
Statistical analysis
Data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation. Two-sided Student t-test was performed for comparisons between two groups. Multi-group comparisons of continuous data were performed using analysis of variance. Non-continuous data were analyzed by χ2 test. Pearson’s correlation test was performed to assess the correlation between continuous data, and Spearman’s correlation test was performed to assess the correlation between continuous data and non-continuous data. To identify predictors of different glucose metabolic disorders, univariate and multivariate ordinal logistic regression models were performed. Probability for stepwise was 0.1 for entry and 0.15 for removal. Statistical significance was set at p < 0.05.
Results
Demographics and clinical information
Among 180 HIV patients, 124 (68.9%) were male and 56 (31.1%) were female. The main route of infection was homosexual transmission (115 patients, 63.9%), followed by heterosexual transmission (35 patients, 19.4%). One hundred and fifty-three patients underwent HAART for an average of 6.46 ± 5.12 y. With respect to therapeutic regimens, 37 patients took 2 nucleoside reverse transcriptase inhibitors (NRTIs) containing 1 protease inhibitor (PI), 68 patients took 2 NRTIs containing 1 non-nucleoside reverse transcriptase inhibitor (NNRTI), 28 patients took 2 NRTIs containing 1 integrase inhibitor (II), and 20 patients followed other regimens. The mean age of patients with HAART was 48.21 ± 10.84 y, and the mean age of HAART-naive patients was 44.70 ± 11.96 y. Viral load, lg(VL), was significantly lower in the HAART group (1.62 ± 1.43) than in the HAART-naive group (4.03 ± 1.41, p < 0.05). Mean CD4 + T cells count was 529.34 ± 280.38/μl in the HAART group and 556.11 ± 412.08/μl in the HAART-naive group. Mean CD8 + T cells count was 844.56 ± 441.07/μl in the HAART group and 867.63 ± 379.97/μl in the HAART-naive group. CD4 to CD8 ratio was significantly higher in the HAART group (0.74 ± 0.44) than in the HAART-naive group (0.48 ± 0.58, p < 0.05; Table ).
Table 1 Demographic and clinical characteristics of HIV patients
Serum glucose levels in HIV patients
Among 180 HIV patients, 41 (22.8%) were diagnosed with euglycemia, 104 (57.8%) were diagnosed with IFG, and 35 (19.4%) were diagnosed with DM. In the HAART-naive group, 12 patients (44.4%) had euglycemia, 13 patients (48.1%) had IFG, and 2 patients (7.4%) had DM. Serum glucose levels were higher in DM patients than in other patients (133.00 ± 41.52 mg/dl vs. 85.61 ± 9.68 mg/dl or 133.00 ± 41.52 mg/dl vs. 98.51 ± 16.37 mg/dl; p < 0.05, Fig. ). In the HAART group, 29 patients (19.0%) had euglycemia, 91 (59.5%) had IFG, and 33 (21.6%) had DM. Serum glucose levels were significantly different among the three sub-groups of HAART patients, which were considerably higher in DM and IFG patients (133.18 ± 42.50 and 98.23 ± 16.41 mg/dl, respectively) than in euglycemic patients (84.14 ± 9.36 mg/dl; p < 0.05, Fig. ). There were no differences in serum glucose levels between HAART and HAART-naive groups for patients with euglycemia, IFG, or DM. DM prevalence was higher in HIV-1 patients receiving HAART containing PIs than in HAART-treated patients without PIs (29.7% vs. 14.7, 14.3, 15%; p = 0.000, Table ). There was no significant association between serum glucose levels and HAAR duration (r = –0.012, p > 0.05) or HIV duration (r = –0.049, p > 0.05).
Figure 1 Serum glucose levels in HIV-infected patients with or without HAART. HAART group represents HIV patients undergoing HAART; HAART-naive group represents HIV patients without HAART; total group represents all HIV patients; non-dysglycemia; IFG: impaired fasting glucose; DM, diabetes mellitus; GLU, glucose. The horizontal lines represent mean values, the boxes represent standard deviations, and the whiskers represent ranges. *p < 0.05.
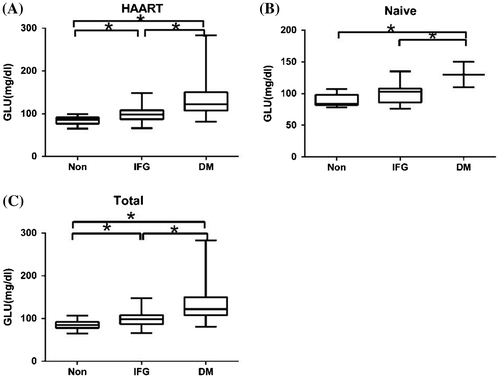
Table 2 Glucose metabolic disorders in HIV-1 patients with different HAART regimens
Serum lipid levels in HIV patients
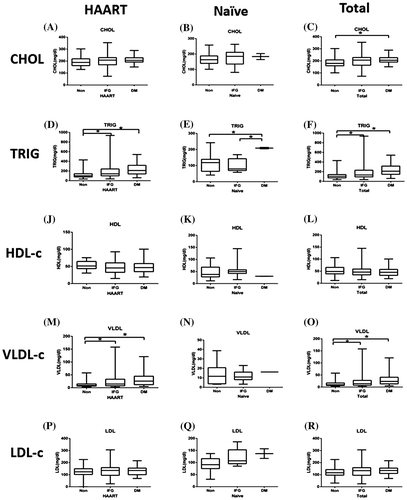
We measured serum levels of CHOL, TRIG, and lipoprotein cholesterol (including HDL-c, LDL-c, and VLDL-c) in patients with euglycemia, DM, or IFG. We separately analyzed data from HAART patients and HAART-naive patients because HAART has been reported to affect lipid metabolism.Citation16 Serum TRIG levels in HAART patients with euglycemia, IFG, and DM were 118.41 ± 71.87, 197.86 ± 167.81, and 233.15 ± 125.91 mg/dl, respectively. Serum VLDL-c levels in HAART patients with euglycemia, IFG, and DM were 13.31 ± 12.18, 22.77 ± 23.67, and 30.56 ± 24.11 mg/dl, respectively (Fig. ). Serum TRIG and VLDL-c levels were significantly higher in hyperglycemic patients than in euglycemic patients (p < 0.05). Compared to euglycemic patients, hyperglycemic patients had higher CHOL levels and lower HDL-c levels; however, the difference was not statistically significant. Serum TRIG levels in HAART-naive patients with euglycemia, IFG, and DM were 121.75 ± 64.39, 100.69 ± 40.41, and 208.50 ± 6.36 mg/dl, respectively. Serum TRIG levels were significantly higher in the DM group than in the other patients (p < 0.05, Fig. ). For total HIV-1 patients, serum levels of CHOL, TRIG, and VLDL-c were considerably higher in hyperglycemic patients than in euglycemic patients (p < 0.05).
Figure 2 Serum lipid levels in HIV patients with or without HAART. HAART group represents HIV patients undergoing HAART; HAART-naive group represents HIV patients without HAART; total group represents all HIV patients; non-dysglycemia; IFG: impaired fasting glucose; DM, diabetes mellitus; CHOL, cholesterol; TRIG, triglyceride; HDL, high density lipoprotein; VLDL-c, very low-density lipoprotein; LDL-c, low-density lipoprotein. The horizontal lines represent mean values, the boxes represent standard deviations, and the whiskers represent ranges. *p < 0.05.
Immune activation in HIV patients with different glucose metabolism
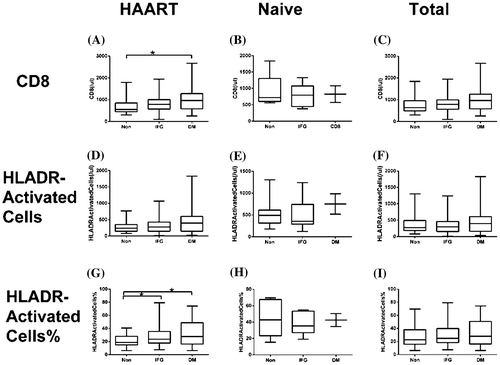
The human leukocyte antigen-DR (HLA-DR) is an immune activation marker expressed on T cells and a predictor of HIV disease progression.Citation17,18 We analyzed the levels of immune activation in HIV patients with different glucose metabolism using CD8 + T cell count and percentage and CD8 + HLA-DR + cell count and percentage. Within the HAART group, the CD8 + T cell count was significantly higher in patients with DM (1044.36 ± 579.69 counts/μl) than in patients with euglycemia and IFG (707.21 ± 390.21 and 815.87 ± 374.38 counts/μl, respectively; p < 0.05). CD8 + HLA-DR + T cell count and percentage were 477.64 ± 451.07 counts/μl and 31.34 ± 18.32% in DM patients, respectively, and 320.21 ± 231.08 counts/μl and 27.96 ± 15.76% in IFG patients, respectively, which were higher than in euglycemic patients (273.24 ± 161.20 and 20.88 ± 9.37%, respectively; p1 = 0.053 and p2 < 0.05, respectively, Fig. ). Immune activation has been reported to be related with CD4 + nadir.Citation19,20 In our study, we obtained a negative correlation between percentage of CD8 + HLA-DR + and CD4 + T cell nadir.
Figure 3 CD8 + and CD8 + HLA-DR + T cell counts and percentages in HIV patients with or without HAART. HAART group represents HIV patients undergoing HAART; HAART-naive group represents HIV patients without HAART; total group represents all HIV patients; non-dysglycemia; IFG: impaired fasting glucose; DM, diabetes mellitus; HLADR, Human leukocyte antigen-DR (HLA-DR). The horizontal lines represent mean values, the boxes represent standard deviations, and the whiskers represent ranges. *p < 0.05.
Univariate and multivariate ordinal logistic regression analyses to evaluate predictors of glucose metabolic disorders
The diagnosis of glucose metabolic disorders was used as the dependent variable. The independent variables, including HAART, HAART regimens, sex, and routes of infection, were incorporated into the ordinal logistic regression model as factor variables. Age, duration of HIV infection, duration of HAART, levels of serum lipid (CHOL, TRIG, HDL-c, VLDL-c, and LDL-c), LDL/HDL ratio, viral load, count of CD4+, CD8+, and activated CD8 + HLA-DR + T cells were incorporated as continuous variables. The results of the ordinal regression analyses are presented in Table . From the univariate ordinal logistic regression model, HAART, age, and levels of TRIG, VLDL-c, and CD8 + T cells were statistically associated with glucose metabolic disorders. Multivariate ordinal logistic regression analysis revealed that TRIG, VLDL-c, CD8 + T cells, and HAART were predictors of glucose metabolic disorders.
Table 3 Univariate and multivariate ordinal logistic regression analysis to evaluate predictors of glucose metabolic disorders
Discussion
DM is of particular concern in HIV infection because of its association with myocardial infarction and stroke, which are elevated in HIV patients.Citation21,22 Even though the risk of DM and IFG in HAART-naive HIV patients may be similar to or slightly higher than in the general population,Citation23 studies have found an increased incidence of DM and IFG in HIV-infected individuals on cART.Citation24 Quin et al.Citation23 reported that the prevalence rate of Type 2 DM was four times higher in HAART patients than in the HIV-negative cohort. HAART contributes to insulin resistance, which is an important pathological mechanism of DM.Citation25 In our study, ordinal logistic regression analysis revealed that HAART was closely associated with serum glucose metabolism. Serum glucose levels of HIV patients were not associated with HAART duration, but were associated with different HAART regimens. A high DM prevalence was obtained in patients receiving HAART containing PIs. Considering that HAART regimens for HIV-1 patients were based on two NRTIs, we attributed the different effects of HAART on glucose metabolism to the PIs. It has been reported that PIs may cause glucose metabolic disorders, e.g. impairment of insulin secretion and increased endogenous glucose production.Citation26 PIs and structurally related oligopeptides can reversibly bind and inactivate the insulin-responsive facilitative glucose transporter 4 (GLUT4),Citation27 leading to insulin resistance. Even though PIs are the main drug class implicated in insulin resistance, some studies have shown increased diabetes risk with cumulative NRTI exposure.Citation26 Inconsistent with our results, Tien et al. reported that the HAART with PI group had lower DM rates than the non-PI HAART group (2.50/100 person-years vs. 2.89/100 person-years).Citation28 The effect of HAART on glucose metabolism remains to be elucidated.
Regression analysis revealed that serum glucose levels were associated with lipid levels and CD8 + T cell counts. Insulin resistance, hyperglycemia, and DM have a close relationship with lipid metabolic disorders.Citation29 Mohammed et al.Citation30 reported that most HIV patients with DM have higher serum CHOL, TRIG, and LDL-c levels and lower serum HDL-c levels compared to healthy subjects. Moreover, the researchers concluded that serum LDL-c levels, HAART duration, and HIV infection were significantly correlated with DM. Additionally, serum LDL-c level was significantly associated with DM after adjusting for the other variables.Citation30 It has been reported that HAART affects lipid metabolism. Stavudine, a thymidine analog, reduces limb fat.Citation31 Additionally, ritonavir and, to a lesser extent, efavirenz affect serum triglyceride levels.Citation32 In our study, we separately analyzed data from HAART patients and HAART-naive patients. The results showed that serum TRIG and VLDL-c levels were significantly higher in hyperglycemic HAART patients than in euglycemic HAART patients. Among HAART-naive patients, serum TRIG levels were significantly higher in the DM group than in the other patients. Our results revealed that lipid metabolic disorders were more severe in HIV patients with glucose metabolic disorders regardless of whether they were undergoing HAART or not. According to Araujo et al.,Citation33 insulin resistance is related to serum TRIG levels and HAART duration, but not to baseline serum CHOL, LDL-c, or HDL-c levels. Even though the cause of glucose and lipid metabolic disorders is complex, our results revealed that dyslipidemia is closely related with glucose metabolic disorders in HIV patients. More attention should be paid to lipid metabolism in HIV patients with glucose metabolic disorders. Furthermore, plasma glucose levels should be monitored and controlled in HIV patients to decrease the incidence of DM, dyslipidemia, and CVD.
Chronic extensive immune activation is a feature of HIV infection. HIV patients exhibit persistent inflammation and immune activation, even after years of effective HAART.Citation15,34 CD8 + T cells are extensively activated, and CD8 + HLA-DR + T cells are suitable markers of immune activation in HIV patients.Citation35,36 Persistent immune activation could accelerate the development of Type 2 DM.Citation37 Pro-inflammatory cytokines and T cells are involved in the pathogenesis of DM. Pancreatic beta cells are the targets of cytotoxic CD8 + T cells.Citation38 We analyzed levels of CD8 + T cells and activated CD8 + HLA-DR + T cells in HIV patients with different glucose metabolisms to investigate the association between immune activation and glucose metabolism. Immune activation was higher in HIV patients with hyperglycemia who received HAART, especially in patients with DM. However, there were no differences in immune activation between HAART-naive patients with hyperglycemia and HAART-naive patients with euglycemia, which could be due to the small case number of HIV-1-naive patients, especially for DM patients (only four patients). In addition, there was a negative correlation between immune activation and CD4 + T cell nadir, which was consistent with previous studies.Citation19,20 Immune activation of HIV patients is associated with HIV viral load.Citation36 The initiation of antiretroviral treatments at an early disease stage with minimum effects on glucose metabolism would be beneficial for inhibiting viral replication, reducing immune activation, and decreasing the prevalence rate of glucose metabolic disorders.
In conclusion, our study revealed that HAART regimen is an important factor that affects serum glucose levels of HIV-1 patients. HIV patients with hyperglycemia have severe lipid metabolic disorders and immune activation. More attention should be paid to glucose and lipid metabolism of HIV patients with DM, especially those undergoing antiretroviral therapy.
Disclaimer statements
Contributors
CJ, SJ, TX, SH, NB, and NW were involved in study conception or design. CJ, WF, XL, HW, LC, and AS participated in acquisition, analysis, and interpretation of data. CJ, SJ, and NW wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Zhejiang Provincial Science & Technology Foundation [grant number 2015C33183], the National Key Technologies R&D Program for the 12th Five-year Plan of China [grant number 2012ZX10001–004], and the National Natural Science Foundation of China [grant number 81402726].
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval
This study was conducted in compliance with the Declaration of Helsinki and all Good Clinical Practice Guidelines established by the International Conference on Harmonisation. The final protocol, amendments, and informed consent documentation were reviewed and approved by the Ethics Review Boards of St. Josef Hospital, Ruhr University Bochum and the First Affiliated Hospital, School of Medicine, Zhejiang University. All subjects provided written, informed consent.
References
- Beltran S, Lescure FX, Desailloud R, Douadi Y, Smail A, Esper I, et al. Increased prevalence of hypothyroidism among human immunodeficiency virus--infected patients: A need for screening. Clin Infect Dis. 2003;37(4):579–583.10.1086/376626
- McManus KA, Pinkerton R, Dillingham R. Effects of recent virginia AIDS drug assistance program policy changes on diabetes and hyperlipidemia control in people living with HIV. SAGE Open Med. 2014;2:2050312114532809.
- Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;3499(21):1993–2003.
- Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–1849.10.1097/QAD.0b013e32832d3b85
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512.10.1210/jc.2006-2190
- Sobieszczyk ME, Hoover DR, Anastos K, Mulligan K, Tan T, Shi Q, et al. Prevalence and predictors of metabolic syndrome among HIV-infected and HIV-uninfected women in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2008;48(3):272–280.10.1097/QAI.0b013e31817af461
- Paruthi J, Gill N, Mantzoros CS. Adipokines in the HIV/HAART-associated lipodystrophy syndrome. Metabolism. 2013;62(9):1199–1205.10.1016/j.metabol.2013.04.014
- Freitas P, Carvalho D, Santos AC, Madureira AJ, Martinez E, Pereira J, et al. Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis. 2014;14:347.
- Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184.10.1001/archinte.165.10.1179
- Estrada V, Geijo P, Fuentes-Ferrer M, Alcalde ML, Rodrigo M, Galindo MJ, et al. Dyslipidaemia in HIV-infected women on antiretroviral therapy. Analysis of 922 patients from the Spanish VACH cohort. BMC Womens Health. 2011;11:36.10.1186/1472-6874-11-36
- Adewole OO, Eze S, Betiku Y, Anteyi E, Wada I, Ajuwon Z, et al. Lipid profile in HIV/AIDS patients in Nigeria. Afr Health Sci. 2010;10(2):144–149.
- De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new-onset diabetes in hiv-infected patients: The data collection on adverse events of Anti-HIV drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–1229.10.2337/dc07-2013
- Zhang C, Chow FC, Han Y, Xie J, Qiu Z, Guo F, et al. Multicenter cohort study of diabetes mellitus and impaired fasting glucose in HIV-infected patients in China. J Acquir Immune Defic Syndr. 2015;68(3):298–303.10.1097/QAI.0000000000000474
- Fan M, Li Y, Zhang S. Effects of Sitagliptin on Lipid Profiles in Patients With Type 2 Diabetes Mellitus: A Meta-analysis of Randomized Clinical Trials. Medicine (Baltimore). 2016;95(2):e2386.10.1097/MD.0000000000002386
- Younas M, Psomas C, Reynes J, Corbeau P. Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med. 2016;17(2):89–105.10.1111/hiv.2016.17.issue-2
- Blázquez D, Ramos-Amador JT, Saínz T, Mellado MJ, García-Ascaso M, De José MI, et al. Lipid and glucose alterations in perinatally-acquired HIV-infected adolescents and young adults. BMC Infect Dis. 2015;15:119.10.1186/s12879-015-0853-8
- Eggena MP, Barugahare B, Okello M, Mutyala S, Jones N, Ma Y, et al. T cell activation in HIV‐seropositive Ugandans: Differential associations with viral load, CD4 + T cell depletion, and coinfection. J Infect Dis. 2005;191(5):694–701.10.1086/jid.2005.191.issue-5
- Gascon RL, Narváez AB, Zhang R, Kahn JO, Hecht FM, Herndier BG, et al. Increased HLA-DR expression on peripheral blood monocytes in subsets of subjects with primary HIV infection is associated with elevated CD4 T-cell apoptosis and CD4 T-cell depletion. J Acquir Immune Defic Syndr. 2002;30(2):146–153.10.1097/00042560-200206010-00002
- Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, et al. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS. 2010;24(13):1991–2000.10.1097/QAD.0b013e32833c93ce
- Lange CG, Valdez H, Medvik K, Asaad R, Lederman MM. CD4+ T-lymphocyte nadir and the effect of highly active antiretroviral therapy on phenotypic and functional immune restoration in HIV-1 infection. Clin Immunol. 2002;102(2):154–161.10.1006/clim.2001.5164
- Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non–HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–358.10.1097/QAI.0b013e31825c7f24
- Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003.
- Bonfanti P, Giannattasio C, Ricci E, Facchetti R, Rosella E, Franzetti M, et al. HIV and metabolic syndrome: A comparison with the general population. J Acquir Immune Defic Syndr. 2007;45:426–431.10.1097/QAI.0b013e318074ef83
- Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184.10.1001/archinte.165.10.1179
- Quin J. Diabetes and HIV. Clin Med (Lond). 2014;14(6):667–669.10.7861/clinmedicine.14-6-667
- Das S. Insulin resistance and diabetes in HIV infection. Recent Pat Antiinfect Drug Discovery. 2011;6(3):260–268.10.2174/157489111796887846
- Hresko RC, Kraft TE, Tzekov A, Wildman SA, Hruz PW. Isoform-selective inhibition of facilitative glucose transporters: Elucidation of the molecular mechanism of HIV protease inhibitor binding. J Biol Chem. 2014;289(23):16100–16113.10.1074/jbc.M113.528430
- Tien PC, Schneider MF, Cole SR, Levine AM, Cohen M, DeHovitz J, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the women’s interagency HIV study. AIDS. 2007;21(13):1739–1745.10.1097/QAD.0b013e32827038d0
- Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351(9119):1881–1883.10.1016/S0140-6736(98)03391-1
- Mohammed AE, Shenkute TY, Gebisa WC. Diabetes mellitus and risk factors in human immunodeficiency virus-infected individuals at Jimma University Specialized Hospital. Southwest Ethiopia. Diabetes Metab Syndr Obes. 2015;8:197–206.10.2147/DMSO
- Nix LM, Tien PC. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr HIV/AIDS Rep. 2014;11(3):271–278.10.1007/s11904-014-0219-7
- Grunfeld C. Dyslipidemia and its treatment in HIV infection. Top HIV Med. 2010;18(3):112–118.
- Araujo S, Bañón S, Machuca I, Moreno A, Pérez-Elías MJ, Casado JL. Prevalence of insulin resistance and risk of diabetes mellitus in HIV-infected patients receiving current antiretroviral drugs. Eur J Endocrinol. 2014;171(5):545–554.10.1530/EJE-14-0337
- Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17(13):1907–1915.10.1097/00002030-200309050-00009
- Jin C, Zhang F, Wu L, Xie T, Cheng Y, Tang Z, et al. Immune activation and CD127 expression on T lymphocyte subsets of a Chinese cohort of pediatric AIDS patients with different viral responses. Curr HIV Res. 2012;10(7):584–591.10.2174/157016212803305961
- Rosso R, Fenoglio D, Terranova MP, Lantieri F, Risso D, Pontali E, et al. Relevance of CD38 expression on CD8 T cells to evaluate antiretroviral therapy response in HIV-1-infected youths. Scand J Immunol. 2010;71(1):45–51.10.1111/sji.2009.71.issue-1
- Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286–1292.10.1007/s001250050822
- Wali JA, Trivedi P, Kay TW, Thomas HE. Measuring death of pancreatic beta cells in response to stress and cytotoxic T cells. Methods Mol Biol. 2015;1292:165–176.10.1007/978-1-4939-2522-3
