Abstract
Background: The use of combination antiretroviral therapy (cART) and cytotoxic chemotherapy for HIV-associated lymphoma runs the risks of inducing HIV drug resistance. This study examined two possible mechanisms: altered expression of membrane drug transporter protein (MTP) and acquisition of mutations in pro-viral DNA.
Methods: Expression levels of MTP and pro-viral DNA resistance mutation analysis were performed on peripheral blood mononuclear cells (PBMC) before, during, and after chemotherapy.
Results: Twenty nine patients completed the three time point estimations. There were no significant variations before, during, and after chemotherapy in the expression of four MTPs: ABCB1, ABCC1, ABCC2, and SLCO3A1 (OATP3A1). Pro-viral DNA sequencing revealed that only one patient developed a new nucleos/tide reverse transcriptase inhibitor-associated mutation (184V) during the course of the study, giving a mutation rate of 0.0027 per person per year.
Conclusions: In conclusion, concomitant administration of cytotoxic chemotherapy and cART does not induce expression of MTP. Furthermore, no significant changes in viral resistance were observed pre- and post-chemotherapy, suggesting mutagenic cytotoxic chemotherapy seems not to induce mutations in HIV pro-viral DNA.
Introduction
Despite improved HIV-related outcomes following the introduction of combination antiretroviral therapy (cART), cancer remains a significant cause of morbidity and mortality for people living with HIV (PLWH).Citation1 Clinical management involving use of cART with chemotherapyCitation2,3 has produced survival ratesCitation4 similar to those seen in HIV negative individuals.Citation5 The simultaneous use of cART and chemotherapy medication requires careful monitoring to avoid potential pharmacokinetic interactions, including exacerbation of toxicity and loss of drug efficacy.
The pharmacokinetic fate of an ingested substance is governed by absorption, distribution, metabolism, and excretion. As the majority of cART medications are orally administered, carrier-mediated mechanisms present on the surface of gut enterocytes transport these medications to the bloodstream for systemic distribution.Citation6 Medications are actively moved into cells by membrane transporter proteins (MTP) which chaperone the passage of these molecules across cell surface membranes.
MTPs are present on many of the body’s epithelial barriers, and regulate the movement of solutes across body fluid compartments. There are two main transporter ‘superfamilies’ – the solute carrier transporters (SLC), which include the subfamily of organic anion-transporting polypeptides (OATP), and adenosine triphosphate (ATP)-binding cassette transporters (ABC). All antiretroviral medications, apart from entry inhibitors, utilize these transporters to gain entry to HIV-infected cells in order to suppress viral replication.Citation6 The abundance and activity of MTP may vary individually due to the presence of genetic polymorphisms.Citation7 MTP activity can also be influenced by medications. Molecules may compete for binding to the active transport site, or may alter a transporter’s activity.
MTP and cancer
Some forms of cancer, including blood and solid tumor malignancies, may develop an over-expression of certain MTP which actively expel chemotherapeutic agents from cancer cells. Two ABC MTP, P-glycoprotein and multidrug resistance-associated protein (MRP), have been identified, and their presence may lead to failure of chemotherapy.Citation8 In patients with lymphoma, MDR expression is relatively low at 10–20%, but rises to 50–70% in cases of disease recurrence,Citation9 but may be higher in HIV-associated lymphomas.Citation10
MTP and chemotherapy
Expression of MTP can be altered with medication. P-glycoprotein is up regulated by drugs including rifampicin.Citation11 Anticancer chemotherapeutic agents inhibit MDR activity to prevent treatment failure.Citation12
MTP and ARVs
Hepatic organic anion transport proteins (OATP) may be inhibited by certain antiretroviral medications including protease inhibitors and non-nucleoside reverse transcriptase inhibitors. Renal OATP may also be inhibited by these medications. Other antiretrovirals including integrase inhibitors and chemokine receptor antagonists can affect MTP activity; with ABCB1 inhibited by elvitegravir and vicriviroc (of which they are substrates), and ABCC2 stimulated by maraviroc and vicriviroc.Citation13
Both anticancer chemotherapy agents and antiretroviral drugs are substrates of MTP, and altered expression or function may contribute to variable efficacy and possible drug resistance.Citation14,15
Genotypic HIV mutations have been detected at viral rebound in PWLH treated with chemotherapy for lymphoma, but did not confer virological resistance.Citation16 Anticancer chemotherapy causes DNA damage and may lead to subsequent mutations.Citation17 This raises the question – could development of mutations in host DNA and integrated proviral HIV DNA give rise to novel HIV mutations?
The purpose of this study was to investigate two potential mechanisms for chemotherapy induced failure of cART leading to loss of viral control in PLWH. The first was the evolution of chemotherapy-induced cellular resistance through variation of lymphocyte MTP expression, and the second was chemotherapy-induced mutagenesis of pro-viral HIV DNA leading to acquisition of novel resistance mutations.
Methods
Patients receiving concomitant cART and systemic cytotoxic chemotherapy for HIV-associated lymphoma were asked to participate in the study. Ethical approval was obtained from the West London Research Ethics Committee ([email protected]), and the study was conducted in accordance with Good Clinical Practice.
This was an observational pilot non-drug study, aimed at investigating intra-individual molecular changes among HIV-positive individuals with lymphoma. To show an increase in MTP expression by 40 at 5% level significance and 90% power, 30 patients were needed to study for significance. A drop-out rate of approximately 15% was expected. 31 HIV-positive patients with a diagnosis of lymphoma attending HIV oncology services at Chelsea and Westminster Hospital, London, were approached and asked to provide written consent and participate in the study over a two-year-study period. Five HIV-infected subjects on cART without a diagnosis of lymphoma and with an undetectable viral load, who did not receive chemotherapy, were also enrolled as controls, to correctly develop laboratory techniques and monitor possible molecular changes not due to lymphoma and its treatment.
Inclusion criteria included patients’ ability to understand and sign written informed consent prior to participation in screening procedures, and willingness to comply with all study requirements. Participants had documented HIV-1 infection, a diagnosis of lymphoma (with exclusion of the five control subjects), and taking or starting cART as part of routine clinical care prior to initiation of chemotherapy. Exclusion criteria involved patients receiving antituberculosis treatment, and one patient initially recruited was excluded from the final analysis for this reason.
The study included three scheduled visits. The first was before chemotherapy, the second was 6–8 weeks later during the second cycle of chemotherapy, and the third was one month following cessation of chemotherapy. At each visit, blood was drawn for peripheral blood mononuclear cell (PBMC) isolation and evaluation for MTP expression, and HIV pro-viral resistance.
The MTPs reviewed included ABC subfamily B members 1 (ABCB1) and C members 1 (ABCC1) and 2 (ABCC2), and SLC subfamily member OATP3A1, which have previously been reported as being present on lymphocytes,Citation18 and may modulate cellular efflux of certain antiretroviral medications.Citation19,20 ABCB1, ABCC1, ABCC2, and SLCO3A1 transporter expression was measured by flow cytometry as described previously.Citation21 PBMC samples were inactivated by suspension in formalin solution (10%, Sigma Aldrich, UK) overnight at room temperature. Following inactivation PBMC samples were washed three times in ice cold phosphate buffered saline (PBS), centrifuged at 2000 rpm for 5 min, supernatant removed, prior to being resuspended in antibody working solutions. Antibodies for ABCB1 and ABCB1 isotype control (IC) were directly conjugated to phycoerythrin (PE) and made to a final dilution of 1:20. Primary antibodies for ABCC1, ABCC2, and SLCO3A1 were used at a dilution of 1:40 in saponin (0.1mg/mL) solution with matched isotype controls. PBMC were incubated with antibodies for 60 minutes prior to three additional wash steps. Cells stained for ABCB1 were transferred to flow cytometry running buffer and stored in the dark at 4 °C until analysis. Following wash steps cells were resuspended in with respective secondary antibody solution and incubated for a further 60 min followed by three final wash steps. PBMC were finally resuspended in flow cytometry running buffer and analyzed on a MACSQuant flow cytometer equipped with three air-cooled lasers at 488, 633, and 405 nm. Side scatter outcome was set to logarithmic and detected at a scattering angle of 90°. Flow rates were chosen such that less than 2000 events/s are recorded. For each measurement, a total number of 30,000 events were recorded. Data were expressed as the median relative fluorescence of the test antibody sample minus the median fluorescence of the isotype control.
HIV pro-viral resistance analysis was performed on isolated PBMCs using commercial assay systems (pro-viral DNA, TruGene HIV-1 Assay GeneKit & Open Gene system, mutation analysis by Stanford database).
Data were tested for normality using the Shapiro–Wilk test. For continuous data, analysis was conducted using simple linear regression. For non-normally distributed data, a log transformation was first conducted. Statistical analysis of the intra-individual (before and after chemotherapy) variation in transmembrane transporter expression was performed by calculating geometric mean ratios and 90% confidence intervals. The effect of exposure to different chemotherapeutic agents or antiretroviral drugs was also explored when five or more patients were exposed. For this, a univariate and a multivariate analyses against log change in expression of each transporter were conducted. Pre- and post-chemotherapy qualitative analysis (i.e. emergence of new HIV mutations) was performed by McNemar’s test. Data analyses were carried out using SAS version 9.1 statistical software.
Results
Thirty-one subjects and 5 controls were initially recruited. One subject was taking antituberculosis therapy and was withdrawn, another patient returned to their original center of care and did not complete all study procedures. Thus, 29 subjects (22 male, 7 female) and 5 controls (all male) completed the study and were included in the final analysis. The median age was 49 (range: 30–71) years and the median interval from initial HIV diagnosis to lymphoma diagnosis was 122 (range: 0–613) months (Table ).
Table 1 table showing baseline patient characteristics of those who completed all three study visits
Of the 29 participants who completed the study, 12 patients had Hodgkin’s lymphoma (HL) and 17 had non-Hodgkin lymphoma (10 diffuse large B cell lymphoma (DLBCL), 4 Burkitt’s lymphoma (BL), 2 overlap gray zone B-cell lymphoma with features intermediate between DLBCL and BL, 2 extra cavity primary effusion lymphoma (ePEL), and 1 mantle cell lymphoma). All 12 patients with HL were treated with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) chemotherapy. Thirteen patients were treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy and 6 with R-CODOX-M/IVAC (rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate, etoposide, ifosfamide, cytarabine) chemotherapy (Table ).
Of the 29 participants who completed all study visits, antiretroviral therapy was as follows (TDF tenofovir, FTC emtricitabine, ABC abacavir, 3TC lamivudine, EFV efavirenz, NVP nevirapine, RPV rilpivirine, ETR etravirine, RAL raltegravir, MVC maraviroc, ATV atazanavir, DRV darunavir, RIT ritonavir): TDF/FTC/EFV 8, TDF/FTC + NVP 1, TDF/FTC/RPV 1, TDF/FTC + ETR 1, TDF/FTC + RAL 8, TDF/FTC + DRV + RIT + MVC 1, TDF/FTC + DRV + RIT 3, TDF/FTC + DRV + RIT + ETR 1, TDF/FTC + ATV + RIT 2, ABC/3TC + DRV + RIT 1, ABC/3TC + RAL 1, 3TC + DRV + RIT + MVC 1 (Table ).
Overall, 29 subjects who completed all 3 study visits received a total 154.9 months of chemotherapy plus cART.
Their HIV parameters are described here. The baseline pre chemotherapy median CD4 count was 273 (32–740) cells/mm3, and CD4 percentage median was 18.3% (4.2–41.0%). At visit 1, HIV viral load information was as follows: nine patients had suppressed plasma HIV viral loads (below 40 copies/mL); two patients had no baseline viral load available. The median plasma HIV viral load for the 18 patients with detectable viraemia was 104 copies/mL (range: 68–1,975,269). At visit 3, 22 participants (76%) had an undetectable plasma HIV viral load (median <40 copies/ml, range: <40–8822).
Regarding HIV genomic resistance, at the first visit 24 participants had wild type HIV (or non-significant resistance mutations according to http://hivdb.stanford.edu), and while 5 participants had significant HIV baseline mutations. These included the following: 4 with nucleos/tide reverse transcriptase inhibitor mutations (3 M41L, 1T69N), 4 with non-nucleos/tide reverse transcriptase inhibitor mutations (1: A98G, K103N, V108I, H221Y, 2: V108I, Y181C, H221Y, 3: V106I, 4: K103N, Y181C, 5: K103N, Y181C) and 2 with major protease inhibitor mutations (1: M46L, I84V, 2: M46L, I50V, I54V, V82A).
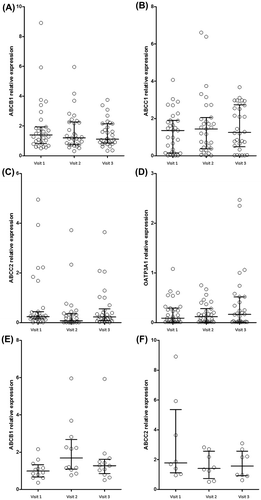
Drug transporter expression varied over the three study visits but none of these achieved statistical significance (Figure ). Expression of ABCB1 and ABCC2 was lower at V2 (15.4%; p = 0.39 and 48.2%; p = 0.14, respectively) and V3 (20.3%; p = 0.64 and 67.3%; p = 0.19, respectively) compared to V1 (Figure A) and (Figure C). ABCC1 expression was higher at V2 (24.2%; p = 0.62) and V3 (13.9%; p = 0.50) compared to V1 (Figure B). SLCO3A1 expression was lower at V2 compared to V1 (12.9%; p = 0.79), however, at V3 expression was higher than V1 (73.7%; p = 0.97; Figure D). In multivariate analysis adjusted for chemotherapy and antiretroviral regimens, ABVD use was significantly associated with higher ABCB1 expression at V2 (p = 0.04; Figure E) and use of antiretroviral agent raltegravir was significantly associated with higher ABCC2 expression at V1 (p = 0.01; Figure F).
Four weeks after completing chemotherapy, six subjects had significant viral mutations shown by the pro-viral DNA analysis; five pre-existing, and one patient who developed a new nucleos/tide reverse transcriptase inhibitor-associated mutation (184V) during the course of the study. This was not associated with a detectable viral load. Overall, 29 subjects received a total 154.9 months of chemotherapy plus cART, yielding a mutation rate of 0.0027 per person per year.
Discussion
This pilot study is the first to review cell membrane transporter expression variation in HIV-infected individuals on cART receiving chemotherapeutic combinations for the treatment of different lymphomas, in addition to investigations on emergence of genotypic HIV mutations. The mechanisms reviewed included alterations in the expression and activity of membrane drug transporter proteins (MTP) and the induction of mutations in integrated pro-viral DNA. This study sought to evaluate these two mechanisms in vivo.
The majority of patients in this study achieved undetectable viral loads. One patient developed a new resistance mutation – M184V. This mutation is selected by emtricitabine (FTC) or lamuvidine (3TC), and also by abacavir (ABC) and didanosine (DDI) and reduces susceptibility to FTC and 3TC, and also to ABC and DDI.Citation22 All participants in this study received cART containing either emtricitabine or lamuvidine. This rate of M184V emergence is less than previously published rates of resistance development in patients not undergoing chemotherapy.Citation23 The clinical significance of this new mutation is unknown, but is unlikely to be clinically significant, given the majority (22 of 29) of study patients achieved and maintained undetectable plasma HIV loads at end of study.
Membrane transport proteins ABCB1, ABCC1, ABCC2, and SLCO3A1 (OATP3A1) were all shown to be expressed in PBMC from patients and the levels of expression varied over the course of the chemotherapy. However, there was wide inter-individual variability and differences in the cohort were not found to be statistically significant. In particular, there was no progressive increase in MDR-associated transmembrane transporters during the course of chemotherapy. ABCB1 expression during chemotherapy was found to be positively correlated with ABVD chemotherapy use, and doxorubicin has been previously described as an inducer of ABCB1 mRNA expression in vitro.Citation24 In addition, pre-chemotherapy raltegravir use was associated with higher ABCC2 expression. ABCC1 expression has been shown to be induced by raltegravir use, and ABCC3 induced by elvitegravir use,Citation18 but no previous studies exist for comparison of ABCC2 expression and raltegravir use.
The limitations of this study include small numbers of participants, variability in demographics, oncological diagnosis and antiretroviral regimen. However, the main objective of this study was to assess variation in expression of MTP and development of HIV genomic resistance in the setting of chemotherapy co-administration. We also did not assess individuals for the development of the MDR MTP phenotype, however, all participants responded well to treatment with no disease relapses reported to date. Further work is needed to fully evaluate for these variables, with larger more in-depth studies powered for greater detail.
Our study suggests that concomitant administration of chemotherapy and cART does not induce expression of MTP that could lead to cellular resistance to antiretrovirals. Similarly, evaluation of pro-viral integrated DNA before and after completion of chemotherapy confirmed that despite the mutagenesis of cytotoxic chemotherapy, it did not appear to contribute to the acquisition of antiretroviral resistance mutations in vivo. The development of mutations in HIV during chemotherapy has been reported previously and again was not thought to contribute to antiretroviral resistance or virological failure.Citation25
In conclusion, concomitant administration of cytotoxic chemotherapy and cART does not induce expression of MTP that could lead to cellular resistance to antiretrovirals and no significant changes in viral resistance were observed pre- and post-chemotherapy, suggesting mutagenic cytotoxic chemotherapy seems not to induce mutations in HIV pro-viral DNA.
Disclaimer statements
Contributors
Katie McFaul, PhD student and clinical research fellow, Centre for Immunology and Vaccinology, Imperial College London. Her research is focused on immunology of acute HIV infection, HIV in MSM. Alison Cox, specialist scientist, Infection and immunity laboratory, Imperial College Healthcare NHS Trust, London, UK. Department of Molecular and Clinical Pharmacology, University of Liverpool Faculty Members include Neill Liptrott, Phillip Martin, Deirdre Egan,Andrew Owen. Research Nurses include Sarah Kelly (Oncology specialist nurse), Zeenat Karolia (Research specialist nurse), and Kate Shaw (Oncology specialist nurse). Mark Bower, medical oncologist at Chelsea & Westminster Hospital and professor at Imperial College. His research interests include treatment of AIDS-related malignancies and epidemiology, etiology, pathogenesis, and management of these tumors. Marta Boffito is the associate director of the St Stephen's AIDS Trust Research Unit. Her research interests include pharmacokinetics of antiretroviral medication and HIV in aging populations.
Conflict of Interest
I have no conflict of interest to declare.
Funding statement
This work was supported by research grants from the British HIV Association (BHIVA), UK and Janssen UK. The St. Stephen’s AIDS Trust (reg. charity no. 1134163) also provided funding support.
Acknowledgments
We would like to thank the patients and staff of St Stephen’s AIDS Trust.
References
- Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet [Internet]. 2014;384(9939):241–248. http://www.ncbi.nlm.nih.gov/pubmed/2504223410.1016/S0140-6736(14)60604-8
- Bower M, Collins S, Cottrill C, et al. British HIV association guidelines for HIV-associated malignancies 2008. HIV Med [Internet]. 2008;9(6):336–388. http://www.ncbi.nlm.nih.gov/pubmed/1870575910.1111/hiv.2008.9.issue-6
- Bower M, Stebbing J, Tuthill M, et al. Immunologic recovery in survivors following chemotherapy for AIDS-related non-Hodgkin lymphoma. Blood [Internet]. 2008;111(8):3986–3990. http://www.ncbi.nlm.nih.gov/pubmed/1817200410.1182/blood-2007-10-115659
- Spagnuolo V, Galli L, Salpietro S, et al. Ten-year survival among HIV-1-infected subjects with AIDS or non-AIDS-defining malignancies. Int J Cancer [Internet]. 2012;130(12):2990–2996. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2179663310.1002/ijc.26332
- Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol [Internet]. 2012;30(33):4111–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23045581
- Minuesa G, Huber-Ruano I, Pastor-Anglada M, Koepsell H, Clotet B, Martinez-Picado J. Drug uptake transporters in antiretroviral therapy. Pharmacol Ther. 2011;132(3):268–279.10.1016/j.pharmthera.2011.06.007
- Nigam SK. What do drug transporters really do? Nat Rev Drug Discov [Internet]. 2015 Jan;14(1):29–44. Available from: http://dx.doi.org/10.1038/nrd4461
- Persidis A. Cancer multidrug resistance. Nat Biotech.. 1999 Jan;17(1):94–5. PMID: 9920278. doi: 10.1038/5289
- Yuen AR, Sikic BI. Multidrug resistance in lymphomas. J Clin Oncol. 1994 Nov;12(11):2453–9.
- Tulpule A. Multidrug resistance in AIDS-related lymphoma. Curr Opin Oncol [Internet]. 2005;17(5):466–468. http://www.ncbi.nlm.nih.gov/pubmed/1609379710.1097/01.cco.0000172825.85942.d6
- Schuetz EG, Beck WT, Schuetz JD. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996 Feb;49(2):311–8.
- Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40Suppl: S13–S19.10.1007/s002800051055
- Zembruski NC, Buchel G, Jodicke L, Herzog M, Haefeli WE, Weiss J. Potential of novel antiretrovirals to modulate expression and function of drug transporters in vitro. J Antimicrob Chemother [Internet]. 2011;66(4):802–812. http://www.ncbi.nlm.nih.gov/pubmed/2139317410.1093/jac/dkq501
- Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med [Internet]. 2014;7:53–64. http://www.ncbi.nlm.nih.gov/pubmed/24523596
- Giraud C, Manceau S, Treluyer JM. ABC transporters in human lymphocytes: expression, activity and role, modulating factors and consequences for antiretroviral therapies. Expert Opin Drug Metab Toxicol [Internet]. 2010;6(5):571–589. http://www.ncbi.nlm.nih.gov/pubmed/2036710910.1517/17425251003601953
- Simonelli C, Zanussi S, Cinelli R, et al. Virological efficacy in HIV-infected patients affected by non-hodgkin lymphoma, treated with antiblastic chemotherapy and highly active antiretroviral therapy. Scand J Infect Dis Suppl. 2003;35:49–53.10.1080/03008870310009678
- O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015 Nov;60(4):547–560.10.1016/j.molcel.2015.10.040
- Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci [Internet]. 2010;31(1):22–35. http://www.ncbi.nlm.nih.gov/pubmed/2000448510.1016/j.tips.2009.10.001
- Owen A, Chandler B, Back DJ. The implications of P-glycoprotein in HIV: friend or foe? Fundam Clin Pharmacol. 2005 Jun;19(3):283–296.10.1111/fcp.2005.19.issue-3
- Janneh O, Hartkoorn RC, Jones E, et al. Cultured CD4T cells and primary human lymphocytes express hOATPs: intracellular accumulation of saquinavir and lopinavir. British J Pharmacol. 2008;155:875–883.10.1038/bjp.2008.320
- Chandler B, Detsika M, Khoo SH, Williams J, Back DJ, Owen A. Factors impacting the expression of membrane-bound proteins in lymphocytes from HIV-positive subjects. J Antimicrob Chemother. 2007 Sep;60(3):685–689.10.1093/jac/dkm230
- No Title. Stanford University Drug Resistance database. http://hivdb.stanford.edu/DR/NRTIResiNote.html. Accessed November 13, 2014.
- McColl DJ, Margot N, Chen S-S, Harris J, Borroto-Esoda K, Miller MD. Reduced emergence of the M184V/I resistance mutation when antiretroviral-naïve subjects use emtricitabine versus lamivudine in regimens composed of two NRTIs plus the NNRTI efavirenz. HIV Clin Trials. 2011;12(2):61–70.10.1310/hct1202-61
- Chaudhary PM, Roninson IB. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst [Internet]. 1993;85(8):632–639. http://www.ncbi.nlm.nih.gov/pubmed/809687510.1093/jnci/85.8.632
- Simonelli C, Zanussi S, Cinelli R, et al. Impact of Concomitant antiblastic chemotherapy and highly active antiretroviral therapy on human immunodeficiency virus (HIV) viremia and genotyping in HIV‐infected patients with non‐hodgkin lymphoma. Clin Infect Dis [Internet]. 2003;37(6):820–827. http://www.ncbi.nlm.nih.gov/pubmed/1295564410.1086/cid.2003.37.issue-6