Abstract
Background: Endothelial progenitor cells (EPCs) are bone marrow-derived cells that contribute to vascular repair. EPCs may be reduced in HIV-infected (HIV+) persons, contributing to cardiovascular disease (CVD). Telmisartan is an angiotensin receptor blocker that increases EPCs in HIV-uninfected adults.
Objective: To assess telmisartan’s effects on EPC number and immunophenotype in older HIV + adults at risk for CVD.
Methods: HIV + persons ≥50 years old with HIV-1 RNA < 50 copies/mL on suppressive antiretroviral therapy and ≥1 CVD risk factor participated in a prospective, open-label, pilot study of oral telmisartan 80 mg daily for 12 weeks. Using CD34 and CD133 as markers of early maturity and KDR as a marker of endothelial lineage commitment, EPCs were quantified via flow cytometry and defined as viable CD3−/CD33−/CD19−/glycophorin− cells of four immunophenotypes: CD133+/KDR+, CD34+/KDR+, CD34+/CD133+, or CD34+/KDR+/CD133+. The primary endpoint was a 12-week change in EPC subsets (NCT01578772).
Results: Seventeen participants (88% men, median age 60 years and peripheral CD4+ T lymphocyte count 625 cells/mm3) enrolled and completed the study. After 6 and 12 weeks of telmisartan, frequencies of all EPC immunophenotypes were higher than baseline (all p < 0.10 except week 12 CD133+/KDR+ EPC, p = 0.13). Participants with lower baseline EPC levels had the largest gains. Additionally, the percentage of CD34+ cells with endothelial commitment (KDR+) increased.
Conclusions: Our data suggest that telmisartan use is associated with an increase in circulating EPCs in older HIV + individuals with CVD risk factors. Further controlled studies are needed to assess whether EPC increases translate to a reduction in CVD risk in this population.
Introduction
The balance between endothelial damage and repair determines progression of endothelial dysfunction and cardiovascular disease (CVD). Endothelial progenitor cells (EPCs) are bone marrow-derived cells that are released in response to vascular injury and participate in vascular repair.1 Specifically, EPCs are involved in re-endothelialization and neo-angiogenesis following vascular damage.Citation1–4 Endogenous mobilization and/or systemic infusion of EPCs following vascular injury leads to improved re-endothelialization, improved endothelial function, and reduced development of atherosclerosis in animal and human studies.Citation4–7 Additionally, perturbations in circulating EPC levels have been associated with traditional CVD risk factors, atherosclerotic burden, and cardiovascular events, with most studies associating lower EPC levels with increased current and future CVD risk.Citation8–13
HIV-1-infected individuals are at increased risk of CVD events compared to HIV-uninfected individuals,Citation14,15 but the contribution of EPCs to this risk is unclear.Citation16 While decreased EPC quantityCitation17 or functionality may contribute, the mechanisms by which the virus and/or antiretroviral therapy (ART) may modulate EPCs are not fully understood. However, it is known that HIV-1 can infect EPCs,Citation18–20 and cumulative nucleoside reverse transcriptase inhibitor (NRTI) and protease inhibitor (PI) exposures have been cited as potential risk factors for EPC depletion.Citation21 Additionally, HIV-1 can be found in bone marrow and infect hematopoietic stem cells.Citation22 Most mechanistic studies of CVD risk in HIV-infected individuals have assessed endothelial dysfunction using subclinical or clinical cardiovascular imaging modalities or inflammatory biomarker surrogates,Citation23,24 leaving the effects of clinical interventions on EPCs under-explored.
Telmisartan, an angiotensin receptor blocker (ARB) and peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonist approved for the treatment of essential hypertension, has been shown to improve insulin glucose homeostasis and fasting lipid parameters,Citation25,26 vascular inflammation and vascular function in predominantly HIV-uninfected adults with essential hypertension.Citation25–28 Importantly, while telmisartan has been shown to increase circulating EPC numbers in predominantly HIV-uninfected adults with CVD risk factors,Citation29–31 its effects on EPCs in HIV-1-infected persons are unknown. We explored the effect of standard dose telmisartan on EPC subsets among older HIV-1-infected adults on suppressive ART with traditional CVD risk factors, to test the hypothesis that telmisartan would increase circulating EPC numbers in this population.
Methods
Participants
Participants were enrolled into a 12-week, prospective, open-label, pilot study between October 2012 and July 2013 at the University of California, Los Angeles (UCLA) Center for Clinical AIDS Research and Education under a protocol approved by the UCLA institutional review board. All subjects provided written informed consent prior to initiation of study procedures. Inclusion criteria included: HIV-1 infection, age ≥50 years, plasma HIV-1 RNA < 50 copies/mL at screening and for 12 weeks prior to entry, stable ART for 12 weeks prior to entry, systolic blood pressure (SBP)>110 mm Hg and one or more traditional CVD risk factor (smoking, controlled hypertension, hyperlipidemia, diabetes mellitus). Family history of CVD alone was not sufficient for entry. Exclusion criteria included: uncontrolled hypertension (defined as SB p > 140 mm Hg or diastolic blood pressure [DBP] > 90 mm Hg); current use of any other ARB, nelfinavir, or etravirine in the ART regimen (due to potential interactions with telmisartan); untreated renal artery stenosis; unstable heart disease; active, untreated opportunistic, and/or AIDS-defining illness; absolute neutrophil count < 750 cells/mmCitation3; hemoglobin <10 g/dL; creatinine clearance <30 mL/min; aspartate transaminase; or alanine transaminase greater than three times the upper limit of normal; need for ongoing potassium supplementation; and history of intolerance to any member of the ARB class of agents. Participants on stable doses of angiotensin converting enzyme inhibitors, lipid-lowering agents, thiazolidinediones, and/or insulin-sensitizing agents for at least twelve weeks prior to entry were permitted to enroll, but asked not to titrate these medications during the study period. The study protocol is registered at clinicaltrials.gov (NCT01578772).
Intervention
Enrolled participants received open-label telmisartan 80 mg by mouth daily for 12 weeks. Blood was collected for EPC assessment at weeks 0, 6, and 12. All assessments occurred in the fasting state (nothing to eat or drink except water or medications for at least eight hours).
Flow cytometry
EPCs were quantified using multicolor flow cytometryCitation32,33 performed on freshly isolated peripheral blood mononuclear cells (PBMCs) using the following fluorochrome-conjugated antibodies: anti-CD45 FITC (clone HI30), anti-CD3 PerCP-Cy5.5 (clone UCHT1), anti-CD33 PerCP-Cy5.5 (clone P67.6), anti-CD19 (clone HIB19), anti-CD34 PE-Cy7 (clone 8G12), all from BD Biosciences (San Jose, CA); anti CD235ab (Glycophorin) PerCP-Cy5.5 (clone HIR2) from Biolegend (San Diego, CA); anti-CD133/2 PE (clone 293C3) from Beckman-Coulter (Brea, CA); and anti-VEGF R2 (KDR/Flk-1) APC (clone 89106) from R&D (Minneapolis, MN). Using CD34 and CD133 as markers of EPC early maturity and KDR as a marker of endothelial lineage commitment,Citation16 EPCs were defined as viable CD45−(or dim)/CD3−/CD33−/CD19−/glycophorin− cells with one of the following four immunophenotypes: CD133+/KDR+, CD34+/KDR+, CD34+/CD133+ or CD34+/KDR+/CD133+. These marker combinations were chosen as they provided the best balance of sensitivity and specificity during extensive methodology validation in our lab. While CD34+/KDR+ EPCs are commonly reported in the literature, EPCs can be defined by a variety of cellular markers. Additionally, CD34+/KDR+/CD133+ cells are rare in circulation but highly specific for EPC lineage, and were measured to ensure stringency of technique. Finally, the subset of CD34+ cells was further examined to determine whether telmisartan therapy preferentially altered frequency of cells with progenitor capacity/immaturity (CD133+), cells with endothelial commitment (KDR+) or both (CD133+KDR+).
An LSR-II flow cytometer (BD) was used to analyze ≥500,000 cells per sample. Cell Preparation Tube beads (BD Biosciences, San Jose, CA) were used for instrument setup prior to each analysis, and Rainbow beads (Spherotech, Lake Forest, IL) to standardize instrument settings between sampling runs. Fluorescence Minus One controls were prepared for each run to ensure between run gate consistency. All samples had > 75% viability; only live cells (defined by negative 7-aminoactinomycin D staining) were included. Data were analyzed in FlowJo software, version 9.3.3 (Treestar, Ashland, OR). EPCs values are expressed per 105 PBMC.
Outcomes and adverse events
The primary endpoint was the median, within-person, 12-week change in EPC subset number. Secondary endpoints were the absolute 6- and 12-week EPC changes, and 6-week changes in EPC subset number. Secondary safety endpoints included reporting of all Grade ≥3 clinical events and Grade ≥2 lab abnormalities as adverse events. Grades were determined using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (Version 1.0, December 2004).
Statistical analyses
A descriptive analysis of baseline characteristics and clinical events is presented. Continuous variables are reported as median and interquartile range (IQR), and nominal data as absolute values and percentages. Statistical analyses were performed using the Mann–Whitney U-test to determine association between clinical and demographic data and outcomes variables, and using the Wilcoxon signed rank test for pairwise comparisons. Due to the exploratory nature of the analyses, statistical significance was defined using a two-sided alpha level of 0.10. Similarly, all analyses were exploratory without adjusting for multiple testing. Due to the small sample size (n = 17), multivariate analysis was not feasible.
Results
Study population
Twenty-three potential participants screened, and seventeen met inclusion criteria and were enrolled. All participants completed the 12-week study period. Baseline demographic, clinical, and biological characteristics are detailed in Table . Participants were predominantly male (88%). Median age was 60 years, with time since diagnosis of HIV-1 infection 19 years and CD4+ T lymphocyte count 625 cells/mmCitation3. Twenty-nine percent had a pre-existing AIDS diagnosis. Regarding ART use, 71% were receiving a PI, 29% a non-NRTI (NNRTI), 29% abacavir, and 65% tenofovir. CVD risk factor profile included 18% current tobacco use, 12% diabetes mellitus, 65% hypertension, and 82% hyperlipidemia.
Table 1 Baseline demographic and clinical characteristics
Of note, participants were predominantly normotensive at baseline and had no changes in their CVD risk profile during the study period. Baseline CVD risk factor prevalence was not consistently associated with EPC number. While current smokers did have fewer CD133+/KDR+, CD34+/KDR+ and CD34+/KDR+/CD133+ EPCs, only three participants reported current smoking (data not shown).
No treatment discontinuations or adverse events related to telmisartan therapy occurred over the 12-week study period. Due to scheduling issues, 12 of 17 participants had their week 12 fasting blood sampling performed after the end of study treatment (average time off telmisartan prior to blood draw 8.7 days). One subject was excluded from the week 12 analyses due to an escalation of statin dose between weeks 6 and 12. However, a supplemental analysis including this participant at week 12 was performed, and did not change results (data not shown).
Changes in EPC subsets after 12 weeks of telmisartan administration
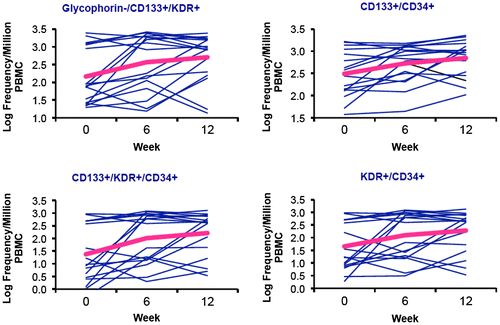
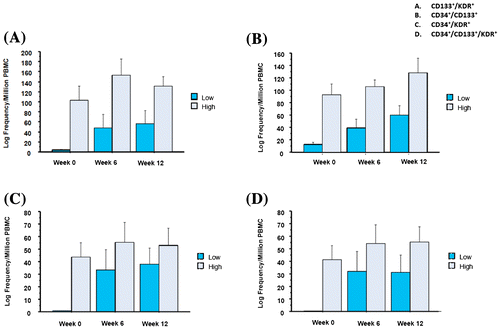
Absolute values and median within-person changes of EPC subsets over 12 weeks are reported in Table . After six weeks, all EPC subsets increased from baseline, including when changes were calculated both as absolute frequencies and as percentages of total PBMCs (p < 0.10). After 12 weeks, EPCs remained increased from baseline, with 12-week changes retaining statistical significance except for CD133+/KDR+ EPCs (p = 0.13). Twelve-week changes in EPC subsets are illustrated in Fig. . Of note, 12-week EPC increases were greater among participants with baseline EPC values below the median (p < 0.05 vs. participants with baseline EPCs above the median for all EPC subtypes except CD133+/KDR+, Fig. ).
Table 2 EPC changes over 12 weeks of telmisartan therapy
Figure 2 Twelve-week changes in EPC number stratified by baseline EPC number above (high) vs. below (low) the median value. *Results shown as mean ± standard error.
Additionally, among CD34+ cells, the frequency of the maturity marker CD133 remained stable, while an increase in the frequency of the endothelial commitment marker KDR was observed (Table ). Participants had similar median 12-week EPC increases regardless of telmisartan continuation or discontinuation at the time of the blood analysis (data not shown), suggesting that EPC bone marrow mobilization was not immediately affected by telmisartan cessation. Log transformation of EPC changes resulted in similar findings. Finally, in subgroup analyses, neither 6- nor 12-week changes in EPC numbers varied by race or CVD risk factor profile, although variability was high and sample sizes were small (data not shown).
EPC subset analyses by ART type
Although this study was not powered for subset analyses, abacavir-treated participants had higher baseline numbers of KDR+ EPCs than non-abacavir-treated participants (CD133+KDR+: 9.1 vs. 5.2 per 105 PBMCs, p = 0.33; CD34+KDR+: 15.8 vs. 0.9 per 105 PBMCs, p = 0.44; CD133+34+KDR+: 4.1 vs. 0.6 per 105 PBMCs, p = 0.28), a finding not present when data were stratified by tenofovir vs. non-tenofovir use. These absolute differences, while large in effect size, did not reach statistical significance in the small numbers of participants within subsets. Abacavir users also had smaller 12-week increases in KDR+ EPCs with telmisartan therapy than non-abacavir users (CD133+KDR+: 1.2 vs. 30.6 per 105 PBMCs, p = 0.45; CD34+KDR+: 5.6 vs. 10.3 per 105 PBMCs, p = 0.95; CD133+34+KDR+: 4.2 vs. 7.8 per 105 PBMCs, p = 0.78), although this finding was also present when tenofovir and non-tenofovir users were compared (CD133+KDR+: 13.1 vs. 147.5 per 105 PBMCs, p = 0.44; CD34+KDR+: 2.7 vs. 44.8 per 105 PBMCs, p = 0.07; CD133+34+KDR+: 1.4 vs. 55.5 per 105 PBMCs, p = 0.91). Additionally, only one participant was on an NRTI-sparing regimen, creating difficulty in discerning NRTI effects from third agent effects.
PI-treated participants had more similar baseline EPC numbers to non-PI-treated participants (CD133+KDR+: 8.2 vs. 8.0 per 105 PBMCs, p = 0.80; CD34+KDR+: 2.7 vs. 1.0 per 105 PBMCs, p = 0.80; CD133+34+KDR+: 1.3 vs. 0.3 per 105 PBMCs, p = 0.65). However, 12-week increases in KDR+ EPCs with telmisartan therapy were smaller for PI than non-PI users (CD133+KDR+: 5.6 vs. 45.1 per 105 PBMCs, p = 0.51; CD34+KDR+: 4.7 vs. 15.9 per 105 PBMCs, p = 0.91; CD133+34+KDR+: 3.7 vs. 11.2 per 105 PBMCs, p = 0.83).
Discussion
In this small, single-arm, pilot study, increased circulating EPCs were observed following 12 weeks of telmisartan therapy in older HIV-1-infected adults with traditional CVD risk factors on suppressive ART. To our knowledge, our study is the first to demonstrate therapeutic EPC modulation in HIV-1-infected persons. Our data are also unique in that they target an older subset of HIV-1-infected persons with both a long median duration of HIV-1 infection (as opposed to most other EPC studies in HIVCitation16) and traditional CVD risk factors but without known CVD. Importantly, we observed the greatest EPC increases among the CD34+/CD133+/KDR+ subpopulation, which are rare in circulation, highly specific for endothelial lineage, and the most stringent of our four panels for defining EPCs.
We also observed larger EPC increases among participants with baseline EPC numbers below vs. above the median. While lower EPC numbers have been associated with CVD morbidity and mortality in the general population, our ability to modulate EPCs in older HIV-1-infected adults at risk for but without known CVD is notable and suggests that, if confirmed in a controlled study, telmisartan therapy could reduce CVD risk in this population. Finally, we observed an increase in endothelial lineage commitment (KDR+) among EPC subsets during telmisartan therapy. This finding is consistent with data on telmisartan in HIV-1-uninfected persons: Pellicia et al.Citation30 demonstrated increases only in EPC subtypes with endothelial commitment among participants with known coronary artery disease treated with telmisartan vs. placebo. Other ARBs have also been reported to increase CD34+/KDR+ and CD34+/CD133+/KDR+ cell numbers.Citation34,35 Notably, the effects of ARBs on EPCs is not believed to be wholly attributable to their anti-hypertensive properties, as EPC effects also occur in normotensive patients (as in our study).Citation30 Specifically, telmisartan may delay EPC senescence via angiotensin receptor blockadeCitation36 and promote EPC proliferation, migration Citation37 and differentiationCitation38 by serving as a PPAR-ɣ agonist.Citation39–43
While this study was not powered to address this issue, we hypothesized that participants on different ART agents would have varying EPC responses to telmisartan therapy. Specifically, we hypothesized that abacavir- and PI-treated participants would have smaller increases due to potential associations of these drugs with CVD risk.Citation44–49 Interestingly, abacavir-treated participants had higher baseline numbers of EPCs with endothelial lineage commitment than non-abacavir-treated participants, and both abavacir- and PI-treated participants had smaller EPC increases with telmisartan therapy than non-abacavir- and non-PI-treated participants (even when comparing subsets of PI-treated participants treated with and without abacavir to assess overlap, data not shown). A potential explanation is that abacavir- and/or PI-induced endothelial toxicity create a stimulus for KDR+ EPC production that cannot be altered by telmisartan. While these findings give only preliminary insight into possible relationships between ART and EPCs, they provide a direction for future interventions and investigations.
Our pilot study has several limitations, including its small sample size, the wide range of observed EPC values, the poorly understood biologic variability of EPCs over time in HIV-infected persons, and the lack of concomitant clinical estimates of CVD (such as arterial flow-mediated dilatation or carotid intima media thickness) to provide a clinical correlate of CVD risk. Moreover, the lack of a placebo-controlled comparator arm prevents direct comparison of the 12-week effects of telmisartan therapy to the natural history of EPC variation in older HIV-1-infected persons on suppressive ART with known CVD risk factors. Additionally, our study was not designed to explore the effects of telmisartan on EPC function, although our data suggesting quantitative EPC increases underscores the importance of addressing this question as a next step. Finally, EPCs are a heterogeneous population of cells, and methods for EPC isolation and identification lack standardization in the published literature, limiting comparisons to other studies that used different techniques (for example, culture-derived EPCs).Citation16,50 Despite these limitations, we demonstrated both a consistent increase in EPC number and commitment to endothelial lineage during exposure to telmisartan, and begin to explore possible ART effects on EPCs, both of which are important to developing CVD prevention and treatment strategies for HIV-1-infected persons.
Conclusions
This pilot study demonstrated that 12 weeks of standard dose telmisartan therapy was associated with increased EPC numbers and EPC commitment to endothelial lineage in older HIV-1-infected individuals with traditional CVD risk factors. This study is the first to assess telmisartan’s effects on EPCs in HIV infection, and suggests that vascular reparative capacity can be modulated in chronic, treated HIV infection. This intervention may represent a strategy for CVD prevention by enhancing vascular reparative capacity and slowing atherosclerosis progression in HIV-1-infected adults. Larger, randomized studies are needed to confirm our findings and associate observed effects with clinical surrogates of CVD burden, with the goal of defining telmisartan’s role as an agent for endothelial preservation in treated HIV infection.
Declarations of interest
Jordan E. Lake has served as a consultant to Gilead Sciences and GlaxoSmithKline. Sophie Seang, Theodoros Kelesidis, Judith S. Currier and Otto O. Yang has no conflicts of interest to report.
Funding
This work was supported by the Campbell Foundation to JEL, the John A. Hartford Foundation to JEL and the National Institutes of Health [grants K23 AI110532 to JEL, P30 AG028748, UL1 TR000124 and 5P30 AI028697], and also supported by a seed grant and the flow cytometry core facility funded by the UCLA CFAR NIH/NIAID AI028697.
Notes on contributors
Jordan E. Lake was an assistant professor in the UCLA Division of Infectious Diseases when this work was conducted. She is now an associate professor at the University of Texas Health Science Center in Houston. Her research focuses on understanding the contribution of chronic inflammation to comorbid metabolic disease in treated HIV infection.
Sophie Seang is a clinician researcher and infectious disease specialist at Assistance Publique-Hôpitaux de Paris in Paris, France. She recently completed a two-year research position at UCLA focused on understanding cardiovascular disease in treated HIV infection.
Theodoros Kelesidis is an assistant professor in the UCLA Division of Infectious Diseases. He is a clinician researcher whose laboratory focuses on understanding the role of oxidized lipids in HIV-1 pathogenesis.
Judith S. Currier is a professor of Medicine in the UCLA Division of Infectious Diseases. As a clinician researcher, her research focuses on understanding the pathogenesis of and optimal management for the metabolic complications of treated HIV infection.
Otto O. Yang is professor of Medicine in the UCLA Division of Infectious Diseases. He has a background in clinical infectious diseases, and his laboratory focuses on understanding T cell immunology in HIV infection.
Acknowledgments
The authors would like to thank the study staff and participants for their time and commitment to this project. We would also like to thank: Ms Diana Liao from UCLA for her help with database development and analysis; Ms Stephanie Koh for her assistance with data entry and cleaning; and Sangeun Park and Diana Huynh from UCLA for their assistance with flow cytometry.
References
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–966.10.1126/science.275.5302.964
- Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438.
- Asahara T, Masuda H, Takahashi T, et al. Bone Marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228.10.1161/01.RES.85.3.221
- Werner N, Priller J, Laufs U, et al. Bone Marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22(10):1567–1572.10.1161/01.ATV.0000036417.43987.D8
- Kuliczkowski W, Derzhko R, Prajs I, Podolak-Dawidziak M, Serebruany VL. Endothelial progenitor cells and left ventricle function in patients with acute myocardial infarction: potential therapeutic considertions. Am J Ther. 2012;19(1):44–50.10.1097/MJT.0b013e3181e0cab3
- Leone AM, Rutella S, Bonanno G, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26(12):1196–1204.10.1093/eurheartj/ehi164
- Krankel N, Luscher TF, Landmesser U. “Endothelial progenitor cells” as a therapeutic strategy in cardiovascular disease. Curr Vasc Pharmacol. 2012;10(1):107–124.10.2174/157016112798829832
- Chironi G, Walch L, Pernollet MG, et al. Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007;191(1):115–120.10.1016/j.atherosclerosis.2006.02.041
- Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–e7.10.1161/hh1301.093953
- Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987.10.1161/CIRCULATIONAHA.104.504340
- Fadini GP, Coracina A, Baesso I, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37(9):2277–2282.10.1161/01.STR.0000236064.19293.79
- Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007.10.1056/NEJMoa043814
- Hill JM, Zalos G, Halcox JP, et al. Circulating Endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600.10.1056/NEJMoa022287
- Currier JS. Update on cardiovascular complications in HIV infection. Top HIV Med. 2009;17(3):98–103.
- Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-Infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35.10.1161/CIRCULATIONAHA.107.189624
- Costiniuk CT, Hibbert BM, Simard T, Ghazawi FM, Angel JB, O’Brien ER. Circulating endothelial progenitor cells in HIV infection: a systematic review. Trends Cardiovasc Med. 2013;23(6):192–200.10.1016/j.tcm.2012.12.002
- López M, Vispo E, San Román J, et al. Short Communication high risk of endothelial dysfunction in HIV individuals may result from deregulation of circulating endothelial cells and endothelial progenitor cells. AIDS Res Hum Retroviruses. 2012;28(7):656–659.10.1089/aid.2011.0152
- Teofili L, Iachininoto MG, Capodimonti S, et al. Endothelial progenitor cell trafficking in human immunodeficiency virus-infected persons. AIDS. 2010;24(16):2443–2450.10.1097/QAD.0b013e32833ef79d
- McNamara LA, Onafuwa-Nuga A, Sebastian NT, Riddell Jt, Bixby D, Collins KL. CD133+ hematopoietic progenitor cells harbor HIV genomes in a subset of optimally treated people with long-term viral suppression. J Infect Dis. 2013;207(12):1807–1816.10.1093/infdis/jit118
- Bordoni V, Bibas M, Abbate I, et al. Bone marrow CD34+ progenitor cells may harbour HIV-DNA even in successfully treated patients. Clin Microbiol Infect. 2015;21(3):e5–e8.
- Gómez-Garre D, Estrada V, Ortega-Hernández A, et al. Association of HIV-infection and antiretroviral therapy with levels of endothelial progenitor cells and subclinical atherosclerosis. J Acquir Immune Defic Syndr. 2012;61(5):545–551.10.1097/QAI.0b013e31826afbfc
- Carter CC, Onafuwa-Nuga A, McNamara LA, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16(4):446–451.10.1038/nm.2109
- Beltrán LM, Muñoz Hernández R, de Pablo Bernal RS, et al. Reduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infection. PLoS ONE. 2014;9(3):e90541.10.1371/journal.pone.0090541
- Bernal E, Verdu JM, Vera F, et al. Improvement of endothelial function after switching previously treated HIV-infected patients to an NRTI-sparing bitherapy with maraviroc. J Int AIDS Soc. 2014;17(4 Suppl 3):19726.
- Wago T, Yoshimoto T, Akaza I, et al. Improvement of endothelial function in patients with hypertension and type 2 diabetes after treatment with telmisartan. Hypertens Res Official J Jpn Soc Hypertens. 2010;33(8):796–801.10.1038/hr.2010.107
- Perl S, Schmölzer I, Sourij H, et al. Telmisartan improves vascular function independently of metabolic and antihypertensive effects in hypertensive subjects with impaired glucose tolerance. Int J Cardiology. 2010;139(3):289–296.10.1016/j.ijcard.2008.10.048
- Benndorf RA, Appel D, Maas R, Schwedhelm E, Wenzel UO, Böger RH. Telmisartan Improves endothelial function in patients with essential hypertension. J Cardiovasc Pharmacol. 2007;50(4):367–371.10.1097/FJC.0b013e31811dfbe7
- Jung AD, Kim W, Park SH, et al. The effect of telmisartan on endothelial function and arterial stiffness in patients with essential hypertension. Korean Circulation J. 2009;39(5):180–184.10.4070/kcj.2009.39.5.180
- Bahlmann FH, de Groot K, Mueller O, Hertel B, Haller H, Fliser D. Stimulation of Endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension. 2005;45(4):526–529.10.1161/01.HYP.0000159191.98140.89
- Pelliccia F, Pasceri V, Cianfrocca C, et al. Angiotensin II receptor antagonism with telmisartan increases number of endothelial progenitor cells in normotensive patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Atherosclerosis. 2010;210(2):510–515.10.1016/j.atherosclerosis.2009.12.005
- Endtmann C, Ebrahimian T, Czech T, et al. Angiotensin II impairs endothelial progenitor cell number and function in vitro and in vivo: implications for vascular regeneration. Hypertension. 2011;58(3):394–403.10.1161/HYPERTENSIONAHA.110.169193
- Fadini GP, Losordo D, Dimmeler S. Critical Reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–637.10.1161/CIRCRESAHA.111.243386
- Blann AD, Woywodt A, Bertolini F, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93(2):228–235.
- Calò LA, Dal Maso L, Pagnin E, et al. Effect of olmesartan medoxomil on number and survival of circulating endothelial progenitor cells and calcitonin gene related peptide in hypertensive patients. J Hypertens. 2014;32(1):193–199.10.1097/HJH.0b013e32836522c3
- Tan H, Li X, Chen W, et al. Effects of losartan on the mobilization of endothelial progenitor cells and improvement of endothelial function. Ann Clin Lab Sci. 2013;43(4):402–406.
- Li H, Liu Q, Wang N, Xu J. Correlation of different NADPH oxidase homologues with late endothelial progenitor cell senescence induced by angiotensin II: effect of telmisartan. Intern Med. 2011;50(16):1631–1642.10.2169/internalmedicine.50.5250
- Esposito K, Ciotola M, Maiorino MI, et al. Circulating CD34+KDR+ Endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med. 2009;6(1):107–114.10.1111/j.1743-6109.2008.01042.x
- Wang CH, Ciliberti N, Li SH, et al. Rosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropy. Circulation. 2004;109(11):1392–1400.10.1161/01.CIR.0000123231.49594.21
- Amano Y, Yamaguchi T, Ohno K, et al. Structural basis for telmisartan-mediated partial activation of PPAR gamma. Hypertens Res. 2012;35(7):715–719.10.1038/hr.2012.17
- Spigoni V, Picconi A, Cito M, et al. Pioglitazone improves in vitro viability and function of endothelial progenitor cells from individuals with impaired glucose tolerance. PLoS ONE. 2012;7(11):e48283.10.1371/journal.pone.0048283
- Esposito K, Maiorino MI, Di Palo C, et al. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes–a randomized controlled trial. Diabetes Obes Metab. 2011;13(5):439–445.10.1111/dom.2011.13.issue-5
- Cao Z, Yang Y, Hua X, et al. Telmisartan promotes proliferation and differentiation of endothelial progenitor cells via activation of Akt. Chin Med J (Engl). 2014;127(1):109–113.
- Honda A, Matsuura K, Fukushima N, Tsurumi Y, Kasanuki H, Hagiwara N. Telmisartan induces proliferation of human endothelial progenitor cells via PPARγ-dependent PI3K/Akt pathway. Atherosclerosis. 2009;205(2):376–384.10.1016/j.atherosclerosis.2008.12.036
- Esplugues JV, De Pablo C, Collado-Díaz V, Hernández C, Orden S, Álvarez Ángeles. Interference with purinergic signalling: an explanation for the cardiovascular effect of abacavir? AIDS. 2016;30(9):1341–1351.10.1097/QAD.0000000000001088
- Auclair M, Afonso P, Capel E, Caron-Debarle M, Capeau J. Impact of darunavir, atazanavir and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin. Antivir Ther. 2014;19(8):773–782.10.3851/IMP2752
- Jiang B, Khandelwal AR, Rogers LK, et al. Antiretrovirals induce endothelial dysfunction via an oxidant-dependent pathway and promote neointimal hyperplasia. Toxicol Sci. 2010;117(2):524–536.10.1093/toxsci/kfq213
- Young J, Xiao Y, Moodie EE, et al. Effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV cohort study. J Acquir Immune Defic Syndr. 2015;69(4):413–421.10.1097/QAI.0000000000000662
- Sabin CA, Reiss P, Ryom L, et al. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14(1):1417.10.1186/s12916-016-0588-4
- Tripathi A, Liese AD, Winniford MD, et al. Impact of clinical and therapeutic factors on incident cardiovascular and cerebrovascular events in a population-based cohort of HIV-infected and non-HIV-infected adults. Clin Cardiol. 2014;37(9):517–522.10.1002/clc.2014.37.issue-9
- Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–637.10.1161/CIRCRESAHA.111.243386