Abstract
Background: Tenofovir disoproxil fumarate (TDF) is a component of many combinations of antiretroviral treatment (ART) regimens. Although potent and generally well tolerated, TDF may cause renal and bone toxicity. The magnitude of off-target side effects is proposed to be related to tenofovir plasma concentrations, which are affected by food and drug–drug interactions with concomitant antiretrovirals.
Objective: To perform a systematic literature review and qualitatively report on renal and bone safety outcomes associated with efavirenz (EFV), emtricitabine (FTC), and TDF (EFV+FTC+TDF) ART.
Methods: Embase and PubMed databases were searched for randomized clinical trials and observational cohort studies reporting on HIV treatment with EFV+FTC+TDF. Relevant articles were hand-searched for renal (Grade 3–4 serum creatinine/estimated glomerular filtration rate elevations, renal adverse events [AEs], discontinuation due to renal AEs, and urinary biomarkers) and bone outcomes (bone mineral density [BMD] reductions, bone turnover markers, and fracture), and results compiled qualitatively.
Results: Of 337 retrieved articles, 29 reporting renal and 11 reporting bone outcomes met the review criteria. EFV+FTC+TDF was associated with a low frequency of renal AEs and treatment discontinuations due to renal AEs. Renal AEs were more frequent when TDF was taken with protease inhibitor (PI)- or cobicistat-containing ART. EFV+FTC+TDF was associated with reduced BMD and increased bone turnover markers, but BMD reductions were less than with PI-containing ART. No treatment-related bone fractures were identified.
Conclusions: EFV+FTC+TDF appeared to have a more favorable renal safety profile than TDF administered with a PI or cobicistat. BMD decreased with EFV+FTC+TDF, but no treatment-related fractures were identified.
Introduction
Antiretroviral treatment (ART) has significantly improved the life expectancy of patients living with HIV infection. However, this increased survival has led to an increased incidence of chronic age-related complications,Citation1 including renal impairmentCitation2 and osteoporosis.Citation3 Potential drivers of these complications in the aging HIV population include: (1) an over-representation of “traditional” risk factors such as diabetes and hypertension; (2) direct effects of HIV infection and associated immune dysfunction (low CD4 count and high HIV-1 RNA levels); (3) co-infection with hepatitis C; and (4) antiretroviral toxicity.Citation1–3
Tenofovir disoproxil fumarate (TDF), a prodrug of tenofovir, was approved by the US Food and Drug administration (FDA) in 2001 and is a common component of many ART regimens. It is also included in three single-tablet regimens (STRs): efavirenz (EFV)/emtricitabine (FTC)/TDF; rilpivirine (RPV)/FTC/TDF; and elvitegravir (EVG)/cobicistat (COBI)/FTC/TDF.
While early randomized controlled trials (RCTs) did not reveal significant renal or bone toxicity of TDF,Citation4–6 cohort studies, systematic reviews, and meta-analyses of RCTs have now indicated an association between TDF-based regimens and an increased risk of renal impairment and bone demineralization,Citation7–10 with longer term studies suggesting incremental risk with cumulative exposure to tenofovir.Citation7,11–13
While the mechanism by which tenofovir causes renal impairment, increased bone turnover, and bone demineralization is not fully understood, tenofovir accumulation within proximal renal tubular cells leading to local mitochondrial damage and associated proximal tubular dysfunction and hypophosphatemic osteomalacia is proposed to underlie these adverse outcomes.Citation14,15
Tenofovir levels are significantly increased when TDF is taken with food and with concomitant administration of protease inhibitors (PIs), RPV, and COBI. The bioavailability of tenofovir is increased by more than 40% following a period of once-daily dosing of TDF 300 mg in the fed state.Citation16 Notably, RPV/FTC/TDF, EVG/COBI/FTC/TDF, and PIs are recommended to be taken with food.Citation17–19 In addition, tenofovir exposure has been shown to increase by 37, 32, 24, 23, and 22% when TDF was co-administered with atazanavir (ATV)/ritonavir (RTV), lopinavir/RTV, RPV, darunavir/RTV, and COBI, respectively.Citation16,20,21 Thus, increased tenofovir exposure with these TDF-containing regimens might lead to a greater risk of renal and bone toxicity.
Conversely, EFV+FTC+TDF is taken in the fasted state, and no clinically relevant drug–drug interactions have been reported between TDF and either EFV or FTC.Citation16,22,23 Moreover, compared with other ART, cumulative exposure to EFV is associated with a lower risk of proteinuria and chronic kidney diseaseCitation7 and lower levels of the urinary biomarkers interleukin-18 and kidney injury molecule-1.Citation24 Therefore, EFV+TDF+FTC may possess a more favorable renal and bone safety profile than other TDF-containing regimens.
Tenofovir alafenamide (TAF), a novel prodrug of tenofovir, is associated with a 91% lower plasma tenofovir concentration than that following TDF administration while maintaining high intracellular concentrations for HIV suppression.Citation25 Renal and bone biomarker profiles appear to be more favorable with TAF-containing regimens compared with TDF-containing regimens.Citation26–28 However, switching from EFV+FTC+TDF to a TAF-containing regimen has shown relatively less improvement than switching from EVG/COBI/FTC/TDF or ATV/RTV+FTC/TDF,Citation29–32 suggesting that EFV+FTC+TDF already has a comparatively favorable renal and bone safety profile.
Given the ongoing widespread use of EFV+FTC+TDF and EFV+lamivudine (3TC)+TDF worldwide and the absence of an EFV co-formulation with TAF, there is a need to characterize the renal and bone safety of FTC+TDF when combined with EFV. The primary aim of this report was to document the renal and bone safety of EFV+FTC+TDF (either given as separate drugs or as a fixed-dose combination) by conducting a qualitative systematic review of RCTs and observational studies in patients with HIV infection treated with this antiretroviral combination. Where data were available, a secondary aim was to compare the renal and bone safety of EFV+FTC+TDF with that of comparator TDF-containing regimens used in reviewed studies.
Methods
Literature search strategy
We searched PubMed and Embase databases to identify RCTs and observational studies that reported on clinical renal and bone safety outcomes in patients with HIV infection treated with EFV+FTC+TDF (either given as separate drugs or as a fixed-dose combination). The PubMed search was conducted on 18 December 2015, using the search strategy HIV AND ((Atripla OR (efavirenz AND emtricitabine AND tenofovir)) searching both “All Fields” and using “Medical Subject Heading” terms where available (Supplement 1). A similar strategy was employed for the Embase search, which was conducted on 11 January 2016 (Supplement 2). Neither search strategy employed a date range, i.e. all publications were retrieved up to the date that the search was conducted. The results of both the PubMed and Embase searches were combined and de-duplicated. Because reports of renal and bone adverse outcomes were often not reported in abstracts, all publications were hand-searched for relevant renal and bone data.
Additional inclusion and exclusion criteria
Studies were selected for inclusion if they were RCTs or observational studies conducted in adult patients infected with HIV and included an EFV+FTC+TDF treatment group. Articles were excluded if they were not written in English, the primary endpoint was non-clinical (e.g. cost-effectiveness), they were case studies, case series, expert opinion, or review articles. Because a large number of studies with renal outcomes were identified, the inclusion criteria for renal outcomes were modified to include only articles where at least 100 patients received EFV+FTC+TDF. All articles with bone outcomes were evaluated, regardless of the number of patients receiving EFV+FTC+TDF. Conference abstracts were considered for inclusion if they met the review criteria and were presented at major HIV conferences in the 2015 calendar year: Conference on Retroviruses and Opportunistic Infections: International AIDS Society Conference; or European AIDS Clinical Society Conference.
Outcomes
Renal adverse events (AEs) were defined in the review protocol as on-treatment onset of any of the following: acute or chronic kidney disease; toxic nephropathy; Fanconi syndrome; renal insufficiency or failure; nephrotic syndrome; Grade 3–4 increases in serum creatinine or Grade 3–4 decreases in creatinine clearance (CrCl)/estimated glomerular filtration rate (eGFR) as defined by the Division of AIDS (DAIDS) rating criteria;Citation33 or treatment discontinuation due to an unspecified renal AE. A grade of ≥3 for creatinine-based AEs was set owing to the fact that a number of antiretrovirals increase serum creatinine without any demonstrable effect on glomerular filtration rate as assessed by iohexol clearance.Citation34 We also documented changes in urinary biomarkers, including proteinuria, β-2 microglobulin (B2M), retinol-binding protein (RBP), and N-acetyl-β-D-glucosaminidase (NAG), which could indicate proximal tubule damage.Citation35 Bone outcomes included bone mineral density (BMD) as measured by dual-energy X-ray absorptiometry, occurrence of osteoporotic fracture (noting spine/vertebra, hip and wrist/forearm fractures as likely due to osteoporosis), bone fracture risk using the World Health Organization (WHO) fracture risk assessment tool, decreases in vitamin D levels, and increases in bone turnover markers (C-terminal telopeptide, bone-specific alkaline phosphatase [BSAP], procollagen type 1 amino-terminal propeptide, and osteocalcin).
Data synthesis
Articles were classified based on weighting criteria established by the Oxford Centre for Evidence-Based Medicine Levels of Evidence.Citation36 Articles presenting Level 1 evidence (blinded or partially blinded RCTs) or Level 2 evidence (open-label RCTs and observational cohort studies) were evaluated. As per exclusion criteria, publications with Level 3 (case control studies), Level 4 (case series), or Level 5 (expert opinion) evidence were excluded. The search results were compiled qualitatively by the authors in accordance with recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.Citation37 A quantitative meta-analysis was considered but not feasible owing to the wide variation in reported outcomes, which were also not consistently reported across reviewed studies to allow for quantitative comparisons. For all articles, sources of potential bias were assessed according to established methods.Citation38–40
Results
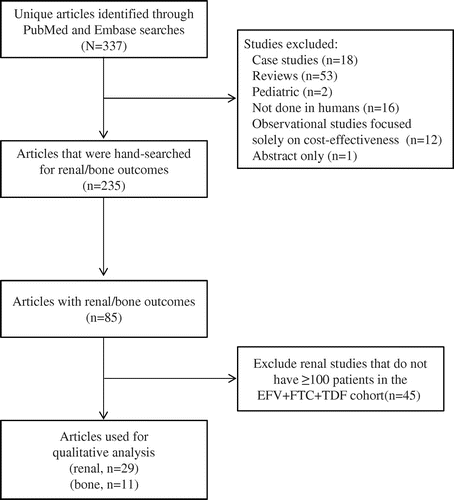
The initial de-duplicated search identified 337 individual studies that evaluated EFV+FTC+TDF in the treatment of HIV. Of these, 102 were excluded for protocol-defined reasons (Fig. ) and 235 were hand-searched for renal/bone outcomes. A total of 29 articles reporting renal outcomes and 11 articles reporting bone outcomes were identified for qualitative synthesis.
Figure 1 PRISMA flow diagram
Renal outcomes
Level 1 evidence
These included 15 articles describing seven blinded or partially blinded RCTs and including a total of 2682 patients on EFV+FTC+TDF (Table ). They reported a total of eight unique protocol-defined renal AEs (0.3%) and four treatment discontinuations due to these AEs (0.1%) with EFV+FTC+TDF over a follow-up period of 1–5 years. Renal AEs with EFV+FTC+TDF included one patient each in STARTMRK,Citation41 ECHO/THRIVE,Citation42 and SINGLECitation43 with a Grade 3–4 serum creatinine abnormality, one patient in STARTMRK with renal failure,Citation44 one patient in SINGLE with renal failure that was considered possibly related to study treatment,Citation45 and three patients in AIDS Clinical Trials Group (ACTG) A5202 with a diagnosis of either Fanconi syndrome, toxic nephropathy, proteinuria, or renal failure.Citation46
Table 1 Level 1 blinded and partially blinded RCTs reporting renal outcomes
The aggregate frequencies of renal AEs and discontinuation due to renal AEs for comparator FTC+TDF-containing regimens were 15/1643 (0.9%) and 14/1643 (0.9%), respectively. Among these comparator regimens, renal AEs (all of which led to discontinuation) were reported more frequently when TDF was taken with ATV/RTV (6/464; 1.3%) or with COBI (8/348; 2.3%), whereas only one renal AE (not leading to discontinuation) occurred with RPV+FTC/TDF and none with raltegravir (RAL)+FTC/TDF. In ACTG A5202, six patients treated with FTC/TDF+ATV/RTV had a renal diagnosis of Fanconi syndrome, toxic nephropathy, proteinuria, or renal failure, and all of these patients discontinued treatment. In GS-US-236-6-0102, eight renal AEs were reported in the EVG/COBI/FTC/TDF arm: at Week 48, five patients discontinued due to renal AEs (renal failure, Fanconi syndrome, and two due to elevated serum creatinine levels);Citation47 at Week 96, one patient had renal failure and another had an abnormal eGFR that led to study discontinuation; andCitation48 at Week 144, one patient with a persistent Grade 1 serum creatinine elevation discontinued.Citation49
No significant worsening of CrCl/eGFR was reported in patients treated with EFV+FTC+TDF (Table ), in contrast to those treated with TDF and a PI or COBI. For example, in the ACTG A5202 study, EFV+FTC+TDF-treated patients had modestly improved median CrCl at Weeks 48 and 96.Citation46 In contrast, patients treated with FTC/TDF+ATV/RTV had median CrCl levels at Weeks 48 and 96 that were lower than baseline levels; these differences were significant when compared with EFV+FTC+TDF-treated patients (p < 0.001 for both follow-up periods). Similarly, patients treated with EVG/COBI/FTC/TDF had significantly higher median levels of serum creatinine and lower eGFR at Week 48 in the GS-US-236-0102 trial (p < 0.001)Citation47; similar differences were observed at Week 96, although statistical significance was not reported for this follow-up period.Citation48 However, the interpretation of these findings may be complicated by the potential for agents to alter serum creatinine levels independently of glomerular filtration. COBI, for example, is known to inhibit the tubular secretion of creatinine without having an effect on glomerular filtration as assessed by iohexol clearance.Citation34 RTV, dolutegravir, and RPV also inhibit creatinine secretion through various mechanisms and to varying degrees.Citation50
Level 2 evidence
Renal AEs associated with EFV+FTC+TDF were reported at a higher frequency in Level 2 evidence studies than in Level 1 evidence studies. A total of 30 unique renal AEs, which included 11 treatment discontinuations due to these AEs, were identified in the 14 articles describing the eight open-label RCTs and two observational studies that were reviewed (Tables and ). This represented a frequency of renal AEs of 30/2503 (1.2%) and treatment discontinuations due to these AEs of 11/2503 (0.4%) with EFV+FTC+TDF over a follow-up period of 3 months to 3 years and 8 months. The higher frequency of renal AEs in the Level 2 group of studies was largely driven by a higher frequency of renal AEs in ACTG PEARLS,Citation51,52 which was of the longest duration and was conducted in patients with HIV infection living in resource-limited settings. In ACTG PEARLS,Citation51,52 15 patients treated with EFV+FTC+TDF had a renal abnormality (defined as a serum creatinine level ≥ 1.9 mg/dL [168 μmol/L] or a calculated CrCl of <50 mL/min, which is equivalent to DAIDS Grade ≥ 3 AEs), 1 patient had acute glomerulonephritis, and 1 patient discontinued treatment due to renal failure. In a trial where virologically suppressed HIV patients were switched to EFV+FTC+TDF or remained on their current ART,Citation53 two patients who switched to EFV+FTC+TDF discontinued due to increases in serum creatinine (grade undisclosed). In the TEMPRANO study, two patients treated with EFV+FTC+TDF discontinued treatment due to renal insufficiency or nephrotic syndrome.Citation54 In the STaR trial, one patient receiving EFV+FTC+TDF discontinued treatment due to renal failure.Citation55 In a single-arm, single-center study of generic EFV+FTC+TDF, four patients discontinued treatment due to Grade 3–4 renal toxicity (three of these patients had renal comorbidities at baseline); however, renal toxicity was not defined in this study.Citation56
Table 2 Level 2 open-label studies reporting renal outcomes
Table 3 Level 2 observational studies reporting renal outcomes
Among Level 2 studies, frequencies of renal AEs for the comparator arms could not be meaningfully assessed, as patients receiving TDF-containing comparator regimens were too few in number.
Across all studies reporting Level 2 evidence, the median eGFR of EFV+FTC+TDF-treated patients generally remained near baseline levels. In STaR, improvements in eGFR were reported in the EFV+FTC+TDF-treated patients while declines were reported in RPV/FTC/TDF-treated patients.Citation55 Proteinuria was detected in 6% of EFV+FTC+TDF-treated patients at 144 weeks in GS-01-934.Citation57 In ASSERT, the mean percentage change from baseline for B2 M, RBP, and NAG ratios to creatinine was −2%, +55%, and −6% at 96 weeks.Citation58
Finally, renal safety was evaluated in a study of patients co-infected with HCV and HIV treated with ledipasvir/sofosbuvir (LDV/SOF) and either EFV+FTC+TDF, RAL+FTC/TDF, or RPV/FTC/TDF for 12 weeks.Citation59 Because elevated levels of tenofovir have been observed when TDF is coadministered with LDV/SOF,Citation60 extensive renal and pharmacokinetic monitoring was performed. Notably, tenofovir exposures were lowest in the EFV+FTC+TDF arm (Table ). Three patients receiving LDV/SOF and EFV+FTC+TDF and one patient receiving LDV/SOF and RAL+FTC/TDF, all of whom had baseline renal impairments, had confirmed treatment-emergent increases of 0.4 mg/dL (35.4 μmol/L) in serum creatinine related to the study drug (Table ).
Bone outcomes
Fewer articles reported on bone than renal outcomes. Of the 235 articles that were hand-searched, 12 articles from nine different studies reported on bone safety outcomes in patients treated with EFV+FTC+TDF and met the criteria for inclusion in this review.
Level 1 evidence
BMD loss after ART initiation was universally observed in all studies reviewed. Specifically, EFV+FTC+TDF therapy was associated with BMD loss in six articles reporting results from five clinical trials over a follow-up period of 1 year to 2 years and 8 months (Table ). In ACTG A5202, a trial comparing 3TC/abacavir (ABC) to FTC/TDF, each combined with either EFV or ATV/RTV, there was a trend for smaller BMD declines in patients receiving EFV+FTC/TDF than in those receiving ATV/RTV+FTC/TDF for both spine BMD (−2.5% vs. −4.4%, respectively) and hip BMD (−3.7% vs. −4.3%, respectively); however, the study did not test for the statistical significance of these differences. In the combined sub-analysis of ECHO/THRIVE, where patients were treated with FTC/TDF and either EFV or RPV, respective reductions in total body BMD from baseline were similar for both treatment groups at Week 48 (−1.4% vs. −1.5%) and Week 96 (−1.7% vs. −1.8%).Citation61
Table 4 Level 1 blinded and partially blinded RCTs reporting bone outcomes
Declines in BMD on ART can be mitigated by ancillary therapy (calcium and vitamin D supplementation or bisphosphonates). In A5280, the magnitude of reductions in hip BMD at 48 weeks was significantly less in patients treated with EFV+FTC+TDF plus vitamin D/calcium supplementation compared with patients treated with EFV+FTC+TDF plus placebo (−1.5% vs. −3.2%, respectively, p = 0.001), and for lumbar spine BMD at 48 weeks was numerically less (−1.4% vs. −2.9%, respectively; p = 0.085).Citation62 Furthermore, patients who received supplementation had significantly increased vitamin D levels and reduced levels of bone turnover markers at both Weeks 24 and 48 (Table ).
Increases in markers of bone turnover are also universally seen with initiation of ART (Table ). However, treatment-related bone fractures were not described in any of the articles reviewed. Bone fractures were described in ACTG A5202 (n = 80), but the fracture type (i.e. traumatic, pathologic, study drug-related) was not specified (Table ). In ACTG A5224s, a sub-study of A5202, all bone fractures (n = 15, included in the total number for the parent study) were classified as trauma related with no significant differences between treatments arms for the sub-study or parent study.Citation63
Level 2 evidence
Similar to Level 1 studies, use of EFV+FTC+TDF was associated with: BMD loss in all five articles reporting results from three clinical trials; increases in bone turnover markers in three articles reporting results from two clinical trials; and decreased vitamin D levels in two articles reporting results from two clinical trials (Table ). In a small study (n = 30) of patients continuing on EFV+FTC+TDF vs. those switching to RAL+FTC+TDF, decline of BSAP levels at Week 24 was greater in those switched to RAL+FTC/TDF (–12.46 mg/dL) than in patients continuing on EFV+FTC+TDF (–4.92 mg/dL; p < 0.05).Citation64 None of these studies reported the occurrence of fracture.
Table 5 Level 2 open-label studies evaluating bone outcomes
Discussion
Drug–food and drug–drug interactions with TDF-containing regimens leading to significant variations in tenofovir levels have been well documented, which might impact the rates of renal and bone side effects associated with these regimens. However, we note that early long-term studies of up to 5 years in patients treated with EFV+FTC+TDF reported no discontinuations due to renal AEsCitation65 and a low frequency of renal serious AEs or serum creatinine elevations.Citation66 Thus, while systematic reviews have reported on the safety of TDF, this is the first report of a systematic review of the renal and bone safety of the antiretroviral combination of EFV+FTC+TDF. This review not only presents the renal and bone safety data of EFV+FTC+TDF but also compares this to other TDF-containing regimens (that were also non-EFV containing) where data were available. Since its first introduction in 1998, EFV has been extensively used in combination with 3TC/TDF, FTC/TDF, and other nucleoside reverse transcriptase inhibitors. In the US alone, over 550,000 patients have been treated with an STR of EFV/FTC/TDF since its first US FDA approval in July 2006.Citation67 The FDA has granted “tentative approval” status to a number of generic STRs containing either EFV/FTC/TDF (from August 2009) or EFV/3TC/TDF (from September 2009) for purchase and use only as part of the President’s Emergency Plan for AIDS Relief in resource-limited countries.Citation68 The WHO HIV treatment guidelines have considered EFV/3TC (or FTC)/TDF to be a preferred first-line regimen for adults and adolescents with HIV since 2010.Citation69
Across 29 publications that reported renal outcomes in 5185 patients treated with EFV+FTC+TDF with a maximum follow-up period of 5 years, protocol-defined renal AEs (including protocol-defined renal impairments and decreased eGFR) occurred at a low frequency (0.3% in Level 1 studies and 1.2% in Level 2 studies). Across 12 publications that reported bone outcomes in 2154 patients treated with EFV+FTC+TDF with a maximum follow-up period of 2 years and 9 months, despite decreases in BMD and increases in bone turnover markers, no treatment-related fractures were reported. These publications spanned a period of 10 years (2006–2015) and included treatment-naïve as well as experienced patients.
While this systematic review was not designed to make quantitative comparisons, a higher frequency of renal AEs was reported in patients treated with TDF in combination with a PI or with COBI (i.e. EVG/COBI/FTC/TDF). Drug–food interactions as well as PI–TDF and COBI–TDF drug–drug interactions can lead to increases in tenofovir exposures, thus increasing risk of renal AEs. While there is limited evidence for an independent effect of PIs on renal function decline,Citation70 an increased risk of decline in eGFR has been demonstrated when PIs are co-administered with TDF, which remains significant after multivariate adjustment.Citation71,72 COBI also increases tenofovir exposures, which is thought to be due to inhibition of intestinal P-glycoprotein-mediated efflux of TDF.Citation73 This mechanism specific to COBI could potentially explain the higher frequency of renal AEs observed with EVG/COBI/FTC/TDF than with EFV/FTC/TDF.
The association between TDF and BMD loss has been well documented in the literature. A recent quantitative systematic review across multiple different regimens found that TDF-containing regimens were associated with greater declines in hip and lumbar spine BMD than comparator regimens after 48 weeks of treatment.Citation8 In all articles reviewed here, ART was associated with decreases in hip and lumbar spine BMD. BMD losses were comparable between EFV+FTC/TDF and RVP+FTC/TDF,Citation61 and numerically smaller in magnitude with EFV+FTC/TDF than with ATV/RTV+FTC/TDF.Citation63
Recent findings that administration of vitamin D/calcium or zoledronic acid at ART initiation can mitigate ART-associated BMD loss are of interest. ACTG A5280 demonstrated mitigation of EFV+FTC+TDF-associated BMD losses by vitamin D/calcium supplementation at ART initiation and continued over 48 weeks.Citation62 A small proof-of-concept study demonstrated that a single infusion of zoledronic acid at initiation of ATV/RTV+FTC/TDF can prevent ART-induced BMD loss over 48 weeks.Citation74
This systematic review has a number of limitations. First, formal quantitative data synthesis using meta-analytic methods was not performed. Thus, while numeric trends in data might be observed, an overall estimate of effect size and associated significance could not be made. Related to this point, while estimates of the frequency of renal and bone adverse outcomes with EFV+FTC+TDF were as complete as the literature search allowed, frequencies in the comparator arms should be treated with caution as only a small subset of trials involving the respective comparators were selected. Second, the lack of consistency with which AEs were reported across studies limits the generalizability of these findings; different classes of renal AEs were often reported in different trials, and some studies did not provide specific details on renal AEs that led to treatment discontinuation. Third, patient populations were different across studies and likely contributed to the variability of individual study results. For example, the Level 2 studies examining renal outcomes were conducted in both treatment-naïve and experienced patients; thus, preexisting renal disease or previous ART nephrotoxicity may have been more likely in these patients. Fourth, while Level 1 RCT evidence is considered to provide the best clinical information, many of these trials employ restrictive inclusion criteria (e.g. a CrCl above a threshold level) and have limited follow-up periods. Thus, many of the reviewed articles may not have reflected real-world clinical conditions. Long-term observational studies sufficiently powered to detect uncommon safety events are needed to fully characterize the safety of ART regimens in clinical practice.
This systematic review has a number of strengths. To our knowledge, this is the first systematic review to examine renal and bone safety in the context of EFV+FTC+TDF. This is important because TDF is subject to drug–drug and drug–food interactions, which lead to varying tenofovir exposures across differing TDF-containing regimens. Thus, when reviewing the safety profile of TDF-containing regimens, it is important to consider the co-administration context. In addition, we only included studies that were likely to contain the highest quality data—RCTs and observational cohort studies—and specifically excluded studies with lower quality of evidence.
Conclusions
In this systematic review of RCTs and observational studies, EFV+FTC+TDF, which is devoid of relevant tenofovir drug interactions with EFV and FTC and drug–food interactions, was found to be associated with a low frequency of renal AEs and treatment discontinuations due to renal AEs (renal AEs occurred more frequently when TDF was taken with a PI or with COBI). EFV+FTC+TDF was found to be associated with reduced BMD (although reductions were less than with ATV/RTV+FTC/TDF) and increased markers of bone turnover, which have been shown to be mitigated by vitamin D/calcium supplementation. No treatment-related bone fractures were identified.
In summary, the renal and bone safety profile of EFV+FTC+TDF has been well demonstrated in RCTs and appears to be favorable compared to regimens associated with higher tenofovir plasma concentrations such as those containing a boosted PI or COBI.
Notes on contributors
Roger Bedimo is an associate professor of Medicine at the University of Texas Southwestern Medical Center (UTSW) and the chief of Infectious Diseases at the VA North Texas and the president of the national VA Specialty Providers in Infectious Diseases (VASPID). He is a member of the Department of Health and Human Services (DHHS) Antiretroviral Treatment Guidelines committee and the IAS-USA Editorial Board for Cases on the Web. His research interests include the analysis of rates and mechanisms of chronic complications of HIV disease and antiretroviral therapy, as well as the contribution of HCV co-infection on HIV-associated morbidity.
Lisa Rosenblatt received her medical degree from McGill University and her master’s of Public Health in Epidemiology from Columbia University. She has worked in the field of Health Economics and Outcomes Research since 2002. She joined Bristol-Myers Squibb in 2006 and is currently the US HEOR lead for the Virology and Immunology therapeutic areas.
Joel Myers received his master’s of Pharmacy from the University of Bath. He was awarded a postgraduate diploma in Pharmacy Practice from University College London School of Pharmacy and an independent prescribing certificate from King’s College London. He has provided clinical care to patients with HIV and has experience in conducting clinical trials in HIV medicine and other fields. He joined Bristol-Myers Squibb in 2012 and is currently working within the Research and Development organization as the US Medical Lead for HIV.
Declaration of interest
RB has received research grants from Bristol-Myers Squibb and Merck & Co. He has also served on scientific advisory board from Bristol-Myers Squibb, Merck & Co., Theratechnologies, and Gilead Sciences. LR and JM are employees of and own stock in Bristol-Myers Squibb.
Funding
This work was supported by Bristol-Myers Squibb.
Supplementary material
The supplemental data for this article can be accessed here http://dx.doi.org/10.1080/15284336.2016.1243363.
YHCT_1243363_Supplementary_Material.zip
Download Zip (13.6 KB)Acknowledgments
The authors wish to thank Dr. Angelina Villasis-Keever who reviewed and provided feedback on the study protocol and results. Medical writing assistance was provided by Ben Dale and Julian Martins of inScience Communications, Springer Healthcare, which was funded by Bristol-Myers Squibb.
References
- Winston A, Underwood J. Emerging concepts on the use of antiretroviral therapy in older adults living with HIV infection. Curr Opin Infect Dis. 2015;28(1):17–22.10.1097/QCO.0000000000000117
- Ando M, Yanagisawa N. Epidemiology, clinical characteristics, and management of chronic kidney disease in human immunodeficiency virus-infected patients. World J Nephrol. 2015;4(3):388–395.10.5527/wjn.v4.i3.388
- Cazanave C, Dupon M, Lavignolle-Aurillac V, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22(3):395–402.10.1097/QAD.0b013e3282f423dd
- Schooley RT, Ruane P, Myers RA, et al. Tenofovir DF in antiretroviral-experienced patients: results from a 48-week, randomized, double-blind study. AIDS. 2002;16(9):1257–1263.10.1097/00002030-200206140-00008
- Izzedine H, Isnard-Bagnis C, Hulot JS, et al. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS. 2004;18(7):1074–1076.10.1097/00002030-200404300-00019
- Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs. stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201.10.1001/jama.292.2.191
- Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–875.10.1097/QAD.0b013e328351f68f
- Hemkens LG, Ewald H, Santini-Oliveira M, et al. Comparative effectiveness of tenofovir in treatment-naïve HIV-infected patients: systematic review and meta-analysis. HIV Clin Trials. 2015;16(5):178–189.10.1179/1945577115Y.0000000004
- Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta‐analysis: renal safety of tenofovir disoproxil fumarate in HIV‐infected patients. Clin Infect Dis. 2010;51(5):496–505.10.1086/652979
- Winston J, Chonchol M, Gallant J, et al. Discontinuation of Tenofovir disoproxil fumarate for presumed renal adverse events in treatment-naïve HIV-1 patients: meta-analysis of randomized clinical studies. HIV Clin Trials. 2014;15(6):231–245.10.1310/hct1506-231
- Fafin C, Pugliese P, Durant J, et al. Increased time exposure to tenofovir is associated with a greater decrease in estimated glomerular filtration rate in HIV patients with kidney function of less than 60 ml/min/1.73 m. Nephron – Clin Pract. 2012;120(4):c205–c214.
- Laprise C, Baril J-G, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567–575.10.1093/cid/cis937
- Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26(7):825–831.10.1097/QAD.0b013e32835192ae
- Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009;89(5):513–519.10.1038/labinvest.2009.14
- Jafari A, Khalili H, Dashti-Khavidaki S. Tenofovir-induced nephrotoxicity: incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur J Clin Pharmacol. 2014;70(9):1029–1040.10.1007/s00228-014-1712-z
- Gilead Sciences. Prescribing information for VIREAD®. 2015. Available at http://www.gilead.com/~/media/files/pdfs/medicines/hiv/viread/viread_pi.pdf?la=en. Accessed November 4, 2015.
- Bristol Myers Squibb GS. Prescribing information for ATRIPLA®. 2015. Available at http://packageinserts.bms.com/pi/pi_atripla.pdf. Accessed November 4, 2015.
- Gilead Sciences. Prescribing information for COMPLERA®. 2015. https://gilead.com/~/media/files/pdfs/medicines/hiv/complera/complera_pi.pdf. Accessed November 4, 2015.
- Gilead Sciences. Prescribing information for STRIBILD®. 2015. Available at http://www.gilead.com/~/media/files/pdfs/medicines/hiv/stribild/stribild_pi.pdf?la=en. Accessed November 4, 2015.
- Hoetelmans R, Van Heeswijk R, Kestens D. Pharmacokinetic interaction between the novel nonnucleoside reverse transcriptase inhibitor (NNRTI) TMC278 and tenofovir disoproxil fumarate (TDF) in healthy volunteers. 3rd IAS Conference on HIV Pathogenesis, Treatment and Prevention, Brazil, July 2005, abstract WePe3.3C15.
- European Commission Health and Food Safety. Tybost product characteristics. Available at http://ec.europa.eu/health/documents/community-register/2015/20150210131020/anx_131020_en.pdf. Accessed May 2, 2016.
- Droste JA, Kearney BP, Hekster YA, Burger DM. Assessment of drug-drug interactions between tenofovir disoproxil fumarate and the nonnucleoside reverse transcriptase inhibitors nevirapine and efavirenz in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41(1):37–43.10.1097/01.qai.0000191997.70034.80
- Gilead Sciences. Prescribing information for emtricitabine/tenofovir disoproxil fumarate tablets (Truvada®). Available at http://www.gilead.com/~/media/files/pdfs/medicines/hiv/truvada/truvada_pi.pdf. Last updated December, 2013. Accessed November, 2015.
- Jotwani V, Scherzer R, Estrella M, et al. Associations of tenofovir disoproxil fumarate with urine biomarkers of kidney damage. Conference on Retroviruses and Opportunistic Infections (CROI): February 22–25, 2016. Boston, MA, USA2016.
- Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–455.10.1097/QAI.0b013e3182965d45
- Mills A, Crofoot G Jr, McDonald C, Shalit P, Flamm JA, Gathe J Jr, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2015;69(4):439–445.10.1097/QAI.0000000000000618
- Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615.10.1016/S0140-6736(15)60616-X
- Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67(1):52–58.10.1097/QAI.0000000000000225
- Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52.10.1016/S1473-3099(15)00348-5
- Rijnders B, Stephan C, Lazzarin A, et al. Switching from boosted atazanavir plus emtricitabine /tenofovir disoproxil fumarate to a tenofovir alafenamide-based single-tablet regimen: week-48 data in virologically suppressed adults [abstract 577]. 15th European AIDS Conference, October 21–24, 2015 Bacelona, Spain2015.
- Shamblaw D, Lunzen JV, Orkin C, et al. Switching from atripla (ATR) to a tenofovir alafenamide (TAF)-based single tablet regimen: week 48 data in virologically suppressed adults. ICAAC, Septemeber 17–21, 2015. San Diego, CA, USA2015.
- Thompson M, Morales-Ramirez J, McDonald C, et al. Switching from a TDF to a TAF-based single tablet regimen: week 48 data in HIV-1 infected virologically suppressed adults [abstract 725]. IDSA / ID Week, October 7–11, 2015 San Diego, CA, USA2015.
- Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. Version 2.0. Last updated November, 2014. Available at http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_Grading_Table_v2_NOV2014.pdf. Accessed March 16, 2016.
- German P, Liu HC, Szwarcberg J, et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr. 2012;61(1):32–40.10.1097/QAI.0b013e3182645648
- Del Palacio M, Romero S, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 2012;14(3):179–187.
- OCEBM Levels of Evidence Working Group. The Oxford levels of evidence 1: Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=1025. Accessed February 15, 2016.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annal Int Med. 2009;151(4):264–269, W264.10.7326/0003-4819-151-4-200908180-00135
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.10.1136/jech.52.6.377
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain. 1996;66(2):239–246.10.1016/0304-3959(96)03033-3
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412.10.1001/jama.1995.03520290060030
- Rockstroh JK, Lennox JL, DeJesus E, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53(8):807–816.10.1093/cid/cir510
- Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378(9787):238–246.10.1016/S0140-6736(11)60936-7
- Walmsley S, Baumgarten A, Berenguer J, et al. Dolutegravir Plus abacavir/lamivudine for the treatment of HIV-1 Infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr. 2015;70(5):515–519.10.1097/QAI.0000000000000790
- Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63(1):77–85.10.1097/QAI.0b013e31828ace69
- Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818.10.1056/NEJMoa1215541
- Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154(7):445–456.10.7326/0003-4819-154-7-201104050-00316
- Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–2448.10.1016/S0140-6736(12)60917-9
- Zolopa A, Sax PE, DeJesus E, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;63(1):96–100.10.1097/QAI.0b013e318289545c
- Wohl DA, Cohen C, Gallant JE, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65(3):e118–e120.10.1097/QAI.0000000000000057
- Gutierrez F, Fulladosa X, Barril G, Domingo P. Renal tubular transporter-mediated interactions of HIV drugs: implications for patient management. AIDS Rev. 2014;16(4):199–212.
- Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9(8):e1001290.10.1371/journal.pmed.1001290
- Touzard Romo F, Smeaton LM, Campbell TB, et al. Renal and Metabolic toxicities following initiation of HIV-1 Treatment regimen in a diverse, multinational setting: a focused safety analysis of ACTG PEARLS (A5175). HIV Clin Trials. 2014;15(6):246–260.10.1310/hct1506-246
- DeJesus E, Young B, Morales-Ramirez JO, et al. Simplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified antiretroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr. 2009;51(2):163–174.10.1097/QAI.0b013e3181a572cf
- Ouattara E, Danel C, Moh R, Gabillard D, Peytavin G, Konan R, et al. Early upper digestive tract side effects of zidovudine with tenofovir plus emtricitabine in West African adults with high CD4 counts. J Int AIDS Soc. 2013;16:18059.
- Cohen C, Wohl D, Arribas JR, et al. Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected adults. AIDS. 2014;28(7):989–997.10.1097/QAD.0000000000000169
- Pujari S, Dravid A, Gupte N, Joshix K, Bele V. Effectiveness and safety of generic fixed-dose combination of tenofovir/emtricitabine/efavirenz in HIV-1-infected patients in Western India. J Int AIDS Soc. 2008;10(8):196.10.1186/1758-2652-10-8-196
- Arribas JR, Pozniak AL, Gallant JE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47(1):74–78.10.1097/QAI.0b013e31815acab8
- Moyle GJ, Stellbrink HJ, Compston J, et al. 96-Week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antivir Ther. 2013;18(7):905–913.10.3851/IMP2667
- Naggie S, Cooper C, Saag M, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373(8):705–713.10.1056/NEJMoa1501315
- German P, Pang PS, Fang L, Chung D, Mathias A. Drug-drug interaction profile of the fixed-dose combination tablet ledipasvir/sofosbuvir. Presented at the 65th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, November 7–11, 2014. [ Abstract.]
- Nelson MR, Elion RA, Cohen CJ, et al. Rilpivirine versus efavirenz in HIV-1-infected subjects receiving emtricitabine/tenofovir DF: pooled 96-week data from ECHO and THRIVE studies. HIV Clin Trials. 2013;14(3):81–91.10.1310/hct1403-81
- Overton ET, Chan ES, Brown TT, et al. Vitamin D and Calcium attenuate bone loss with antiretroviral therapy initiation: A randomized trial. Ann Intern Med. 2015;162(12):815–824.10.7326/M14-1409
- McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–1801.10.1093/infdis/jir188
- Gupta SK, Mi D, Moe SM, Dubé MP, Liu Z. Effects of switching from efavirenz to raltegravir on endothelial function, bone mineral metabolism, inflammation, and renal function: a randomized, controlled trial. J Acquir Immune Defic Syndr. 2013;64(3):279–283.10.1097/QAI.0b013e3182a97c39
- Lazzarin A, Johnson M, Ribera E, Weitner L, Chen SS, Warren DR. 5-year safety and efficacy of the once-daily antiretroviral regimen of efavirenz (EFV)/emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF). J Int AIDS Soc. 2010;13(Suppl 4):P6.10.1186/1758-2652-13-S4-P6
- Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21(10):1273–1281.10.1097/QAD.0b013e3280b07b33
- Symphony Health Solutions. PatientSource APLD and Source® PHAST prescription monthly, equivalized counts, January 2007–December 2015. http://symphonyhealth.com/product/pharmaceutical-audit-suite-phast/.
- U.S. Food and Drug Administration. Approved and Tentatively Approved Antiretrovirals in Association with the President’s Emergency Plan. http://www.fda.gov/InternationalPrograms/PEPFAR/ucm119231.htm. Accessed July 11 2016.
- World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach - Second edition (2016). http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed October 18 2016.
- Bagnis CI, Stellbrink HJ. Protease inhibitors and renal function in patients with HIV infection: a systematic review. Infect Dis Therapy. 2015;4:15–50.
- Albini L, Cesana BM, Motta D, et al. A randomized, pilot trial to evaluate glomerular filtration rate by creatinine or cystatin C in naive HIV-Infected patients after tenofovir/emtricitabine in combination with atazanavir/ritonavir or efavirenz. J Acquir Immune Defic Syndr. 2012;59(1):18–24.10.1097/QAI.0b013e31823a6124
- Dazo C, Fahey P, Puls R, et al. Small but significant and non-progressive decline in GFR observed in therapy-naïve HIV+ subjects commencing r/ATV, compared to either EFV or ZDV/ABC with TDF/FTC after 48 weeks: a randomized controlled study. Paper #841 presented at the 18th Conference on Retroviruses and Opportunistic Infections (CROI): February 22–March 2, 2011. Boston, MA.
- Lepist EI, Phan TK, Roy A, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56(10):5409–5413.10.1128/AAC.01089-12
- Ofotokun I, Titanji K, Vunnava A, et al. A single dose zoledronic acid prevents antiretroviral-induced bone loss. Presented at the Conference on Retroviruses and Opportunistic Infections, Boston, February 22–25, 2016.
- Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806.10.1016/S0140-6736(09)60918-1
- Lennox JL, DeJesus E, Berger DS, et al. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39–48.10.1097/QAI.0b013e3181da1287
- Sax PE, Tierney C, Collier AC, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204(8):1191–1201.10.1093/infdis/jir505
- Puls R, Amin J, Losso M, et al. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet. 2014;383(9927):1474–1482.
- Carey D, Puls R, Amin J, et al. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis. 2015;15(7):793–802.
- Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3):251–260.10.1056/NEJMoa051871
- Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes – a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43(5):535–540.
- Fisher M, Moyle GJ, Shahmanesh M, et al. A randomized comparative trial of continued zidovudine/lamivudine or replacement with tenofovir disoproxil fumarate/emtricitabine in efavirenz-treated HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2009;51(5):562–568.10.1097/QAI.0b013e3181ae2eb9
- Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55(1):49–57.10.1097/QAI.0b013e3181dd911e
- DeJesus E, Ruane P, McDonald C, et al. Impact of switching virologically suppressed, HIV-1-infected patients from twice-daily fixed-dose zidovudine/lamivudine to once-daily fixed-dose tenofovir disoproxil fumarate/emtricitabine. HIV Clin Trials. 2008;9(2):103–114.10.1310/hct0902-103
- Erlandson KM, Kitch D, Tierney C, et al. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS. 2013;27(13):2069–2079.10.1097/QAD.0b013e328361d25d
- Wohl DA, Orkin C, Doroana M, et al. Change in vitamin D levels and risk of severe vitamin D deficiency over 48 weeks among HIV-1-infected, treatment-naive adults receiving rilpivirine or efavirenz in a Phase III trial (ECHO). Antivir Ther. 2014;19(2):191–200.10.3851/IMP2721
- Tebas P, Kumar P, Hicks C, et al. Greater change in bone turnover markers for efavirenz/emtricitabine/tenofovir disoproxil fumarate versus dolutegravir + abacavir/lamivudine in antiretroviral therapy-naive adults over 144 weeks. AIDS. 2015;29(18):2459–2464.10.1097/QAD.0000000000000863
- Havers FP, Detrick B, Cardoso SW, et al. Change in vitamin D levels occurs early after antiretroviral therapy initiation and depends on treatment regimen in resource-limited settings. PLoS ONE. 2014;9(4):e95164.10.1371/journal.pone.0095164
- Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir‐lamivudine versus tenofovir‐emtricitabine in HIV‐infected adults: 48‐week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972.10.1086/652982
- Grant PM, Kitch D, McComsey GA, Tierney C, Ha B, Brown TT. Differential skeletal impact of tenofovir disoproxil fumarate in young versus old HIV-infected adults. HIV Clin Trials. 2015;16(2):66–71.10.1179/1528433614Z.0000000010
