Abstract
Background: Good efficacy and safety of raltegravir in person living with HIV was demonstrated in clinical trials over five years, but real-life data, particularly about quality of life (QoL), are lacking. QoL was evaluated over time in adult patients first treated or switched to regimens containing raltegravir in an observational cohort study.
Methods: Patient QoL was evaluated using the Fatigue Impact Scale (FIS) and the HIV Symptom Index (HSI). Data were collected at baseline and at 1, 3, 6, 12, 18, and 24 months. Baseline FIS and HSI subscores were compared with the scores at each visit using the paired Wilcoxon test. The impact of time, sociodemographic and medical variables upon patient-perceived fatigue and symptoms was also assessed using mixed multivariate models.
Results: From baseline, all FIS and HSI subscores improved significantly after one month of treatment. In addition, psychosocial FIS subscores and both the frequency of bothersome symptoms and HSI subscores improved significantly at each visit. Physical FIS subscores also improved significantly, except at month 18, whereas both cognitive and total FIS subscores improved only after 6 months and 24 months, respectively. In multivariate analysis, employment was independently associated over time with improved improvement in both FIS and HSI subscores.
Conclusion: Patient QoL improved significantly over a 24-month period of treatment with a raltegravir-containing regimen. FIS and HSI are sensitive tools to measure the impact of new antiretroviral combinations on a patient’s perception of QoL.
Keywords:
Introduction
The integrase inhibitor raltegravir has demonstrated good efficacy in both naïve and treatment-experienced patients with a good tolerability profile, particularly regarding lipid and glucose metabolism.Citation1,2 Moreover, raltegravir exhibits minimal potential for clinically significant drug–drug interactions. (Isentress® label) However, minimal data from real-life settings are available on the quality of life (QoL) of patients treated with antiretroviral combination therapies, including raltegravir.Citation3
Given its safety profile, we hypothesized that patients treated with raltegravir improved health-related QoL despite the need to take raltegravir twice a day.
Few studies are available on fatigue and QoL for the most recent drugs available for HIV treatment. There is a paucity of literature on the impact of antiretroviral therapy (ART) on QoL in HIV-1 infection. Little is known about the effect of ART on QoL, and data demonstrating QoL improvement on ART are limited.Citation4–6
Fatigue is an extremely common symptom in HIV-infected patients. From reviews of 42 studies, Jong et al. estimated that the rate of fatigue varied from 33 to 88% in HIV-1-infected patients,Citation7 and this rate increases as the disease progresses.Citation8 Fatigue is one of the most disabling symptoms and a major factor affecting QoL and the physical, mental, and socio-professional status of patients.Citation9–11 QoL in HIV-infected patients is also compromised by the symptoms resulting from HIV infection itself and the treatment administered to fight it. The impact of fatigue, HIV, and HIV treatment-related symptoms on QoL in HIV-1-infected patients has been demonstrated and evaluated by numerous researchers.Citation10–12 These variables should be of specific importance to maximize patient QoL to potentially enhance adherence to treatment.
One approach to measure QoL is the use of generic health questionnaires that measure physical, social, and mental dimensions of health, and most studies or prospective cohort studies assessing the QoL of patients infected with HIV based on general or overall self-reports use SF-36. However, generic health questionnaires are less effective in demonstrating significant changes in the QoL of HIV-infected patients than measures of the frequency and discomfort of symptoms experienced by patients. These measurements are not sufficiently sensitive to changes in therapy because they are influenced by socioeconomic variables that dilute clinical and symptomatic indicators.Citation13 Symptoms score are more sensitive to changes in regimen switches than QoL scores assessed by generic QoL scales.Citation14 Thus, we utilized a scale of symptoms (HIV Symptom Index [HSI]) and an assessment of fatigue (Fatigue Impact Scale [FIS]).
Methods
Patients and questionnaires
Over two years, QoL was assessed for 221 patients from the RACING cohort.Citation15 Only patients who completed and returned their questionnaire were included in the study.
All patients provided consent for inclusion in the observational studies. Informed consent was approved by the local institutional ethics committees.
The purpose of the QoL analysis was to evaluate the impact of treatment on patient QoL throughout the study using two self-administered questionnaires, the FIS and the HSI, and determine the possible associations between patient demographic, clinical and sociological factors, and the positive or negative impact of fatigue and symptoms related to HIV infection, and its treatment in an HIV-infected population. Patients who were ART-naïve were eligible for the QoL analysis if their self-report questionnaires were fully completed.
The patients’ demographic and clinical data at baseline were retrieved by the investigators from patients’ medical records and included patient age, gender, body mass index, time since HIV diagnosis, types of transmission, HIV subtype and HIV infection CDC (Center for Disease Control and Prevention) classification, presence or absence of lipodystrophies and proliferative disease, medical history of cardiovascular disease and or cardiovascular risk factors, and alcohol consumption.
Data about professional status, education level, marital status, relationship status, the patient’s main partner, type of housing (owned, rented, etc.), housing comfort, and place of birth were collected in a sociodemographic questionnaire completed by the patient at baseline (Appendix A).
To measure the patients’ QoL outcomes, the FIS and HSI were completed by the patients themselves at baseline (M0) and then at months 1 (M1), 3 (M3), 6 (M6), 12 (M12), 18 (M18), and 24 (M24). The questionnaires were administered to the patients by the investigator at each visit. Patients were encouraged to complete and return the questionnaires.
Self-administered questionnaires
HIV Symptom Index (Appendix B)
This questionnaire was validated by Justice et al. and translated to French. This questionnaire measures the patients’ perception of symptoms associated with HIV infection and side effects resulting from treatment using three subscores to evaluate the frequency of perceived symptoms and the bother associated with these symptoms ranging from 0 to 22 and a global score ranging from 0 to 88. Higher values indicate more bothersome symptoms.Citation16
Fatigue Impact Scale (Appendix C)
The fatigue perceived by patients was assessed throughout the course of the study using the FIS, which was validated by Fisck et al.. The FIS was developed to understand the effects of fatigue on QoL. The FIS is a measure of patients’ attribution of functional limitations to symptoms of fatigue. The FIS provides an assessment of the effects of fatigue in terms of physical, cognitive, and psychosocial functioning, and focuses on the ways in which fatigue affects everyday life. The FIS is a 40-item generic fatigue impact scale aggregated into three subscales of cognitive (10 items), physical (10 items), and psychosocial (20 items) fatigue. Each subscale is scored from 0 (no problem) to 4 (extreme problem), providing a continuous scale of 0–160. The sum of the subscales provides the total fatigue impact. Higher scores indicate a greater impact of fatigue on activity.Citation17 The score reflects patients’ perceptions of the functional limitations that fatigue has caused over the past month.
Cognitive function involves concentration, memory, thinking, and organization of thoughts. Physical function reflects motivation, effort, stamina, and coordination. Psychosocial function describes the impact of fatigue on isolation, emotions, workload, and coping.
Statistical methods
The baseline main characteristics of patients who responded to at least one questionnaire were tabulated and examined for imbalances compared with those patients who never responded to the self-administered questionnaires using Chi2, Wilcoxon and Fisher tests. The intervals from the first assessment for both self-administered questionnaires were 1, 3, 6, 12, 18, and 24 months from assessment 2 to 7.
The average scores were calculated for each time point to illustrate the changes in FIS and HSI scores immediately after starting raltegravir (Isentress®) in combination with other antiretroviral drugs and their subsequent evolution over the 24-month observational period.
The changes in the average FIS and HSI scores from baseline to month 24 (intra-group) were measured and analyzed using the paired Wilcoxon test, and a p-value of 0.05 or less was considered statistically significant. This test was performed on the scores from patients who returned their questionnaires for at least one time point.
To assess the impact of time on patient-perceived fatigue and symptoms, we used mixed multivariate models to perform a repeated-measures analysis over a sequence of time points (inclusion and months 1, 3, 6, 12, 18, and 24). Twenty independent covariates including patient sociodemographic and clinical data were selected and added to the model to identify independent factors associated with QoL.
In the univariate models, factors with a p-value of 0.25 or less were retained for multivariate model analysis; characteristics with a p-value of 0.05 or less were considered statistically significant in multivariate analysis. Confidence intervals of 95% were applied for all measures.
Patients who did not complete the self-administered questionnaire at enrollment and those who completed the questionnaire at the first visit while receiving raltegravir were excluded from the analysis.
The analysis was performed in patients who returned their questionnaires at baseline and at least one follow-up time point regardless of whether they were still taking raltegravir (Isentress®) at month 24. Statistical analyses were performed using SAS version 9.2 (Copyright© 2002–2008 by SAS Institute Inc., Cary, NC, USA).
Results
Among the 480 patients included in the RACING study, 221 raltegravir-naïve patients returned completed FIS and HSI questionnaires at baseline, respectively. These numbers dropped to 136 and 135 at month 6 (M6) and to 99 and 97 at M24, respectively (Table ).
Table 1 Baseline sociodemographic and clinical characteristics of patients
There were no notable differences in sex, VL and CD4 count between patients who responded to at least one questionnaire and patients who never responded to the self-administered questionnaires: sex, VL and CD4 count, but patients who responded to at least one questionnaire were older (data not shown).
Efficacy and safety
Virological response was defined as a VL < 50 copies/mL at M24. At baseline, patients were classified into three categories: pre-treated patients with suppressed VL to <50 copies/mL; virological failure with VL > 50 copies/mL and ART-naïve (Table ).
Table 2 Virological and immunological assessment at M24 (number of “responders*” (<50 copies/mL) and change in CD4 cell counts (cells/mm3) after 24 months according to patient inclusion profile
Changes in baseline Fatigue Impact Scale scores over time
As shown in Figure , the longitudinal averages for the total FIS score and the three FIS subscores improved over time.
Figure 1. Evolution of FIS subscores at each time point.
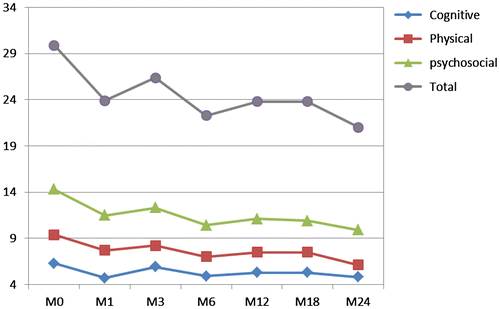
The FIS subscores at each time point were compared with the baseline values to assess statistical significance. Changes were statistically significant at each time point (except at M24 for cognitive dimension). As shown in Table , at M6, all FIS subscores improved significantly compared with the baseline scores [global score (p < 0.001), psychological subscore (p < 0.001), physical subscore (p < 0.001), and cognitive subscore (p = 0.006)]. Between M0 and M24, significant improvements were observed in the FIS psychological (p = 0.011) and physical subscores (p = 0.012). The FIS global score also improved significantly between M0 and M24 (p = 0.012).
Table 3 FIS subscores changes from inclusion to each time point
The cognitive subscore improved significantly from baseline to month 1. The mean change at M1 was statistically significant compared with baseline (p < 0.002), but the mean change at M24 narrowly missed statistical significance compared with baseline (p = 0.622). This change remained stable from M6 to M24.
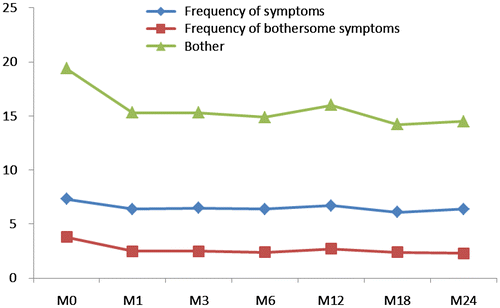
Changes in baseline HIV Symptom Index scores over time
HSI subscores at each time point were compared with baseline for statistical significance. Changes in HSI subscores over time (average values of individual changes) are illustrated in Table . Changes achieved statistical significance at each time points as compared to baseline subscores. A significant improvement in both frequency of bothersome symptoms and bother subscores was observed at M6 (p < 0.001 for both scores) and M24 (p = 0.005 and p = 0.001, respectively). There was no difference in the frequency of symptoms between baseline (M0) and M6 (p = 0.092) and M0 and M24 (p = 0.252). Frequency of symptom subscore improved significantly at month 1 (p = 0.035), month 3 (p = 0.050) and month 18 (p = 0.014). The values did not reach statistical significance at the other times points but remained stable (Figure ).
Table 4 HIS subscores changes from inclusion to each time point
Univariate and multivariate analyses of Fatigue Impact Scale subscales
In univariate analysis, covariates such as patient sex, marital status, living in a couple, main partner, school diploma, housing types, level of housing comfort, stable and comfortable housing, HIV CDC classification, history of cardiovascular disease or risk factors, and alcohol consumption were not associated with any fatigue subscores.
Over time, reporting a high frequency of bothersome symptoms became strongly negatively associated with cognitive, physical and psychosocial FIS dimensions (p < 0.0001, p < 0.0001, and p < 0.0001, respectively). Similarly, the length of time since HIV-seropositive diagnosis (p = 0.0111, p = 0.0013, and p = 0.0151, respectively), mode of transmission (p = 0.0037, p < 0.0001, and p = 0.0003, respectively) and place of birth (p = 0.0037, p = 0.0020, and p = 0.0114, respectively) were also significantly associated with high subscores in the three FIS dimensions. Transmission by blood transfusion, accidental blood exposure or injection drug use had a higher impact on the three FIS subscores than other modes of transmission. Being born in France compared with being born abroad was associated with higher cognitive, physical, and psychosocial FIS subscores. Lower (better) cognitive, physical, and psychosocial FIS subscores were significantly associated with employment and having current or past occupational activity compared with no professional activity (p = 0.0003, p < 0.0001, and p < 0.0001, respectively) and non-B HIV subtype (p = 0.0119, p = 0.0007, and p = 0.0017, respectively). Body mass index was significantly associated with low cognitive (p = 0.0375) and psychosocial (p = 0.0196) FIS subscores. Older age (p = 0.005) and lipodystrophies (p = 0.0078) were associated with reduced improvement in physical FIS subscores, whereas tumoral pathologies were associated with worse psychosocial FIS subscores (p = 0.0295).
Variables related to cognitive, physical, and psychosocial FIS subscores with p ≤ 0.25 included patient age, body mass index, professional status, place of birth, time since HIV diagnosis of seropositivity, mode of transmission, lipodystrophies, frequency of bothersome symptom score, and tumoral pathologies, whereas HIV subtype was related to cognitive and psychosocial FIS subscores. The variables related to psychosocial FIS subscores included marital status, living as a couple, presence of a main partner, and stable and comfortable housing. The variables associated with physical FIS subscores included cardiovascular disease and/or cardiovascular risk factors. All variables identified as possible predictors were retained in the multivariate mixed model. Multivariate analysis revealed an independent positive association over time between employment (having past or current professional activity) and improvement in all three FIS subscores (p = 0.0499, p = 0.0032, and p = 0.0180, respectively). By contrast, a high score for frequency of bothersome symptoms was independently associated with higher (worse) cognitive, physical, and psychosocial FIS subscores (p < 0.0001, p < 0.0001, and p < 0.0001, respectively).
Three covariates were independently associated with one of the three FIS dimensions. Older age was independently associated with worse physical subscores (p = 0.0056), whereas being born in France was independently associated with worse psychosocial subscores (p = 0.0225). By contrast, having stable and comfortable housing was significantly associated with improved psychosocial subscores (p = 0.0208).
The multivariate analysis indicated that time was also significantly associated with improved physical and psychosocial FIS subscores (p = 0.0111 and p = 0.018, respectively), particularly at M6 compared with M24 (coefficient: –1.3273 [CI: –2.527;0.127] and coefficient: –2.491 [CI: –4.411;0.447], respectively) (Table ). Of the factors considered, the psychological dimension of fatigue exhibited the greatest impact in both univariate and multivariate analysis.
Table 5 Univariate and multivariate analysis of FIS subscores (only significant variables)
Univariate and multivariate analyses of HIV Symptom Index subscales
For the HSI questionnaire, univariate analysis over time revealed that low (better) subscores of frequency of bothersome symptoms and bother across time points were significantly associated with male gender (p = 0.0238 and p = 0.0208, respectively), employment (p = 0.0003 and p = 0.0006, respectively), and absence of lipodystrophies (p = 0.0234 and p = 0.0352, respectively), whereas length of time since HIV-seropositive diagnosis (p = 0.0039 and p = 0.0048, respectively) and blood transfusion or accidental blood exposure as the mode of transmission (p = 0.0003 and p = 0.0033, respectively) were both significantly associated with worse scores in both frequency of bothersome symptoms and bother. Non-B HIV subtype was significantly and exclusively associated with a low (better) score for frequency of bothersome symptoms.
Patient age, body mass index, HIV CDC classification, presence of tumoral pathologies, history of cardiovascular disease, presence of cardiovascular risk factors, alcohol consumption, school diploma or other, place of birth, marital status, living in a couple, presence a main partner, housing types, level of housing comfort, and stable and comfortable lodgings did not reach statistical significance regarding the two HSI subscores for frequency of bothersome symptoms and bother.
Patient sex, body mass index, length of time since HIV-seropositive diagnosis, mode of transmission, lipodystrophy, tumoral pathologies, history of cardiovascular disease and/or cardiovascular risk factors, school diploma, professional status, and place of birth exhibited a significance level of ≤0.25 for the subscores for both frequency of bothersome symptoms and bother and were eligible for the multivariate model.
The multivariate analysis indicated that both employment (having a past or current professional activity) and absence of lipodystrophy were independently significantly associated with greater improvement in both HSI subscores of frequency of bothersome symptoms (p = 0.0297 and p = 0.0175, respectively) and bother (p = 0.0177 and p = 0.0093, respectively) over time, whereas the mode of transmission was independently significantly associated with less improvement in both frequency of bothersome symptoms (p = 0.0268) and bother (p = 0.0297) subscores. Time since diagnosis was significantly associated with lower (better) scores of both frequency of bothersome symptoms (p = 0.0001) and bother (p = 0.0101), with the lowest subscores noted at M3 compared with M24 (Table ). Bother exhibited the highest scores for the covariates considered in both univariate and multivariate models.
Table 6 Univariate and multivariate analysis for HSI subscores (only significant variables)
Employment (having a past or current professional activity) and time since diagnosis were independently significantly associated with increased improvement in all dimensions of both FIS and HSI subscores and treatment benefit on QoL over follow-up periods in patients with HIV infection.
Discussion
In this study of patients receiving a raltegravir-containing regimen (pretreatment-naïve patients), improvements in fatigue (cognitive, physical and psychosocial) and perceived symptoms (frequency of bothersome symptoms and bother HIS subscores) were observed. These findings reflect benefits for QoL, as demonstrated by the low FIS and HIS subscores achieved over time, and support previous studies that have reported less fatigueCitation17 and reduced symptom burdenCitation11,18 with ART use using the same scales. In one study, worse FIS scores were independently associated with impaired physical QoL and social relationships in patients co-infected with HIV and hepatitis C virus.Citation12 Some studies have similarly reported lower fatigueCitation19 and reduced HIV symptomsCitation4 in HIV-infected patients receiving ART and demonstrated that ART use improved QoL among HIV-infected patients;Citation5,20 however, these results are not directly comparable to the present study due to differences in the instruments used to measure the fatigue and symptoms caused by treatment and the study populations. By contrast, Michel et al. observed that efavirenz treatment was negatively associated with the three dimensions of the impact of fatigue,Citation11 emphasizing the importance of the availability of potent combinations of antiretroviral drugs that offer long-term efficacy and a good tolerance profile.
The RACING study is unique because it is the first real-world study to assess the QoL of patients receiving raltegravir in combination with other antiretroviral agents. The RACING study is also the first study to use two self-administered questionnaires to evaluate QoL in an HIV-infected population. The FIS and HSI instruments were used in this study to quantify QoL based on the responses of patients regarding their fatigue status and symptom burden, two factors that are considered major components of patient-perceived QoL. Patient-perceived QoL more closely resembles real evaluations.
In addition, independent associations were observed between some patient sociodemographic and clinical characteristics and FIS and HSI outcomes in the present study. Low subscores for the frequency of bothersome symptoms were positively associated with the three FIS dimensions. These findings suggest that switching to raltegravir in combination with other ARTs reduces the impact of fatigue upon cognitive, physical, and psychosocial functioning by improving the frequency of bothersome symptoms. Similarly, Michel et al. reported that the number of self-reported side effects causing discomfort contributes significantly to an increased impact of fatigue on all dimensions of patient function. In one study, self-reported side effects were negative predictors of both Physical and Mental Component Summary scores.Citation11 Preau et al. observed that the absence of experience of AIDS-defining events and difficulties as a result of adverse HIV-treatment reactions were associated with improved physical health-related QoL.Citation21
Employment was significantly associated with a positive impact on the three FIS subscores and two HSI subscores, with the highest impact on the FIS subscore and HSI bother subscore. A number of studies have demonstrated that employment is a strong predictor of better QoL.Citation5,21,22 In their review of the literature, Jong et al. noted an increase in fatigue among HIV-infected patients who are unemployed.Citation7 Pence et al. reported that employment was a predictor of overall lower fatigue scores and was also associated with fatigue remission.Citation23 Working was associated with increased QoL and better adherence to treatment.Citation24
Older age was negatively associated with physical FIS subscores in this study. This finding is in contrast to results reported by Jong et al., who observed no association between age and fatigue in most studies included in their review of the literature.Citation7 Stable and comfortable housing was positively associated with psychosocial FIS subscores, consistent with the observations of Rourke et al.Citation22 By contrast, being born in France was negatively associated with psychosocial FIS subscores compared with being born abroad.
The absence of lipodystrophy was associated with lower HSI subscores for frequency of bothersome symptoms and bother. This finding is consistent with previous work indicating high symptom burden in the patients with lipodystrophy.Citation25
Mode of transmission, particularly blood transfusion or accidental blood exposure, was strongly associated with higher HSI subscores for frequency of bothersome symptoms and bother. Liu et al.Citation26 determined that the route of infection (non-commercial blood donation) was a risk factor related to fatigue in AIDS patients with antiretroviral drug adverse reactions. In the present study, the mode of transmission was not reported as an independent factor related to fatigue, and no association was observed between CDC classifications A, B, or C and fatigue scores by Jong et al.Citation7
The study findings highlight the importance of perceived fatigue and bothersome symptoms in the evaluation and treatment of patients with HIV infection; the contribution of sociodemographic and clinical factors, such as age, unemployment, housing conditions, and the presence of lipodystrophy, to the level of fatigue; the negative impact of fatigue on cognitive, physical, and psychosocial functioning; and the patient’s perception of the bother caused by symptoms related to HIV infection and its treatment. Fatigue in HIV-infected patients has a high prevalenceCitation7 and a negative impact on medication adherence.Citation27 In addition, fatigue interferes with work,Citation9 tends to persist in the absence of intervention and does not resolve on its own.Citation9,18
The prognosis of HIV infection has been transformed by the advent of highly active ARTs with more appropriate treatment protocols and early treatment. Potent ARTs have transformed HIV infection into a chronic disease, and marked improvements in patient morbidity and mortality have been noted. These therapies have extended the lives of patients living with HIV. However, ART is associated with possible co-morbidities and adverse effects.Citation28 Therefore, QoL has emerged as a major consideration in the management of these patients due to their increasing survival rate and lifetime duration of treatment.Citation29 HIV-related fatigue and symptoms and HIV treatment side effects are factors that contribute to reduced QoL in HIV-1-infected patients. To improve QoL in this population, it is important to address such factors.
The limitations of this study are the method of recruitment, which may have introduced selection bias, the lack of a randomly selected sample, and the lack of a sample of healthy controls. The support for the data was patient self-perception, and the self-administered questionnaire was returned at the discretion of the patients, who were more likely to return the questionnaire if they were fatigued. Thus, the patient sample may not be representative, potentially limiting the generalization of these data to other patient groups. However, among this population, independent variables that exhibit improvement from baseline upon switching to raltegravir can be identified.
Conclusion
In this first QoL study of patients treated with raltegravir in a real-life setting in France, the impact of fatigue on cognitive, psychosocial, and physical functioning, as well as the frequency of bothersome symptoms and bother caused by symptoms, were significantly reduced one month after starting raltegravir (Isentress®) in combination with other ARTs. Notably, the improvement of psychosocial and physical functioning was maintained up to month 24. Integrating the assessment of fatigue and symptoms related to HIV infection and HIV treatment may play an important role in guiding choices for ART combination therapy and benefit QoL in HIV-infected patients. FIS and HSI can be considered reliable and validated self-reported questionnaires for outcome measures for QoL clinical follow-up in patients with HIV infection and in future trials.
Adherence to ethics and reporting requirements
This study was an observational survey; it was conducted in compliance with the Declaration of Helsinki and all Good Clinical Practice Guidelines established by the International Conference on Harmonisation. The final protocol, amendments, and informed consent documentation were reviewed and approved by the Institutional Review Board, CNIL (National Commission on Informatics and Liberties) and CCTIRS (Advisory Committee on the treatment of research in the health field information). All subjects provide written informed consent.
Conflicts of interest and financial support
The RACING study was conducted by MSD France. Lella Nait-Ighil was the Project Medical Director and works as MSL for MSD France. Others authors are members of the Scientific Committee for study.
Device status and clinical trials registration number
The RACING study is completed and the Clinical Trials.gov identifier is: NCT01048671.
contributors
BS and LNI wrote the manuscript and designed the analysis plan. PP, IPM, VJ AGM edited the manuscript and gave their input for the analysis plan. EB is the principal investigator (PI) of the clinical study and was associated to the design of the whole RACING study.
Acknowledgments
The authors would like to thank all patients and physicians in clinical sites. They also thank ASCOPHARM team and Kevin Perez who worked on this study.
References
- Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–354.10.1056/NEJMoa0708975
- Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients. J Acquir Immune Defic Syndr. 2013;63(1):77–85.10.1097/QAI.0b013e31828ace69
- Boulet T1, Pavie J, Charreau I, et al. Impact on health-related quality of life of a switch from enfuvirtide to raltegravir among multidrug-resistant HIV-1-infected patients: a randomized open-label trial (EASIER-ANRS 138) HIV Clin Trials. 2010;11(5):283–29310.1310/hct1105-283
- Cohen C, Revicki DA, Nabulsi A, Sarocco PW, Jiang P. A randomized trial of the effect of ritonavir in maintaining quality of life in advanced HIV disease. AIDS. 1998 20;12(12):1495–1502.10.1097/00002030-199812000-00012
- Nglazi MD, West SJ, Dave JA, Levitt NS, Lambert EV. Quality of life in individuals living with HIV/AIDS attending a public sector antiretroviral service in Cape Town, South Africa. BMC Public Health. 2014;14:676. doi: 10.1186/1471-2458-14-676
- Nieuwkerk PT, Gisolf EH, Colebunders R, Wu AW, Danner SA, Sprangers MA. Quality of life in asymptomatic- and symptomatic HIV infected patients in a trial of ritonavir/saquinavir therapy. The Prometheus Study Group. AIDS. 2000 Jan 28;14(2):181–187.
- Jong E, Oudhoff LA, Epskamp C, et al. Predictors and treatment strategies of HIV-related fatigue in the combined antiretroviral therapy era. AIDS. 2010;24(10):1387–1405.10.1097/QAD.0b013e328339d004
- Adinolfi A. Assessment and treatment of HIV-related fatigue. J Assoc Nurses AIDS Care. 2001;12(Suppl):29–34; quiz 5–8.
- Barroso J, Harmon JL, Madison JL, Pence BW. Intensity, chronicity, circumstances, and consequences of HIV-related fatigue: a longitudinal study. Clin Nurs Res. 2014;23(5):514–528.10.1177/1054773813492998
- Barroso J, Voss JG. Fatigue in HIV and AIDS: an analysis of evidence. J Assoc Nurses AIDS Care. 2013;24(1):S5–S14.10.1016/j.jana.2012.07.003
- Michel L, Villes V, Dabis F, et al. Role of treatment for depressive symptoms in relieving the impact of fatigue in HIV-HCV co-infected patients: ANRS Co13 Hepavih, France, 2006–2008. J Viral Hepat. 2010;17(9):650–660.
- Marcellin F, Préau M, Ravaux I, Dellamonica P, Spire B, Carrieri MP. Self-reported fatigue and depressive symptoms as main indicators of the quality of life (QOL) of patients living with HIV and Hepatitis C: implications for clinical management and future research. HIV Clin Trials. 2007;8(5):320–32710.1310/hct0805-320
- Protopopescu C1, Marcellin F, Spire B, et al. Health-related quality of life in HIV-1-infected patients on HAART: a five-years longitudinal analysis accounting for dropout in the APROCO-COPILOTE cohort (ANRS CO-8) Qual Life Res. 2007;16(4):577–59110.1007/s11136-006-9151-7
- Spire B, Marcellin F, Cohen-Codar I, et al. Effect of lopinavir/ritonavir monotherapy on quality of life and self-reported symptoms among antiretroviral-naive patients: results of the MONARK trial. Antivir Ther. 2008;13(4):591–599.
- Pugliese P, Billaud E, Poizot-Martin I, et al. Real life evaluation of HIV-1 infected patients treated with an ARV combination therapy including raltegravir in France the final RACING study results (M24) 14th European AIDS Conference, Brussels, Oct 2013.
- Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(12):S77–S90.10.1016/S0895-4356(01)00449-8
- Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(Suppl 1):S79–S83.10.1093/clinids/18.Supplement_1.S79
- Pence BW, Barroso J, Harmon JL, Leserman J, Salahuddin N, Hammill BG. Chronicity and remission of fatigue in patients with established HIV infection. AIDS Patient Care STDs. 2009;23(4):239–244.10.1089/apc.2008.0175
- Edelman EJ, Gordon K, Rodriguez-Barradas MC, Justice AC. Patient-Reported symptoms on the antiretroviral regimen efavirenz/emtricitabine/tenofovir. AIDS Patient Care STDs. 2012;26(6):312–319.10.1089/apc.2012.0044
- Voss JG. Predictors and correlates of fatigue in HIV/AIDS. J Pain Symptom Manage. 2005;29(2):173–184.10.1016/j.jpainsymman.2004.05.006
- Préau M, Marcellin F, Carrieri MP, Lert F, Obadia Y, Spire B. Health-related quality of life in French people living with HIV in 2003: results from the national ANRS-EN12-VESPA Study. AIDS. 2007;21(Suppl 1):S19–S27.10.1097/01.aids.0000255081.24105.d7
- Rourke SB, Bekele T, Tucker R, et al. Housing characteristics and their influence on health-related quality of life in persons living with HIV in Ontario, Canada: results from the positive spaces, healthy places study. AIDS Behav. 2012;16(8):2361–2373.10.1007/s10461-012-0284-0
- Louwagie GM, Bachmann MO, Meyer K, Booysen Fle R, Fairall LR, Heunis C. Highly active antiretroviral treatment and health related quality of life in South African adults with human immunodeficiency virus infection: a cross-sectional analytical study. BMC Public Health. 2007;7:682.10.1186/1471-2458-7-244
- Bader A, Kremer H, Erlich-Trungenberger I, et al. An adherence typology: coping, quality of life, and physical symptoms of people living with HIV/AIDS and their adherence to antiretroviral treatment. Med Sci Monit. 2006;12(12):CR493–500.
- Guaraldi G, Murri R, Orlando G, et al. Lipodystrophy and quality of life of HIV-infected persons. AIDS Rev. 2008;10(3):152–161.
- Liu Z, Yang J, Liu H, Jin Y. Factors associated with fatigue in acquired immunodeficiency syndrome patients with antiretroviral drug adverse reactions: a retrospective study. J Tradit Chin Med. 2013;33(3):316–321.10.1016/S0254-6272(13)60172-7
- Gay C, Portillo CJ, Kelly R, et al. Self-reported medication adherence and symptom experience in adults With HIV. J Assoc Nurses AIDS Care. 2011;22(4):257–268.10.1016/j.jana.2010.11.004
- Battegay M, Elzi L. Morbidity and mortality in HIV-infected individuals – a shift towards comorbidities. Swiss Med Wkly. 2009;139(39–40):564–570.
- Protopopescu C, Marcellin F, Spire B, et al. Health-related quality of life in HIV-1-infected patients on HAART: a five-years longitudinal analysis accounting for dropout in the APROCO-COPILOTE cohort (ANRS CO-8). Qual Life Res. 2007;16(4):577–591.10.1007/s11136-006-9151-7
Appendix A. Sociodemographic Questionnaire
Appendix B. HIV Symptom Index (HSI)
Self-completed HIV symptom index
Symptoms Distress Module
Please answer the following questions by placing a “x” in the appropriate box.
The following questions ask about symptoms you might have had during the past four weeks. Please check the box that describes how much you have been bothered by each symptom (one box per line)
Thank you very much for completing questionnaire
Appendix C. Fatigue Impact Scale (FIS)
Your fatigue
Instructions: The following questions refer to problems you encounter in your daily life because of your fatigue. Please circle the answer that best suits what you felt during the past four (4) weeks. If a question makes you hesitate, try to answer as best you can.