Abstract
Background: Antiretroviral therapy (ART) regimens for HIV infection are frequently changed. We conducted a systematic review of randomized trials (RCTs) on the benefits and harms of switching to tenofovir disoproxil fumarate (TDF)-based regimens in ART-experienced patients.
Methods: We included RCTs in HIV-infected adults comparing switching to a TDF-containing regimen with maintaining or switching to another regimen. We searched MEDLINE, EMBASE, CENTRAL, LILACS, SCI, and the WHO Global Health Library. We assessed bias with the Cochrane tool and synthesized data using random-effects meta-analyses and Peto’s approach. For further analyses, we added data from a previous systematic review in treatment-naïve patients.
Results: 17 RCTs with 2210 patients were included. All but one study had a high risk of bias. There was no significant association of switching to TDF-based regimens with mortality, fractures, CD4-cell count, body fat, virological failure, LDL-, and HDL-cholesterol. TDF-based regimens decreased total cholesterol (mean difference −12.05 mg/dL; 95% CI −20.76 to −3.34), trigylcerides (−14.33 mg/dL; −23.73 to −4.93), and bone mineral density (BMD; hip: −2.46%; −3.9 to −1.03; lumbar spine −1.52%; −2.69 to −0.34). Effects on estimated glomerular filtration (eGFR) were inconsistent and depended on the measurement. Adding 22 RCTs from 8297 treatment-naïve patients gave consistent results with then significant reductions of LDL (−7.57 mg/dL; −10.37 to −4.78), HDL (−2.38 mg/dL; −3.83 to −0.93), and eGFR (−3.49 ml/min; −5.56 to −1.43).
Conclusions: Switching to TDF-based regimens is associated with reductions of BMD and lipid levels and possibly lowered kidney function. The evidence is limited by the high risk of bias.
Background
Tenofovir disoproxil fumarate (TDF) is a recommended first-line drug for antiretroviral therapy (ART) in HIVCitation1–3 and is frequently used as fixed dose co-formulation containing emtricitabine (TDF/FTC).
In around 40% of HIV-infected adults in Europe and North America, the first line ART regimen is modified within the first 28 months.Citation4 Reasons include side effects, the wish for treatment simplification, patient’s preferences, and virological, immunological, or clinical failure.Citation1,4 Although TDF is widely used as second-line treatment, the randomized trial evidence on efficacy and safety in patients switching to TDF-based regimens has not yet been systematically reviewed. Therefore, our primary objective was to provide a consistent overview of the available randomized trial evidence on benefits and harms of switching to TDF-based treatments. Our secondary goal was to evaluate if treatment effects of TDF differed when only the most commonly used fixed-dose regimens were compared (TDF/FTC and ABC/3TC). Finally, using data from a previous systematic review in ART-naïve patients,Citation5 we aimed to explore if effects are different between ART-naïve and ART-experienced patients. By keeping a wide perspective with broad selection criteria, we aimed to analyze a large body of clinical trial evidence to assess the impact of diverse clinical settings, patient characteristics, or methodological factors on the comparative effectiveness of TDF treatment.Citation6
Methods
We applied widely identical methods as in our previous systematic review and meta-analysis in ART-naïve patients.Citation5
Inclusion criteria
We included RCTs in HIV-infected adults who had received ART not containing TDF (i.e. ART-experienced, TDF-naïve patients). Eligible RCTs compared (1) switching to a TDF-containing regimen with (2) maintaining current or switching to another non-TDF-containing regimen. No other eligibility criteria were applied.
Identification of evidence
We systematically searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, Science Citation Index, and the WHO Global Health Library with the support of an information specialist (last search 01/2015; see Webappendix for details). In addition, we screened clinicaltrials.gov (in 04/2013) and conference proceedings of major international HIV meetings.Citation7–11 We checked clinicaltrials.gov entries and reference lists of included studies for further pertinent publications (in 06/2015). We applied no language restrictions. For all included studies, we systematically sought additional published information on clinicaltrials.gov and systematically contacted authors when analytical details were unclear or missing.
Two reviewers independently screened titles, abstracts, and trial registries. One reviewer screened conference proceedings and reference lists. We obtained any full text that either reviewer deemed to be a potentially eligible article, and two independent reviewers determined their eligibility. Discrepancies were resolved by discussion or with a third reviewer. Agreement between the first two reviewers was measured using a kappa statistic.Citation12
Data extraction and study outcomes
We extracted information on characteristics of the study, patients, interventions, and on the predefined outcomes mortality, AIDS-defining events, virological failure, fractures, cardiovascular events, renal failure, rash, quality of life, CD4-cell count, HDL-, LDL-, total cholesterol, triglycerides, estimated glomerular filtration rate (eGFR), proteinuria, bone mineral density (BMD), and body fat change. For the outcomes death and clinical events, we extracted the latest time-point at which at least 80% of the randomized patients were analyzed to limit impact of attrition bias. For the outcomes CD4-cell count, lipids, eGFR, body fat, BMD, quality of life, and virological failure, we used 48-week results reflecting mid-term effects on outcomes reported as change from baseline.
We extracted baseline HIV-RNA levels and reasons for switching treatment regimens. We assessed three different cutoffs for virological failure (HIV-RNA <50, <200 and <400 copies/mL) and used snapshot analyses if available, otherwise time-to-loss-of-virological-response analyses. Here, we accepted any approach for dealing with incomplete outcome data but for consistency we preferred analyses where missing data was counted as virological failure. We did not differentiate between virological and treatment failure (due to the highly inconsistent reporting). Rash was only evaluated in patients at low risk of developing allergic reactions, i.e. in studies in HLA-B*-5701-negative patients or in studies not containing abacavir (ABC).Citation1–3
We used intention-to-treat-(ITT) data including all randomized patients, where available, otherwise we used results from other reported approaches (e.g. “modified ITT”).Citation13,14
One reviewer extracted the data into pre-piloted electronic extraction forms and another double-checked them. Disagreements were resolved by consensus.
Risk of bias assessment
In teams of two reviewers, we independently used the Cochrane tool for bias assessment to assess the (1) randomization process; (2) blinding; (3) reporting bias (i.e. high risk for abstract publications; no exploration of other sources of reporting bias) and (4) attrition bias.Citation12
Analysis
We combined the reported treatment effects in meta-analyses and calculated summary relative risks or mean differences (between the two groups compared) with 95% confidence intervals (CI).Citation12,15 We used random-effects (DerSimonian and Laird method) to take between-study heterogeneity into account. If an outcome had an event rate of less than 1%, we used Peto’s approach which performs better when events are rare.Citation12 We used a continuity correction of 0.5 and assessed the between-study heterogeneity using the I2-metric.Citation12
If the time-point of outcome assessment was not specified, we used the mean or median study follow-up.Citation12 If for continuous outcomes the number of patients analyzed at 48 weeks was unclear in a study, we imputed it by subtracting the median attrition reported for this outcome in other studies from the number of randomized patients. Where possible, we used the same outcome measures as in our previous meta-analysis,Citation5 i.e. the absolute changes from baseline per group reported as mean with standard deviation (SD).Citation12 Missing means or SDs were converted or approximated from other given statistics or imputed from the remaining studies in the meta-analysis for this outcome.Citation12
If only a few patients of a study did not meet our inclusion criteria, we accepted this and we included two studies with less than 10% patients who had TDF-based ART before randomizationCitation16,17 (we excluded 7 studies with >20% patients being pre-treated with TDFCitation18–29). When studies compared more than two treatment arms, we included only two arms in the main analysis to avoid double-counting patients. This was the case in three studies: In the first two, we excluded the arms with uncommon doses (i.e. stavudine 30mgCitation30 and TDF 75 or 150mgCitation31); in the third, we used the TDF-arm with a backbone treatment that was more similar to the control groupCitation32 (details in Table ).
Table 1 Characteristics of included studies
In secondary analyses, we assessed the comparison of the two fixed dose regimens TDF/FTC vs. ABC/3TC.
We conducted ancillary analyses that combined the studies on ART-experienced patients with studies in ART-naïve patients initiating a TDF containing regimen (reported in our previous meta-analysisCitation5). This larger database improved the imputation of missing information and provided more precise overall estimates, allowed to explore if effects are different between ART-naïve and ART-experienced patients, and maximized statistical power to evaluate clinical outcomes. In these combined meta-analyses, we did not evaluate body fat (because studies in naïve patients reported mostly relative changes, studies in non-naïve patients mostly absolute changes) and virological failure (because clinical circumstances and treatment objectives when initiating ART or when switching ART are both closely related to virological failure and clinically too heterogeneous to be reasonably combined in one analysis).
We used meta-regression analyses to test potentially different effects between ART-experienced and ART-naïve patients and to evaluate whether TDF effects are different when fixed-dose regimens are compared (TDF/FTC vs. ABC/3TC). In a post hoc analysis, we explored baseline lipid levels as modifier of TDF-effects on lipid levels. We conducted none of the pre-specified sensitivity/subgroup analyses on publication status, funding source, risk of bias, sex, pregnancy, breastfeeding, and renal disease because of too few studies per subset.
Stata 13.1 (Stata Corp, College Station, TX, USA) was used for all analyses; p-values are 2-tailed.
Results
We screened 5237 references of which we assessed 421 potentially relevant full texts to determine eligibility. Twenty-one publications were included which reported on 17 RCTs in treatment experienced patients (Fig. ). The agreement between both reviewers was good (kappa = 0.64).
Figure 1 Study selection process
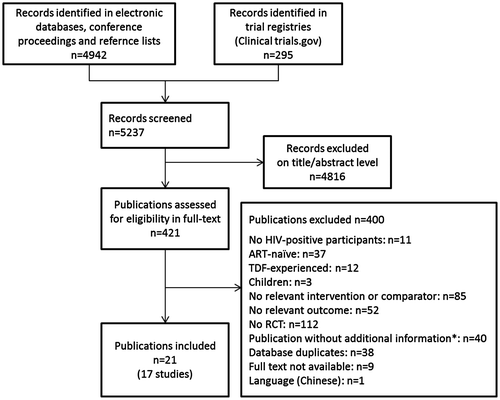
All RCTs were rather small, with the biggest trials including between 200 and 301 patientsCitation17,32–35 (Table ). Study follow-up was between 12 and 79 weeks, with 13 of 17 RCTs reporting data observed at week 48 or later. Four trialsCitation35–39 compared the fixed dose co-formulations TDF/FTC with ABC/3TC, the other studies used various other treatment regimens. Patients switched because of treatment failure in three trials (baseline HIV-RNA levels >1000 copies/mLCitation17,32 or 400 to 100,000 copies/mLCitation30). In the other 14 studies, patients were virologically suppressed (baseline HIV-RNA levels < 50 copies/mL in 8 trials; <200 copies/mL in 4 trials; <400 copies/mL in 2 trials; see Table ) and treatment was changed for other reasons. This included five trials where the indication of treatment switch was related to hyperlipidemia,Citation39 dyslipidemia,Citation40 lipoatrophyCitation41,42, or elevated total cholesterol.Citation38 None of the trials included pregnant women in their analyses.
Thirteen of 17 studies were supported by the pharmaceutical industry, 1 was governmentally funded, no details were reported in 3 (Webappendix). Most studies used ITT- or modified ITT-analysis (14/17), 1 used as treated analysis,Citation42 1 on treatment analysis,Citation43,44 and 1 per protocol analysis.Citation33,34
Risk of bias assessment
All trials, with one exception,Citation30 had a high overall risk of bias due to an open study design which is associated with a high risk of performance and detection bias. The risk of bias in the other domains was mainly low or unclear for all studies (Webappendix).
Outcomes
Deaths
Nine trialsCitation16,17,30,32–35,42,45,46 were included in the analysis. In TDF-based regimens vs. other regimens, the relative risk (RR) for death was 0.69 (95% CI 0.20 to 2.37; median follow-up 48 weeks; range 24 to 79 weeks; Table ).
Table 2 Summary of results
Fractures
Three trialsCitation31,35,46 were included in the analysis. The RR for fractures was 0.65 (95% CI 008 to 518; median follow-up 72 weeks; Table ).
CD4-cell count
Seven trialsCitation16,34,35,39,41,42,46 were included in the analysis. There was no indication that switching to TDF-based regimens had a different impact on CD4-cell count than maintaining or switching to other regimens (mean difference (MD) −13.76 cells/mm3; 95% CI −37.63 to 10.12; changes from baseline to week 48; Table ).
Virological failure
Eight trialsCitation16,32,33,35,39,42,45,46 were included in the analysis. The RR for achieving HIV-1-RNA levels < 50 copies/mL was 1.02 (95% CI 0.98 to 1.07, Table ) at 48 weeks.
Lipid levels
Overall, eight trialsCitation16,34,35,39,41,42,46,47 were included in the analyses for LDL-cholesterol, HDL-cholesterol, total-cholesterol, and triglycerides. All effect estimates indicated decreases of lipid levels (statistically significant for total cholesterol and triglycerides) in TDF-based regimens compared to other regimens (MD (95% CI): LDL-cholesterol −4.71 mg/dL (−9.80 to 0.37); HDL-cholesterol −2.04 mg/dL (−4.68 to 0.59); total cholesterol −12.05 mg/dL (−20.76 to −3.34); triglycerides −14.33 mg/dL (−23.73 to −4.93); changes from baseline to week 48; Table ).
eGFR
Three trialsCitation34,35,44 were included in the analyses. Two trials reported results from the Modification of Diet in Renal Disease (MDRD) formula,Citation34,35 all three trials used the Cockcroft-Gault formula; and in one trial, effects based on the Chronic Kidney Disease Epidemiology Collaboration formula were also reported.Citation44 There was no significant decrease when we used the MDRD-based effects where available and otherwise the Cockcroft-Gault estimate (MD −3.50 ml/min; 95% CI −7.35 to 0.36). Analyzing only the two MDRD-based effects yielded similar results (−3.04 ml/min/1.73 m2; −7.8 to 1.72). Renal function significantly decreased when assessed using Cockcroft-Gault formula estimates (−4.48 ml/min; −6.56 to −2.41) (changes from baseline to week 48; Table 2; Webappendix).
BMD
Three trialsCitation42,43,48 were included in the analyses. They assessed both BMD of the hip and lumbar spine; one trialCitation43 also measured BMD of the femoral neck. Compared to other regimens, TDF-based regimens led to a loss of bone density at the hip (MD −2.46%; 95% CI −3.9 to −1.03) and lumbar spine (−1.52%; −2.69 to −0.34) (changes from baseline to week 48; Table 2, Webappendix).
Body fat
Three trialsCitation41,42,46 were included in the analyses. There was no significant difference of TDF-based vs. other treatments on the absolute changes of trunk fat (MD 218 g; 95% CI −255 to 692) and limb fat (271 g; −207 to 748) (changes from baseline to week 48; Webappendix).
Other outcomes
We did not pool results on AIDS-defining events, cardiovascular events, renal failure, proteinuria, rash, and quality of life as data was inconsistently reported or based on very heterogeneous definitions. Findings are described narratively in the Webappendix.
Secondary analyses in studies comparing only TDF/FTC-based vs. ABC/3TC-based regimens were in line with the primary findings (mortality: RR 0.50, 95% CI 0.05 to 5.49; fractures: RR 0.34, 0.01 to 8.17; CD4-cell count: MD −21.49 cells/mm3, −52.15 to 9.18; HIV-1-RNA levels < 50 copies/mL: RR 1.00, 0.94 to 1.06; LDL-cholesterol: MD -7.98 mg/dL, −13.7 to −2.90; HDL-cholesterol: MD −0.63 mg/dL, −2.27 to 1.01; total cholesterol: MD –12.67 mg/dL, −25.20 to −0.14; for triglycerides −7.27 mg/dL, −22.03 to 7.49; eGFR Cockcroft-Gault: MD −3.99 ml/min, −6.38 to −1.59; eGFR MDRD: MD −5.3 ml/min/1.73m2; −8.83 to −1.77; Details and corresponding Forest plots are shown in the Webappendix). No study comparing TDF/FTC vs. ABC/3TC reported effects on BMD or body fat.
Heterogeneity and evaluation of effect-modifiers
Heterogeneity between studies in ART-experienced patients was low for mortality, fractures, virological failure, triglycerides, and Cockcroft-Gault eGFR (I2 < 40%; Webappendix). It was moderate to high for the other outcomes (I2 ≥ 40%; Webappendix). Among these, estimated heterogeneity was 0% in the subset comparing only TDF/FTC vs. ABC/3TC for the outcomes CD4-cell count, LDL- and HDL-cholesterol explaining some of the statistical heterogeneity between studies as possibly resulting from heterogeneous comparators; it remained high for total cholesterol (I2 = 69.1%) and was not estimable for the other outcomes as there was either none or only one study per subset (Webappendix).
Type of regimen
We found no evidence that the effects of TDF in ART-experienced patients depended on the kind of regimen (i.e. fixed-dose TDF/FTC vs. ABC/3TC compared to other TDF-regimens vs. non-TDF-regimens), although the LDL-reductions were more pronounced with the fixed dose regimen (p for interaction 0.034; Details in Webappendix).
Baseline lipid levels
We detected no association of baseline lipid levels with lipid changes over 48 weeks in post hoc meta-regression analyses (data not shown).
HIV-RNA levels
The results on virological failure were similar in comparisons based on other cut-offs (i.e. 200 and 400 copies/mL), and in analyses excluding trials in patients with baseline HIV-RNA above 200 and 400 copies/mL (Webappendix).
Ancillary analyses of ART-naïve and ART-experienced patients
Combining the treatment effects reported in the 17 RCTs in 2210 ART-experienced patients switching to TDF-based regimens with the effects from previously reviewed 22 RCTs in 8297 ART-naïve patients initiating TDF-based regimens as first-line treatment, yielded very similar effect estimates with smaller confidence intervals and statistical significant reductions of LDL and HDL-cholesterol and eGFR (Table ). Between-study heterogeneity in the ancillary combined analyses was similar to the heterogeneity between studies in treatment-experienced patients (Table ). We found no evidence that the effects of TDF depend on ART-experience, with the exception of triglycerides where greater reductions were seen in studies with ART-naïve patients (p for interaction 0.028).
Discussion
We analyzed the effects of switching to TDF-based regimens in 17 trials with 2210 ART-experienced HIV-patients. This switching was not associated with mortality, fractures, CD4-cell count, body fat, virological failure, LDL- and HDL-cholesterol. Switching to TDF-based regimens, however, decreased total cholesterol, trigylcerides, and BMD. Effects on eGFR were inconsistent and depended on the measurement and effect estimates for indicated LDL- and HDL-cholesterol decreases but were not statistically significant. Findings were similar in trials on switching to fixed-dose TDF/FTC or ABC/3TC-based regimens.
The ancillary analyses in naïve and non-naïve patients using data from 39 RCTs involving over 10,000 patients provided more precise estimates, and in this large body of evidence, effects on eGFR, LDL-, and HDL-cholesterol were now statistically significant. The direction of effect estimates was consistent throughout all analyses (including the naïve, the non-naïve, or both populations combined). We found no evidence that effects of TDF differed between ART-naïve and ART-experienced patients or between fixed dose regimens (TDF/FTC and ABC/3TC) and non-fixed-dose regimens – with only two potential exceptions: Firstly, the lipid-lowering effect on triglycerides appeared to be stronger in treatment-naïve patients (−29.84 mg/dL vs. −14.33 mg/dL). One possible explanation could be that treatment-experienced patients have been exposed to first generation ART over longer periods with persistent adverse effects on lipid levels. We observed lower average baseline lipid levels in studies with naïve patient populations but post hoc meta-regression analyses revealed no association with TDF effects (although the analysis was based on aggregated data and we would need individual patient data to further explore this). Secondly, the difference between effects of TDF and other ART on LDL-reduction seemed to be greater in fixed dose regimens.
The ART-experienced patients were at baseline mostly virologically suppressed with only 3 of 17 studies including patients with previous treatment failure and baseline HIV-RNA levels of 1000 copies/mL and above. In our previous meta-analysis,Citation5 the median baseline values were greater than 58,000 copies/mL HIV-RNA. However, there appears to be no difference between TDF-based regimens and other regimens in treatment-experienced and -naïve patients, irrespective of baseline viral load. Of note, as in our previous review, we found substantial incongruences across trial reports with regard to the definition of treatment failure (i.e. various cut-offs defining virological failure, different approaches for dealing with incomplete outcome data).
This is the first meta-analysis of RCTs exploring the effects of TDF in treatment-experienced patients. The ancillary analysis is, to our knowledge, the largest body of clinical trial evidence on treatment effects of TDF and the first meta-analysis evaluating influences of pretreatment and fixed-dose regime in ART-naïve and ART-experienced patients. Despite the clinically different treatment settings (with or without fixed-dose treatments, various comparators, previous ART or switching to alternative treatment choices) and diverse study populations, the overall findings were largely consistent. The broad perspective with wide selection criteria maximized the statistical power to assess clinical outcomes and the coverage of evidence supporting the clinical use of TDF.Citation49 The between-study heterogeneity introduced some imprecision in the random-effects models, but using all data allowed us to better deal with the problem of missing data which increased the precision of the treatment effect estimates as more data was available for imputation.
We used predefined endpoints relevant to patients and clinicians, used established methodology for meta-analysis throughout all processes and especially when addressing potential bias. Our highly sensitive literature search was developed in collaboration with research librarians to cover the entire evidence on TDF-based regimens.
Some limitations need to be discussed. First, none of the included studies considered mortality or the clinical outcomes as their primary endpoint but often reported them rather unsystematically alongside adverse events. We decided to not extract outcomes that were related to specific events (e.g. to the drug or to withdrawal) as this harbors potential for bias and subjectivity.Citation12,50
Second, for changes of outcome variables from baseline, we only extracted results at 48 weeks because this was the most consistently reported time-point. Still, some studies only reported data for diverse shorter or longer follow-up time-points.
Third, insufficient reporting led to (often unanswered) author queries and required numerous imputations for continuous outcomes. For example, authors rarely provided a measure of dispersion (e.g. 95% CI, standard deviation), the number of analyzed patients, or the follow-up time-point; some results are only reported for all groups together or for one group but not the other. Another reporting issue was missing data. The majority of studies reported ITT or modified-ITT analyses; however, they often do not analyze all randomized or all treated patients. Often, a fair amount of missing patients (e.g. lost-to-follow-up, missing data points, discontinuation, withdrawal) were reported without a statement on how this missing data was dealt with, i.e. if and what kind of imputations were used, or which other methods were applied. For viral load, it was common to report an approach for missing data, e.g. “missing equals failure” but rarely authors reported how many patients were actually missing, so there is no way of knowing how many patients were observed to have a certain outcome event and how many were imputed or assumed to have an event. This lowers the reliability of the reported effects in the primary studies and ultimately limits the clinical interpretation.
Fourth, in some studies, it was not clear which backbone treatments (beyond randomization) were given at discretion of the treating physicians or if they were the same in both treatment groups. This information would have been particularly important in unblinded trials and because some co-administered drugs from different classes are known to influence the lipid profile.Citation51
Fifth, all but one study had a high risk of bias due to lack of blinding. Due to the small number of studies with low risk of bias, we could not assess the impact of risk of bias.
Sixth, the statistical between-study heterogeneity in some analyses could not be explained with the data at hand despite various prespecified strategies to explore if publication status, funding source, sex, pregnancy, breastfeeding, or renal disease potentially modifies the reported effects of TDF. An individual patient-level meta-analysis might be warranted to further explore potential impact of patient-related factors.
We acknowledge these limitations and uncertainties and conclude that there is limited evidence from clinical trials – the majority being of relative short follow-up – indicating that TDF-based regimens have adverse effects on kidney function and bone mineral density but lower lipid levels irrespective of possible pretreatment with non-TDF-based ART. This is in line with current guidelines emphasizing favorable lipid effects of TDF and recommending alternative treatments (such as abacavir or the recently approved tenofovir alafenamide, an oral prodrug of tenofovir) in patients with chronic kidney disease or osteoporosis.Citation2
However, the substantial reporting deficits in numerous trial reports and the high inconsistency of reporting of clinical events did not allow us to assess the effects on such patient-relevant clinical outcomes and increased the clinical uncertainty. This is a substantial waste of existing potentially useful evidence. Since the quality of reporting is elementary for evidence-based decision-making, improved reporting can help closing important knowledge gaps and facilitate treatment decisions in HIV care.Citation52
Disclaimer statement
Contributors
LGH and HCB conceived and designed the study; HE coordinated the review; all authors collected the data; HE and LGH analyzed the data; HE, LGH, and HCB interpreted the results; HE wrote the first draft and all authors made revisions on the manuscript and approved the final version of the paper. HE and LGH are the guarantors.
Declaration of competing interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: HCB has received in 36 months preceeding the submission of this work travel grants, honoraria, and unrestricted research grants from Bristol-Myers-Squibb, Gilead, and ViiV Healthcare. The Basel Institute for Clinical Epidemiology & Biostatistics has received funding from Gilead for a previous project closely related to the current work. MS received travel grants, and is member of advisory boards of abbvie, Bristol-Myers-Squibb, Gilead, Janssen, Merck Sharp & Dohme, and ViiV. All other authors declare no competing interests.
Funding
None.
Ethical approval
Not required for this study.
Acknowledgements
The authors thank Kübra Özoglu for administrative assistance and support with literature management. We thank study authors and the staff of the trial sponsors for providing information on their study results.
Supplemental material
The underlying research materials for this article can be accessed at http://dx.doi.org/10.1080/15284336.2016.1261073.
YHCT_1261073_Supplementary_Material.zip
Download Zip (821.5 KB)References
- European AIDS Clinical Society (EACS). EACS Guidelines Version 8.0 – October 2015; 2015. http://www.eacsociety.org/files/guidelines_8_0-english_web.pdf. Accessed July 7, 2016.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2016. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed September 21, 2016.
- Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults. JAMA. 2016;316(2):191–210.10.1001/jama.2016.8900
- Abgrall S, Ingle SM, May MT, et al. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS. 2013;27(5):803–813.
- Hemkens LG, Ewald H, Santini-Oliveira M, et al. Comparative effectiveness of tenofovir in treatment-naïve HIV-infected patients: systematic review and meta-analysis. HIV Clin Trials. 2015;16(5):178–189.10.1179/1945577115Y.0000000004
- Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–1415.10.1136/bmj.a117
- HIV Drug Therapy in the Americas Congress 13–15 January 2013, São Paulo Brazil. J Int AIDS Soc. 2013;16(1):1–24.
- Raghunathan K, Bonavia A, Nathanson BH, et al. Association between initial fluid choice and subsequent in-hospital mortality during the resuscitation of adults with septic shock. Anesthesiology 2015;123(6):1385–1393.10.1097/ALN.0000000000000861
- 14th European AIDS Conference 16–19 October 2013. Brussels; 2013. http://www.eacs-conference2013.com/fileadmin/templates/eacs/template_FILES/FINAL_EACS13_Final_Program_web.pdf.
- 11th International Congress on Drug Therapy in HIV infection 11–15 November 2012, Glasgow UK. J Int AIDS Soc. 2012;15(4):1–176.
- XIX International AIDS Conference 22–27 July 2012, Washington, DC, USA. J Int AIDS Soc. 2012;15(3):1–287.
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. www.cochrane-handbook.org. Accessed March 2011.
- Nuesch E, Trelle S, Reichenbach S, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ. 2009;339:b3244.10.1136/bmj.b3244
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta-analysis. Int J Epidemiol. 2005;34(1):79–87.
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Internal Med. 1997;127(9):820–826.10.7326/0003-4819-127-9-199711010-00008
- Loutfy MR, Ackad N, Antoniou T, et al. Randomized controlled trial of once-daily tenofovir, lamivudine, and lopinavir/ritonavir versus remaining on the same regimen in virologically suppressed HIV-infected patients on their first PI-containing HAART regimen. HIV Clin Trials. 2007;8(5):259–268.10.1310/hct0805-259
- Bunupuradah T, Chetchotisakd P, Ananworanich J, et al. A randomized comparison of second-line lopinavir/ritonavir monotherapy vs. tenofovir/lamivudine/lopinavir/ritonavir in patients failing NNRTI-regimens: the HIV STAR study. Antivir Ther. 2012;17(7):1351–1361.10.3851/IMP2443
- Wohl DA, Bhatti L, Small CB, et al. Simplification to abacavir/lamivudine + atazanavir maintains viral suppression and improves bone and renal biomarkers in assure, a randomized, open label, non-inferiority trial. PLoS ONE. 2014;9(5):e96187.10.1371/journal.pone.0096187
- Negredo E, Domingo P, Perez-Alvarez N, et al. Improvement in bone mineral density after switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: two-centre randomized pilot study (OsteoTDF study). J Antimicrob Chemother. 2014;69(12):3368–3371.10.1093/jac/dku300
- Fabbiani M, Mondi A, Colafigli M, et al. Safety and efficacy of treatment switch to raltegravir plus tenofovir/emtricitabine or abacavir/lamivudine in patients with optimal virological control: 48-week results from a randomized pilot study (Raltegravir Switch for Toxicity or Adverse Events, RASTA Study). Scand J Infect Dis. 2014;46(1):34–45.10.3109/00365548.2013.840920
- Nishijima T, Gatanaga H, Shimbo T, et al. Switching tenofovir/emtricitabine plus lopinavir/r to raltegravir plus Darunavir/r in patients with suppressed viral load did not result in improvement of renal function but could sustain viral suppression: a randomized multicenter trial. PLoS ONE. 2013;8(8):e73639.10.1371/journal.pone.0073639
- Haskelberg H, Hoy JF, Amin J, et al. Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS ONE. 2012;7(6):e38377.10.1371/journal.pone.0038377
- Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir‐emtricitabine or abacavir‐lamivudine: a randomized, 96‐week trial. Clin Infect Dis. 2009;49(10):1591–1601.10.1086/599193
- Curran A, Martinez E, Podzamczer D, et al. Changes in body composition and mitochondrial DNA in HIV-1-infected patients switching to fixed-dose abacavir/lamivudine or tenofovir/emtricitabine: a substudy of the BICOMBO trial. Antiviral Ther. 2012;17(4):711–718.10.3851/IMP2081
- Martinez E, Arranz JA, Podzamczer D, et al. Long-term outcomes of switching to fixed-dose abacavir/lamivudine (ABC/3TC) or tenofovir/ emtricitabine (TDF/FTC): 3-year results of the BICOMBO study. Paper presented at Conference: 10th International Congress on Drug Therapy in HIV Infection Glasgow United Kingdom. Var.Pagings. 2010.
- Martínez E, Arranz JA, Podzamczer D, et al. A simplification trial switching from nucleoside reverse transcriptase inhibitors to once-daily fixed-dose abacavir/lamivudine or tenofovir/ emtricitabine in HIV-1-infected patients with virological suppression. JAIDS. 2009;51(3):290–297.10.1097/QAI.0b013e3181aa12d5
- Saumoy M, Ordoñez-Llanos J, Martínez E, et al. Low-density lipoprotein size and lipoprotein-associated phospholipase A2 in HIV-infected patients switching to abacavir or tenofovir. Antiviral Ther. 2011;16(4):459–468.10.3851/IMP1785
- Cohen C, Green J, Olivet H, et al. A randomized pilot study of tenofovir/emtricitabine (TDF/FTC) + boosted atazanavir (ATV/r) vs. raltegravir (RAL BID) + ATV/r vs. RAL BID + ATV BID. J Int AIDS Soc. 2012;15(4):18279.
- Martínez E, Larrousse M, Podzamczer D, et al. Abacavir-based therapy does not affect biological mechanisms associated with cardiovascular dysfunction. AIDS. 2010;24(3):F1–F9.10.1097/QAD.0b013e32833562c5
- Schooley RT, Ruane P, Myers RA, et al. Tenofovir DF in antiretroviral-experienced patients: results from a 48-week, randomized, double-blind study. AIDS. 2002;16(9):1257–1263.10.1097/00002030-200206140-00008
- Milinkovic A, Martinez E, López S, et al. The impact of reducing stavudine dose versus switching to tenofovir on plasma lipids, body composition and mitochondrial function in HIV-infected patients. Antiviral Ther. 2007;12:407–415.
- Koulla-Shiro S, Le Moing V, Ndour C, et al. Randomized comparison of three second line art regimens in Africa: the 2 lady/ANRS/EDCTP study. Paper presented at: Conference on Retroviruses and Opportunistic Infections (abstract and poster 541LB). 2014.
- Cooper V, Moyle GJ, Fisher M, et al. Beliefs about antiretroviral therapy, treatment adherence and quality of life in a 48-week randomised study of continuation of zidovudine/lamivudine or switch to tenofovir DF/emtricitabine, each with efavirenz. AIDS Care. 2011;23(6):705–713.10.1080/09540121.2010.534433
- Fisher M, Moyle GJ, Shahmanesh M, et al. A randomized comparative trial of continued zidovudine/lamivudine or replacement with tenofovir disoproxil fumarate/emtricitabine in efavirenz-treated HIV-1-infected individuals. JAIDS. 2009;51(5):562–568.10.1097/QAI.0b013e3181ae2eb9
- Campo R, DeJesus E, Bredeek UF, et al. SWIFT: prospective 48-week study to evaluate efficacy and safety of switching to emtricitabine/tenofovir from lamivudine/abacavir in virologically suppressed HIV-1 infected patients on a boosted protease inhibitor containing antiretroviral regimen. Clin Infect Dis. 2013;56(11):1637–1645.10.1093/cid/cis1203
- Moyle GJ, Orkin C, Fisher M, et al. A randomized comparative trial of continued abacavir/lamivudine plus efavirenz or replacement with efavirenz/emtricitabine/tenofovir DF in hypercholesterolemic HIV-1 infected individuals. PLOS ONE. 2015;10(2):e0116297.10.1371/journal.pone.0116297
- Orkin C, Moyle G, Fisher M, Wang H, Ewan J, ROCKET I Study Group. Switching from Kivexa [KVX] ABC/3TC + Efavirenz [EFV] to Atripla [ATR] (EFV/FTC/TDF) reduces cholesterol in hypercholesterolemic subjects: preliminary results of a 24-week randomized study. Paper presented at: 16th Annual Conference of the British HIV Association; Apr 21–23, 2010.
- Behrens G, Maserati R, Rieger A, et al. Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV-infected adults with raised cholesterol: effect on lipid profiles. Antivir Ther. 2012;17(6):1011–1020.10.3851/IMP2305
- Calza L, Manfredi R, Colangeli V, et al. Efficacy and safety of atazanavir-ritonavir plus abacavir-lamivudine or tenofovir-emtricitabine in patients with hyperlipidaemia switched from a stable protease inhibitor-based regimen including one thymidine analogue. AIDS Patient Care and STDs. 2009;23(9):691–697.10.1089/apc.2009.0039
- Valantin MA, Bittar R, De Truchis P, et al. Switching the nucleoside reverse transcriptase inhibitor backbone to tenofovir disoproxil fumarate+ emtricitabine promptly improves triglycerides and low-density lipoprotein cholesterol in dyslipidaemic patients. J Antimicrob Chemother. 2010;65(3):556–561.10.1093/jac/dkp462
- Moyle GJ, Sabin CA, Cartledge J, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20(16):2043–2050.10.1097/01.aids.0000247574.33998.03
- McComsey GA, O’Riordan M, Choi J, et al. Mitochondrial function, inflammation, fat and bone in HIV lipoatrophy: randomized study of uridine supplementation or switch to tenofovir. Antivir Ther. 2012;17(2):347–353.
- Cotter AG, Vrouenraets SM, Brady JJ, et al. Impact of switching from zidovudine to tenofovir disoproxil fumarate on bone mineral density and markers of bone metabolism in virologically suppressed HIV-1 infected patients; a substudy of the prepare study. J Clin Endocrinol Metab. 2013;98(4):1659–1666.10.1210/jc.2012-3686
- Vrouenraets SM, Fux CA, Wit FW, et al. Persistent decline in estimated but not measured glomerular filtration rate on tenofovir may reflect tubular rather than glomerular toxicity. AIDS. 2011;25(17):2149–2155.10.1097/QAD.0b013e32834bba87
- Gulick RM, Lalama CM, Ribaudo HJ, et al. Intensification of a triple-nucleoside regimen with tenofovir or efavirenz in HIV-1-infected patients with virological suppression. AIDS. 2007;21(7):813–823.10.1097/QAD.0b013e32805e8753
- Ribera E, Larrousse M, Curran A, et al. Impact of switching from zidovudine/lamivudine to tenofovir/emtricitabine on lipoatrophy: the RECOMB study. HIV Med. 2013;14(6):327–336.10.1111/hiv.2013.14.issue-6
- Rasmussen TA, Tolstrup M, Melchjorsen J, et al. Evaluation of cardiovascular biomarkers in HIV-infected patients switching to abacavir or tenofovir based therapy. BMC Infect Dis. 2011;11(1):267.10.1186/1471-2334-11-267
- Rasmussen TA, Jensen D, Tolstrup M, et al. Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS ONE. 2012;7(3):e32445.10.1371/journal.pone.0032445
- Ioannidis JPA, Karassa FB. The need to consider the wider agenda in systematic reviews and meta-analyses: breadth, timing, and depth of the evidence. BMJ. 2010;341:c4875.
- Ioannidis JP, Evans SJ, Gotzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Internal Med. 2004;141(10):781–788.10.7326/0003-4819-141-10-200411160-00009
- Hill A, Sawyer W, Gazzard B. Effects of first-line use of nucleoside analogues, efavirenz, and ritonavir-boosted protease inhibitors on lipid levels. HIV Clin Trials. 2009;10(1):1–12.10.1310/hct1001-1
- Glasziou P, Altman DG, Bossuyt P, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383(9913):267–276.10.1016/S0140-6736(13)62228-X
