Abstract
Background: HIV-infected adults have increased fracture risk.
Objectives: To generate pilot data comparing bone density, structure, and strength between HIV-infected adults with and without a prior fracture.
Methods: Adults with and without a prior fracture after their HIV diagnosis were matched 1:1 based on age, sex, race, and smoking history. Participants underwent dual-energy X-ray absorptiometry (DXA), trabecular bone score (TBS), hip structural analyses (HSA), vertebral fracture assessment (VFA), high-resolution peripheral quantitative tomography (HR-pQCT) and measurement of bone turnover markers. Results were compared between cases and controls, with differences expressed as percentages of control group values.
Results: 23 pairs were included. On DXA, cases had lower areal bone mineral density (aBMD) at the total hip (median difference in T-score −0.25, p = 0.04), but not the lumbar spine (median difference in T-score 0.10, p = 0.68). Cases had greater abnormalities in HSA and most HR-pQCT and HSA measures, by up to 15%. VFA revealed two subclinical fractures among cases but none among controls. TBS, CTX, and P1NP levels were similar between groups, with differences of 1.9% (p = 0.90), 9.7% (p = 0.55), and 10.0% (p = 0.24), respectively. For each parameter, we report the median and interquartile range for the absolute and relative difference between cases and controls, the correlation between cases and controls, and our recruitment rates, to inform the design of future studies.
Conclusions: These pilot data suggest potential differences in bone structure, estimated bone strength, and asymptomatic vertebral fractures among HIV-infected adults with and without fracture, warranting further study as markers of fracture risk in HIV.
Introduction
Numerous studies have reported that persons living with HIV have an increased risk of fracture when compared to the general population.Citation1–6 This risk is likely multifactorial, related to HIV itself, its therapy and traditional osteoporosis risk factors. Because fractures are relatively uncommon, measurement of areal bone mineral density (aBMD) by dual-energy X-ray absorptiometry (DXA) is often used as a surrogate marker for fracture risk, based on literature from the general population.Citation7
However, DXA has several limitations. These include the lack of a clear fracture thresholdCitation8; most people who sustain fragility fractures do not meet criteria for DXA-defined osteoporosis (i.e. they have T-scores above −2.5).Citation9–11 Further, in clinical trials of anti-resorptive drugs for treating osteoporosis, improvements in spinal BMD account for only a portion of reduced vertebral fracture risk.Citation12,13 These observations may in part be because DXA provides only two-dimensional information on three-dimensional structures, rendering it unable to distinguish geometric changes (e.g. thicker bones) from material changes (e.g. increased mineral density).Citation14 Overall, less than 50% of variation in whole-bone strength is attributable to variations in aBMD.Citation12,15 In addition, there is a relative paucity of aBMD data in young men, who comprise most HIV-infected individuals in industrialized world settings, against which to compare individual patient results.
Alternative or complementary bone imaging modalities might aid in fracture risk stratification among HIV-infected persons, and could be useful surrogate outcome measures in clinical trials. For instance, high-resolution peripheral quantitative computed tomography (HR-pQCT) is a novel, non-invasive technology that measures both bone geometric properties (such as cortical thickness, trabecular thickness, trabecular separation, and trabecular number) as well as BMD (including total, cortical, and trabecular volumetric BMD),Citation16 potentially offering insights into bone structure that are not obtainable using DXA. Further, images obtained from routine hip DXA scans can be analyzed using algorithms such as hip structural analysis (HSA) to estimate geometrical and mechanical parameters including buckling ratio (a measure of cortical stability under compressive loads), cross-sectional area (a measure of axial strength), section modulus (a measure of bending strength), and average cortical thickness.Citation17,18 In addition, using standard algorithms, spine DXA scans can be analyzed for trabecular bone score (TBS), an estimate of the spine trabecular connectivity and inhomogeneity, which has been shown to independently predict fractures.Citation19 Finally, vertebral fracture assessment (VFA) is a high-yield method of identifying vertebral fractures using modern bone densitometers during the same session as traditional DXA scanning. Vertebral fractures are important because they represent a significant independent risk factor for all new fractures, yet often go unrecognized.Citation20 Because these tools incur additional expense and are not all widely available, it is important to evaluate their correlation with clinical outcomes and determine their potential additive benefit over traditional DXA.
To aid in the planning of future clinical trials on bone health in HIV, we conducted a pilot case-control study quantifying bone turnover markers, bone structural parameters measured by TBS, HSA, and HR-pQCT, and the results of VFA among HIV-infected adults with and without a history of fracture. The purpose of our pilot study was twofold.Citation21 First, we sought to generate preliminary data on whether these measures correlate with clinically reported fractures among HIV-infected adults, with plans to proceed to a larger comparative study in the future if there was reasonable evidence of differences in these measurements between cases and controls. Second, for each measure we estimated the median and interquartile range for the difference between cases and controls, as well as the correlation between cases and controls, to inform the sample size calculations of future trials that might use these metrics as study outcomes.
Methods
Study design and participants
This was a 1:1 case-control study comparing bone turnover markers, BMD and bone structure among HIV-infected adults aged 18 years or older with a history of fracture (cases) after HIV diagnosis, with HIV-infected matched controls. Fractures resulting from major trauma were excluded. Participants were enrolled from two HIV tertiary care clinics in Toronto, Canada, and matched on age (±5 years), sex, smoking status, and race (White, Black, or other). Consecutive patients were queried during routine clinic visits about ever sustaining a fracture since their HIV diagnosis, and considered for enrollment if the answer was yes. Individuals expected to change their antiretroviral therapy during the study were excluded. To account for seasonal variability in vitamin D levels, participants underwent two study visits for collection of clinical data and bloodwork, one in the spring and one in the autumn, plus an additional visit for DXA (including TBS, HSA, and VFA), and HR-pQCT as soon as possible after enrollment.
Study visits and laboratory testing
Study visits included baseline collection of demographics, medical history, weight, height, and grip strength. Participants also completed the International Physical Activity Questionnaire short form (IPAQ) to quantify physical activity,Citation22 and the Bone Loading History Questionnaire to quantify historical bone loading.Citation23 Blood was collected in the morning after fasting for biochemistry (calcium, phosphate, 25-hydroxy vitamin D, and parathyroid hormone), the bone resorption marker cross-linked carboxyterminal-telopeptide (CTX), and the bone formation marker amino-terminal propeptide of type 1 collagen (P1NP). Values from spring to autumn were averaged, and the correlation between repeated measures is reported. Both bone biomarkers were measured in batches using commercially available test kits (B-CrossLaps for CTX and total P1NP) on an automated system (Elecsys 2010 Immunochemistry Auto-analyzer, Roche Diagnostics).
Bone imaging
aBMD measurements were performed on the Hologic Discovery A (Hologic Inc., Massachusetts, USA) using standard methods at the lumbar spine and proximal femur, with T-scores calculated using reference standards. Technologists followed standard protocols from the manufacturers with attention to accurate positioning and appropriate body size scaling, as both are critical technical considerations in scan acquisition and analysis of results.Citation18 VFA was performed immediately after BMD measurement to obtain single-energy high-definition X-ray images of the AP and lateral spine using the rotating C-arm of the densitometer. Processing and analysis (including TBS, HSA, and VFA) were performed by experienced ISCD-certified technologists and reviewed by an ISCD-certified densitometrist according to standard protocols.
HR-pQCT assessments of the distal radius and distal tibia were performed by an experienced technologist using the Xtreme CT (Scanco, Switzerland), following standard protocols for the positioning of limbs and acquisition of data. Bone strength is a composite parameter and is estimated from all the measured parameters using finite element analyses. Finite element analyses were performed using n88 model generator and FAIM as the solver (Numerics88 Inc., Alberta, Canada). Pre-processing segmentation was done with Scanco’s standard evaluation followed by their standard automatic contouring script and a Scanco custom IPL script to obtain the load sharing results. Participants with a prior history of fracture at the wrist or ankle underwent scans of the contralateral limb. Daily and weekly quality control measurements were performed. Scans were repeated if the fifth rod in the daily phantom exceeded 1% deviation from the established baseline mean. Prior work at our site has shown the intra-operator precision of this test to be excellent, with a root mean square coefficient of variation of 0.52% at the radius and 0.23% at the tibia for volumetric BMD measurements.Citation24
Analysis
Baseline demographic and clinical characteristics and averaged lab values were compared between cases and controls using paired Wilcoxon signed rank tests or exact McNemar’s tests as appropriate. Differences in DXA results, TBS, HSA parameters, HR-pQCT parameters, and averaged bone biomarker levels between cases and controls were expressed both as absolute differences and as percentages of control group values. Absolute and relative differences were tested using paired Wilcoxon signed rank tests. Spearman’s correlation coefficient was used to measure the association of bone parameters between cases and controls, and to estimate the correlation between repeated lab and bone biomarker measurements within individuals.
Conditional logistic regression models were then used to model the relationship between DXA and derived T-scores (hip and lumbar spine separately) and fracture, adjusting for the duration of HIV infection. To explore whether TBS, HSA and HR-pQCT offered additional information independent of DXA, additional models further adjusted for each TBS, HSA, or HR-pQCT parameter, and nested models were compared using the likelihood ratio test.
The primary objective of this pilot study was to ascertain whether there was reasonable evidence of differences in these measurements between cases and controls, even if differences did not reach traditional thresholds of statistical significance. We further conducted two sets of sensitivity analyses; first, excluding pairs in which the case patient had exposure to bisphosphonates, and second, excluding matched pairs in which the case patient’s fracture did not meet criteria for being a fragility fracture (based on the anatomic site involved or the level of trauma). Our secondary objective was to estimate, for each outcome, the absolute and relative differences between cases and controls, a measure of dispersion, and the correlation between cases and controls, to aid in sample size calculations for future trials.
The target sample size of 36 matched pairs was based on feasibility considerations, related to the available funds and recruitment period. With 36 matched pairs, and assuming a standard deviation of 60 mg HA/cm3 based on published data from premenopausal women,Citation16 a correlation coefficient of 0.1–0.5 for tvBMD between cases and controls, a two-sided α = 0.05 and 80% power, the estimated minimum detectable difference in tvBMD within pairs was in the range of 29–39 HA/cm3, or roughly 0.5 standard deviations.
Ethical approval
All participants provided written informed consent, and ethical approval was obtained from the Research Ethics Boards of St. Michael’s Hospital and the University Health Network.
Results
Fifty-six individuals were screened, of which one did not meet matching criteria and one withdrew from the study prior to matching, leaving 27 matched pairs. Of these, four pairs were excluded because one member of the pair subsequently withdrew, leaving 23 pairs for analysis. This number was lower than the target enrollment due to difficulties both in identifying patients with the relatively uncommon outcome of fracture, and in finding controls meeting all four match criteria. The 27 original cases and 27 controls were enrolled over 27 and 28 months, respectively, from 2 tertiary HIV specialty clinics with a combined population of roughly 3000 patients, for a recruitment rate of 12 fracture participants and 12 control participants per year. Ten of the 23 fractures met the traditional definition of a fragility fracture (3 wrist, 2 pelvis, and 1 each of shoulder, hip, rib, spine, and bimalleolar ankle), whereas 7 involved alternative anatomic sites (one finger, three toe, three ankle) and 6 involved more than minimal trauma (e.g. falling off moving bicycles; one arm, three wrist, two feet).
Baseline characteristics of the included participants are shown in Table . Median (interquartile range, IQR) age was 50 (46–56) years, and most participants were white (78%) male (78%) smokers (57%). Baseline immune and virologic status was similar between cases and controls, with an overall median CD4 count of 472 (327-668) cells/mm3 and 85% of participants having HIV RNA <50 copies/mL. The proportion with any prior tenofovir use was similar in both arms, but there was slightly more tenofovir use at baseline and a numerically shorter duration of HIV infection among controls. The median time from enrollment to performance of the radiology studies was 27 (14–53) days.
Table 1 Baseline characteristics of participantsTable Footnotea
Results of the IPAQ and BLHQ suggested comparable levels of physical activity and historical bone loading in the cases and controls, although there was a trend towards less participation in regular weight-bearing exercise in the fracture group. Eight participants had conditions predisposing to osteoporosis, including post-menopausal status, chronic kidney disease, hypogonadism, and rheumatoid arthritis. Hepatitis B and/or C co-infection was more common among cases (22%) than controls (4%), p = 0.22. Four case participants reported ever using osteoporosis medications including two each with ongoing use of risedronate and alendronate, respectively. Fracture participants had lower ionized calcium and correspondingly higher parathyroid hormone levels, albeit all within the normal range. Correlation between repeated lab measurements within individuals was high (vitamin D: ρ = 0.84, ionized calcium: ρ = 0.54, PTH: ρ = 0.58, phosphate: ρ = 0.50).
Outcome measures are shown in Table , according to fracture status. By DXA (Fig. a), fracture patients had lower aBMD at the total hip (median absolute difference in T-score −0.25, p = 0.04), but not at the lumbar spine (median absolute difference in T-score 0.10, p = 0.68). Among those aged 50 and older, 3 (25%) cases, and 4 (36%) controls met traditional World Health Organization BMD criteria for osteoporosis,Citation25 while 5 (42%) and 5 (46%) met criteria for low bone mass, respectively. Among those aged under 50, 1 (10%) and 0 met ISCD definitions for osteoporosis (i.e. low bone mass plus a fragility fracture)Citation26 while 3 (27%) and 3 (25%) had Z-scores below two standard deviations of the expected range for age. TBS was similar between groups (Fig. a).
Table 2 Bone outcomes according to fracture statusTable Footnotea
Figure 1. Percentage differences in bone imaging and biomarker levels in fracture participants relative to matched controls.
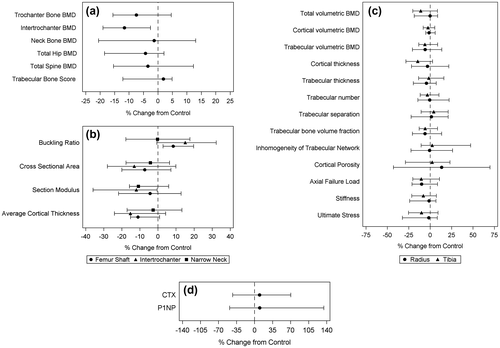
Comparisons in HSA parameters were numerically most pronounced at the intertrochanteric area (Fig. b), where the buckling ratio was 15.1% greater (p < 0.01), cross-sectional area was 13.1% lower (p = 0.09), section modulus was 12.0% lower (p = 0.08) and average cortical thickness was 15.3% lower (p = 0.05) in fracture patients.
VFA revealed no clinically significant vertebral fractures in the control group but two subclinical grade 2 fractures among two individuals in the fracture group, one of whom had borderline low bone mass (T-scores at the hip and lumbar spine −1.12 and −0.71, respectively) and one of whom had normal BMD (T-scores −0.52 and +1.06, respectively).
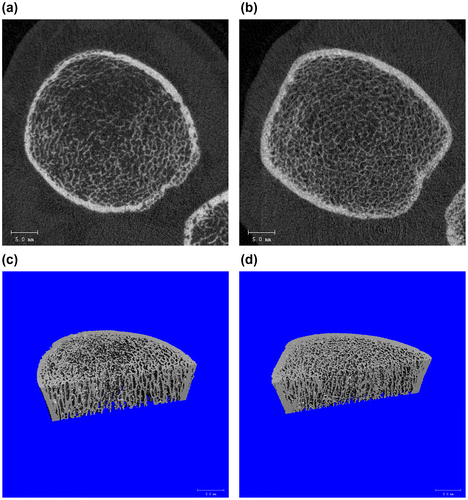
Comparisons of HR-pQCT parameters revealed multiple numerical differences in bone microstructure between cases and controls (Table , Figs. c and ). Although both groups had similar total vBMD at the distal radius (median relative difference −0.09%, p = 0.86), there was a trend towards lower total vBMD at the distal tibia among cases (median relative difference −11.1%, p = 0.21). Indeed, differences in HR-pQCT outcomes were generally more pronounced at the tibia (Fig. c) than the radius. The most dramatic numerical differences were cortical porosity at the radius (13.4% greater in cases than controls, p = 0.20) and cortical thickness at the tibia (14.6% lower in cases, p = 0.22), although some differences in trabecular parameters were also apparent. Considerable numerical differences of roughly −10% were also observed in axial failure load and ultimate stress, both of which provide overall estimates of bone strength.
Figure 2. Microarchitectural differences of HIV-infected patients with and without fracture as shown by HRpQCT.
After exclusion of the four participants using bisphosphonates and their matched pairs, median CTX and P1NP levels were numerically similar among fracture participants and controls with differences of 9.65% (p = 0.55) and 10.0% (p = 0.24), respectively (Fig. d). The correlation between repeated measurements for CTX and P1NP was 0.80 and 0.78, respectively.
In general, TBS, HSA, and HR-pQCT results were not qualitatively different after excluding the four pairs with bisphosphonate exposure (Supplementary Table). Differences in HR-pQCT, HSA, and bone biomarkers between cases and controls were generally more pronounced in the second set of sensitivity analyses restricted to matched pairs in which the case participant had sustained a fragility fracture (Table ).
Conditional logistic regression models adjusted for the duration of HIV infection suggested that higher DXA-derived T-scores may be associated with a lower risk of fractures, with OR = 0.45 (95%CI = 0.17,1.14, p = 0.09) per 1 unit of T-score at the hip, and OR = 0.87 (95%CI = 0.53,1.43, p = 0.58) per 1 unit of T-score at the lumbar spine. After further adjustment for individual HRpQCT, TBS, HSA, or bone biomarker parameters, comparison of nested models using the likelihood ratio test generally did not result in significant differences in model fit (data not shown). These findings suggest that the additional bone imaging and biomarker parameters did not generally result in significantly improved differentiation between fracture and non-fracture patients beyond the information already provided by DXA, potentially due to a lack of sufficient power.
Discussion
In this pilot study, we observed numerically lower aBMD by DXA, greater bone structural abnormalities by HRpQCT, and decreased composite measures of bone strength on HSA and HRpQCT among HIV-infected adults with a reported history of fracture compared to age, sex, race, and smoking status-matched HIV-infected controls. Fracture patients also had more subclinical vertebral fractures and similar TBS and bone biomarker levels. When analyses were restricted to those sustaining fragility fractures and their matched controls, differences were generally more pronounced. However, because the main purpose of this pilot study was to generate preliminary data to inform future work, and because most of our observed differences did not reach statistical significance, these findings should be considered hypothesis-generating only. To our knowledge, this is the first study to directly compare measures of bone strength and architecture in fracture and non-fracture patients in the setting of HIV infection.
Given our modest sample size, many of the differences we observed did not reach traditional levels of statistical significance. We were unable to identify a clear pattern regarding which particular imaging parameters are most important for predicting fractures in this population. Published literature on HR-pQCT and HSA in the general population has similarly been unable to define the relative importance of each parameter, most likely because different disease states and medications affect the various parameters differently. In general, preferred parameters include compartmental (cortical vs. trabecular) volumetric BMD, cortical thickness, cortical porosity and trabecular bone volume. Estimated bone strength, represented by failure load and ultimate stress, is often the preferred composite summary of the various parameters measured by HRpQCT.
Our preliminary findings could guide further investigation into the potential uses of these novel technologies for several reasons. First, the HR-pQCT differences we observed were similar in magnitude, both in absolute and relative terms, to differences in cortical and trabecular HR-pQCT outcomes documented between HIV-infected and uninfected adults in previous studies. For instance, HIV positive individuals were found to have 14.1% lower trabecular density (median 135.0 vs. 157.1 mg HA/cm3) and 13.2% lower trabecular number (1.58 vs. 1.82 mm−1) at the distal tibia, as well as 3.0% lower cortical density at the distal radius (922.5 vs. 951.3 mg HA/cm3) in one study among premenopausal women compared to age- and BMI-matched HIV-uninfected controls.Citation27 Similarly, young males infected with HIV perinatally or during adolescence were found to have decreases in total and trabecular vBMD as well as both cortical and trabecular thickness in the range of 6–19% compared to uninfected controls.Citation28 Relatively fewer differences were seen in a study among Hispanic and African-American postmenopausal women, with HIV-infected participants only having 11–12% lower cortical thickness and area at the tibia than uninfected controls.Citation29 In a recent cross-sectional study, HIV-infected women were found to have greater periosteal and endosteal circumference and lower cortical volumetric BMD z-scores (differences of 0.30, 0.38 and −0.40, respectively) than a reference sample of uninfected women.Citation30 Overall, these data suggest that the differences we observed likely correspond to clinically meaningful differences in bone health. It remains unclear which imaging parameter(s) are most clinically relevant for predicting fracture risk in this population.
Second, by reporting the difference between cases and controls and the correlation between cases and controls for the parameters studied, our results may be helpful to other investigators performing sample size calculations for other studies.
Third, our observed rate of recruitment informs feasibility assessments for future work. Although the HIV-infected population is aging and is at higher risk of fracture than the general population, fragility fractures remain rare events, with an estimated incidence of 1.4–7.4/1000 person-years.Citation5 We recruited patients with prevalent, not just incident fractures, from two clinics that each follow ~1500 HIV patients 3–4 times per year, yet still were unable to meet our target enrollment.
Fourth, we observed larger HR-pQCT differences at the distal tibia than the distal radius, with the differences in several parameters nearly reaching statistical significance. As a weight-bearing site, differences in bone strength tend to be less pronounced at the distal tibia, as seen with reduction in circulating estrogen levels.Citation31 Although case participants reported somewhat less weight-bearing exercise at the time of study participation, their historical levels of physical activity and bone loading were high and similar to controls. Further data are needed to ascertain whether this unexpected finding is reproducible and related to HIV.
Fifth, we did observe significant differences in HSA parameters between cases and controls in this study of up to 10–15%. These differences were most notable at the intertrochanteric region, suggesting the possibility of a predisposition to intertrochanteric hip fracture at this anatomic site in the setting of HIV. To our knowledge, however, there are no data showing this to be the case. Only two previously published studies have reported on HSA parameters in HIV-infected individuals. In one study, HIV, hepatitis C co-infected men had a statistically significantly lower buckling ratio by 5.7% and centroid position by 2.1% at the narrow neck than HIV, HCV-uninfected men, after adjustment for race, age, smoking status, height, and weight.Citation32 In the other study, HIV-infected patients with tenofovir use at baseline had a 12.3% lower section modulus and 8.6% lower cross-sectional area than those with abacavir use, and these differences were statistically significant.Citation33
Previous fracture case-control studies in non-HIV-infected populations have also employed DXA and HR-pQCT and shown differences in HR-pQCT parameters that are of similar magnitude to those we observed in this study. Further, studies among postmenopausal women have demonstrated HR-pQCT parameters to be independently associated with fragility fractures even after adjustment for DXA-derived T-scores,Citation34,35 suggesting an incremental benefit of this testing modality beyond traditional DXA that could be useful prognostically. Indeed, in other secondary forms of osteoporosis such as diabetes, observed fracture risk has been greater than what would be expected based on BMD alone.Citation36 In contrast, we did not observe an improved ability to discriminate fracture and non-fracture patients using either HR-pQCT or HSA, beyond what was possible using DXA alone, perhaps because of our modest sample size. Alternatively, there may be less pronounced differences in fracture-related bone structure in the setting of HIV infection. The multiplicity of factors that combine to increase fracture risk in HIV patients, and how they correlate with changes in bone structure in HIV patients requires further study.
Finally, subclinical vertebral fractures were observed in two cases, one of whom had normal aBMD, and none in controls. In three cross-sectional studies from Italy, the prevalence of vertebral fractures among HIV-infected adults was estimated at 23.3 and 26.9% using lateral chest X-rays,Citation37,38 and 27% using VFA.Citation39 In the latter report, 12% of the 33 participants with normal DXA-derived BMD were affected.Citation39 Vertebral fractures are important because they represent a significant independent risk factor for all new fractures,Citation20 yet often go unrecognized. More data are needed to ascertain how best to integrate vertebral fracture screening into a comprehensive approach to bone health in HIV.
To our knowledge, this is the first fracture case-control study in the setting of HIV infection. Strengths of our study include matching on key variables including age, race, sex, and smoking status, and the comprehensiveness of our radiographic assessments. This study also has limitations that warrant consideration. First, our sample size was modest, and we were unable to control for small numerical imbalances in hepatitis C, baseline tenofovir use, diabetes, and traditional osteoporosis risk factors between groups. Second, four participants in the fracture group were using bisphosphonates, potentially biasing our findings towards the null; these individuals were excluded from analysis of bone turnover markers and from sensitivity analyses of other outcomes. Finally, our participants were relatively young, and HIV-related changes in bone health may become more prominent with age.
In summary, our preliminary data suggest possible differences in bone density, structure and estimated strength, vertebral fractures and bone biomarkers between HIV-infected patients with and without prior fractures using a comprehensive battery of diagnostic techniques. The magnitude of these differences is comparable to those reported with other clinically significant categories, including comparisons based on hepatitis C co-infection, tenofovir use, and HIV status itself. Our parameter estimates and recruitment rates may inform the design of future research in this area. Future work should characterize the bone parameters most closely linked to fracture in the setting of HIV, and consider the use of a broader range of bone imaging modalities as outcome measures in interventional trials.
Contributors
DHST is a clinician scientist at St. Michael’s Hospital and an Assistant Professor of Medicine at the University of Toronto, with research interests in HIV co-morbidities and co-infections. JR is a Professor of Biostatistics at the Dalla Lana School of Public Health, and LS is a research associate at the Toronto General Research Institute; their work focuses on methodologic issues in the design and analysis of HIV clinical trials, and the analysis of longitudinal data. ES is a mechanical engineer and HR-pQCT research analyst. HH is a statistical data analyst, and QW is BMD technologist at the Centre for Excellence in Skeletal Health Assessment, with shared research interests in osteoporosis and post-menopausal women's health. AMC is a professor of Medicine and Medical Imaging at the University of Toronto, and the Director of the Osteoporosis Program and Centre for Excellence in Skeletal Health Assessment at the University Health Network, whose research interests are in the area of post-menopausal osteoporosis, especially in prevention and early diagnosis, and evaluation of new therapies and technologies. SLW is a Professor of Medicine at the University of Toronto, Clinician-Investigator at Toronto General Hospital and the Co-Director of the CIHR Canadian HIV Trials Network, whose work focuses on clinical trials and observational studies in the management of HIV infection and its comorbidities.
Funding
This work was supported by Canadian HIV Trials Network, Canadian Institutes of Health Research [grant number CTN-PT 001].
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/15284336.2016.1266074
YHCT_1266074_Supplementary_Material.zip
Download Zip (17.2 KB)References
- Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504.10.1210/jc.2008-0828
- Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV outpatient study (hops) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52:1061–8.10.1093/cid/ciq242
- Hansen AB, Gerstoft J, Kronborg G, et al. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012;26:285–93. doi:10.1097/QAD.0b013e32834ed8a7.
- Guerri-Fernandez R, Vestergaard P, Carbonell C, et al. HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J Bone Miner Res. 2013;28:1259–63. doi:10.002/jbmr.874.
- Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS. 2013;27:1949–57. doi:10.097/QAD.0b013e328361d241.
- Prieto-Alhambra D, Guerri-Fernandez R, De Vries F, et al. HIV infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr. 2014;66:90–5. doi:10.1097/QAI.0000000000000112.
- Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The study of osteoporotic fractures research group. Lancet. 1993;341:72–5.10.1016/0140-6736(93)92555-8
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9.10.1136/bmj.312.7041.1254
- Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20:1813–9.10.1359/JBMR.050609
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195–202.10.1016/j.bone.2003.10.001
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–12.10.1001/archinte.164.10.1108
- Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281–9.10.1016/S0002-9343(01)01124-X
- Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res. 2002;17:1–10.10.1359/jbmr.2002.17.1.1
- Unnanuntana A, Gladnick BP, Donnelly E, Lane JM. The assessment of fracture risk. J Bone Joint Surg Am. 92:743–53.
- Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah B. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;73:423–32.10.1007/s00223-002-2104-4
- Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–15.10.1210/jc.2005-1258
- Beck TJ, Ruff CB, Warden KE, Scott WW, Jr., Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol. 1990;25:6–18.10.1097/00004424-199001000-00004
- Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Curr Osteoporos Rep. 2007;5:49–55.10.1007/s11914-007-0002-4
- McCloskey EV, Oden A, Harvey NC, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31:940–8. Epub 015 Nov 19. doi:10.1002/jbmr.2734.
- Melton LJ, 3rd, Atkinson EJ, Cooper C, O’Fallon WM, Riggs BL. Vertebral fractures predict subsequent fractures. Osteoporos Int. 1999;10:214–21.10.1007/s001980050218
- Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/471-2288-10-1.
- Craig CL, Marshall AL, Sjostrom M, et al. International physical activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.10.1249/01.MSS.0000078924.61453.FB
- Dolan SH, Williams DP, Ainsworth BE, Shaw JM. Development and reproducibility of the bone loading history Questionnaire. Med Sci Sports Exerc. 2006;38:1121–31.10.1249/01.mss.0000222841.96885.a8
- Cheung AM, Chan C, Demaras A, et al. Intra-operator precision for in vivo HR-pQCT scans. International Society for Clinical Densitometry 14th Annual Meeting; March 12–15, San Francisco, CA: Abstract 184; 2008.
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1–129.
- Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom. 2004;7:17–26.
- Calmy A, Chevalley T, Delhumeau C, et al. Long-term HIV infection and antiretroviral therapy are associated with bone microstructure alterations in premenopausal women. Osteoporos Int. 2013;24:1843–52. Epub 2012 Nov 9. doi:10.007/s00198-012-2189-1.
- Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS. 2014;28:345–53. doi:10.1097/QAD.0000000000000070.
- Yin MT, Shu A, Zhang CA, et al. Trabecular and cortical microarchitecture in postmenopausal HIV-infected women. Calcif Tissue Int. 2013;92:557–65. Epub 2013 Mar 5. doi:10.1007/s00223-013-9716-8.
- Lo Re V, 3rd, Lynn K, Stumm ER, et al. Structural bone deficits in HIV/HCV-coinfected, HCV-monoinfected, and HIV-monoinfected women. J Infect Dis. 2015;212:924–33. Epub 2015 Mar 9. doi:10.1093/infdis/jiv147.
- Cheung AM, Tile L, Cardew S, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. 2012;13:275–84. Epub 2012 Feb 7. doi:10.1016/S470-2045(11)70389-8.
- Walker Harris V, Sutcliffe CG, Araujo AB, et al. Hip bone geometry in HIV/HCV-co-infected men and healthy controls. Osteoporosis Int. 2012;23:1779–87.
- Haskelberg H, Pocock N, Amin J, et al. Hip structural parameters over 96 weeks in HIV-infected adults switching treatment to tenofovir-emtricitabine or abacavir-lamivudine PLoS ONE. 2014;9:e94858. eCollection 2014. doi: 10.1371/journal.pone.0094858.
- Liu XS, Stein EM, Zhou B, et al. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012;27:263–72. doi:10.1002/jbmr.562.
- Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010;25:2572–81. Epub 2010 Jun 18. doi:10.1002/jbmr.152.
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes – a meta-analysis. Osteoporos Int. 2007;18:427–44. Epub 2006 Oct 27.10.1007/s00198-006-0253-4
- Borderi M, Calza L, Colangeli V, et al. Prevalence of sub-clinical vertebral fractures in HIV-infected patients. New Microbiol. 2014;37:25–32. Epub 2014 Jan 15.
- Torti C, Mazziotti G, Soldini PA, et al. High prevalence of radiological vertebral fractures in HIV-infected males. Endocrine. 2012;41:512–7. Epub 2011 Dec 25. doi:10.1007/s12020-011-9586-7.
- Porcelli T, Gotti D, Cristiano A, et al. Role of bone mineral density in predicting morphometric vertebral fractures in patients with HIV infection. Osteoporos Int. 2014;25:2263–9. Epub 2014 Jul 24. doi:10.1007/s00198-014-2760-z.
