Abstract
Background: The objective of this study was to determine the impact of tenofovir or abacavir discontinuation on low-density lipoprotein (LDL) phenotype and lipoprotein-associated phospholipase A2 (Lp-PLA2) activity in HIV-infected patients treated with lopinavir/ritonavir plus 2 nucleos(t)ide reverse transcriptase inhibitors (NRTI).
Methods: Multicenter, open-label study. Patients were randomized to continue with lopinavir/ritonavir plus 2 NRTI (triple therapy) or to switch to lopinavir/ritonavir plus lamivudine (dual therapy). LDL phenotype (by gradient gel electrophoresis) and Lp-PLA2 (by 2-thio-PAF) were determined at baseline and week 48.
Results: Forty-four patients included (triple therapy n = 19, dual therapy n = 25): men 63.6%, age 41.5 years (25–61), Framingham score 4.9% (0.2–22). Tenofovir was part of the regimen in 28 (63.6%) patients. Dual therapy patients were younger (p = 0.013) and had lower baseline apolipoprotein A1 (p = 0.029). At week 48, there were no changes in standard lipid measurements, except ApoA1/Apo B, which increased in dual therapy (p = 0.038) with no differences between arms. At week 48, no change in LDL phenotype was found in either arm. No changes in total Lp-PLA2 activity or the relative distribution of LDL and HDL particles were found at week 48 in either arm.
Conclusions: Discontinuing the third nucleos(t)ide, mainly tenofovir and abacavir, in a lopinavir/ritonavir-containing regimen was not associated with a deleterious effect on LDL phenotype nor in Lp-PLA2 activity.
Introduction
Several studies have investigated switching from standard triple antiretroviral therapy (ART) to dual therapy or monotherapy to reduce toxicity associated with long-term use of nucleos(t)ide reverse transcriptase inhibitors (NRTI) and the cost of therapy.Citation1, 2 Recent studies have reported the efficacy of a ritonavir-boosted protease inhibitor (PI/r) plus lamivudine in naïve and switching scenarios.Citation3, 4 Discontinuation of tenofovir has been associated with mild worsening of several lipid parameters.Citation5, 6 which is consistent with the hypolipidemic effect of tenofovir (even when combined with a PI/r) reported in the TULIP trial.Citation5 Nevertheless, there is no information of the effect of tenofovir or abacavir discontinuation on low density lipoprotein (LDL) phenotype and lipoprotein-associated phospholipase A2 (Lp-PLA2) activity, two biomarkers associated with cardiovascular (CV) risk in general population.Citation6, 7
Methodology
This is a substudy of the OLE study (ClinicalTrials.gov number NCT01471821),Citation5 a multicenter, randomized trial. Patients included in the main study from four hospitals in Barcelona were consecutively enrolled. Inclusion criteria were HIV-infected adults with HIV-1 RNA less than 50 copies/mL for at least 6 months, receiving triple treatment with lopinavir/ritonavir (twice daily) plus lamivudine or emtricitabine and a second NRTI, with no resistance or virological failure to these drugs and negative testing for hepatitis B serum surface antigen. Patients were randomized to continue triple therapy or switch to dual therapy with lopinavir/ritonavir 400/100 mg twice daily and lamivudine 300 mg once daily. The protocol was approved by the central and local ethics committees, and written informed consent was obtained from all participants.
The primary endpoints were changes occurring in LDL phenotype and Lp-PLA2 activity at week 48 in each arm. Secondary endpoints were changes in standard lipid measures, and CV risk estimated by the Framingham score (FS) at week 48.
Clinical and laboratory assessments were performed at baseline and week 48. Cardiovascular risk factors were evaluated according to the National Cholesterol Education Program guidelines (NECP III).
LDL particle size was determined by 3% polyacrylamide gel electrophoresis using a commercial system (Lipoprint, Quantimetrix Co., Redondo Beach, CA) and patients were classified into 3 phenotypes: A (LDL size > 268 Å, predominance of large buoyant LDL particles), intermediate (LDL size 260–268 Å), and B (LDL size < 260 Å, predominance of a small, dense LDL particles). Total Lp-PLA2 activity was measured in serum and lipoproteins using the substrate 2-thio-platelet-activating factor (Cayman Chemical Company, Ann Arbor, MI, USA). The distribution of Lp-PLA2 between lipoprotein fractions was also assessed.
Sample size was not calculated, as this was an exploratory study. A p value < 0.05 was considered biologically relevant. The Student’s t-test or Mann–Whitney U and chi-square or Fisher exact test were used to compare continuous or quanlitatives variables between arms respectively. . Comparisons between baseline and follow-up measures in each arm were carried out with the paired t-test or Wilcoxon signed rank test. The Pearson correlation coefficient was calculated to estimate the strength of associations between continuous variables. Analyses were performed with PASW Statistics 18 (Chicago, Illinois, USA).
Results
The OLE trial included 239 patients. Forty-four patients from OLE (25 switched to dual therapy and 19 continued on triple therapy) were included in this metabolic substudy between December 2011 and December 2012. At baseline, age and apolipoprotein (apo) A-I level differed between arms (p = 0.013 and 0.029, respectively). Tenofovir was part of the regimen in 28 participants (64%) (Table ).
Table 1 Baseline characteristics of the participants
There were no differences in baseline characteristics between participants in the substudy and the general study except in high-density lipoprotein (HDL)-c level (p = 0.002). Three patients were lost to follow-up, Two receiving triple therapy and one dual therapy.
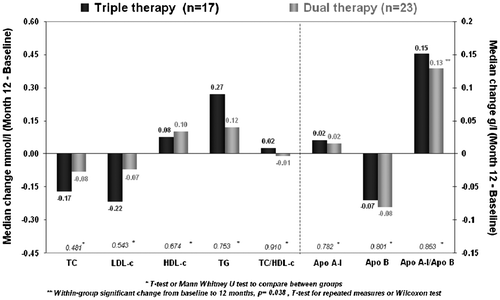
There were no significant changes in total cholesterol (TC), LDL-c, (HDL)-c or trygliceride (TG) concentrations at week 48 in either arm nor between arms, except an increase in the apolipoprotein apo A-I /apo B ratio in dual therapy arm (0.1 g/L [−1.0; 0.8]; p = 0.038), with no significant difference between arms (Fig. ).
Regarding the LDL phenotype at week 48, there were no significant changes in LDL particle size in either arm. At baseline, 86.4% of participants in the dual therapy arm and 86.7% in triple therapy had LDL phenotype A, and there were no changes at week 48 (77.3 and 86.7%, respectively, p = nonsignificant).
We found no significant changes in total Lp-PLA2 activity and no shift in the relative distribution of Lp-PLA2 in LDL or HDL particles in either arm at week 48.
Several correlations were seen at baseline between lipid variables and LDL size (TG r = −0.502, p = 0.002; Apo B r = −0.605, p < 0.001; HDL-c r = 0.364, p = 0.027), or total Lp-PLA2 activity (LDL-c r = 0.508, p = 0.001; TG r = 0.363, p = 0.027; Apo B r = 0.587, p < 0.001; TC/HDL-c r = 0.641, p < 0.001). At week 48, the following correlations were found: changes in TG and the TC/HDL-c ratio were associated with LDL size changes (r = −0.359, p < 0.029 and r = −0.499, p = 0.002, respectively); TC, LDL-c and TC/HDL-c changes were associated with Lp-PLA2 activity changes (r = 0.466, p = 0.004; r = 0.532, p < 0.001 and r = 0.459, p = 0.004; respectively); and LDL size changes were associated with Lp-PLA2 activity (r = −0.375, p = 0.022).
A subanalysis of the 14 patients on dual therapy who discontinued tenofovir showed no changes in any of the lipid variables studied.
There were no changes in CV risk estimation in either arm at week 48.
Discussion
In this study, discontinuation of tenofovir or abacavir from a standard triple ART based on lopinavir/ritonavir was not associated with a deleterious effect on two proatherogenic biomarkers: LDL phenotype and Lp-PLA2 activity. Furthermore, no changes were observed in the standard lipid measurements in contrast of the results of the overall OLE study, in which patients who switched to dual therapy showed increases in some of these variables,Citation3 likely related to loss of the recently described hypolipidemic effect of tenofovir,Citation5 and in accordance with similar studies.Citation4 The small sample included in our study may explain the absence of changes observed, although a considerable number of patients in dual therapy had stopped tenofovir.
Low-density lipoprotein cholesterol particles are heterogeneous and differ in size, density, charge, and lipid and apolipoprotein content. A predominance of small, dense LDL particles has been associated with an increased risk of coronary disease in the general population.Citation6 In the present study, no changes in the phenotype of LDL particles were seen in either arm, in accordance with no changes in standard lipid variables, included triglycerides, the major determinant of LDL size.Citation8
Lp-PLA2 is a specific marker of vascular inflammation. In HIV-infected patients, plasma Lp-PLA2 levels are influenced by ART and cardiovascular risk factors.Citation9 In previous studies, pretreated and naïve patients receiving tenofovir showed a greater decrease in Lp-PLA2 plasma levels than those receiving abacavir.Citation10, 11 In the present study, no changes were observed in Lp-PLA2 activity at week 48, in accordance with the lack of changes in standard lipid measurements related with plasma Lp-PLA2 activity in previous studies.Citation7, 10
The main limitation of our study is the relatively small sample size, which may have influenced the statistical power to detect changes. Nevertheless, we found congruent correlations between the variables studied, indicating consistent results.
In conclusion, the switch to an abacavir- or tenofovir-free regimen was not associated with worsening of LDL-c particle phenotype or Lp-PLA2 activity in this small sample. This information may be of interest because of the increasing use of ART combinations without NRTIs, mainly tenofovir.
| List of abbreviation | ||
| ART | = | Antiretroviral therapy |
| NRTI | = | nucleos(t)ide reverse transcriptase inhibitors |
| PI/r | = | ritonavir-boosted protease inhibitor |
| LDL | = | low-density lipoprotein |
| Lp-PLA2 | = | lipoprotein-associated phospholipase A2 |
| CV | = | cardiovascular |
| FS | = | Framingham score |
| TC | = | total cholesterol |
| LDL-c | = | low-density lipoprotein cholesterol |
| HDL-c | = | high-density lipoprotein |
| TG | = | trygliceride |
| Apo | = | apolipoprotein |
Acknowledgement
We thank all the patients who participated in the study.
Geolocation information
The study has been carried out in 5 hospitals of Barcelona, Spain.
Disclosure statements
Notes on contributors
Maria Saumoy PhD, staff physician of HIV and STD Unit, Infectious Disease Service of Hospital de Bellvitge, Barcelona. The author’s research interests include HIV infection, clinical trials, HIV comorbidities, and cardiovascular risk. Published books/articles: 2/30.
Juan M. Tiraboschi, staff physician of HIV and STD Unit, Infectious Disease Service of Hospital de Bellvitge, Barcelona. The author’s research interests include HIV infection, clinical trials, HIV reservoirs, HIV associated neurocognitive disorders. Published books/articles: 4/20.
Jordi Ordoñez-Llanos, senior consultant, Head Cardiovascular Laboratory of Clinical Biochemistry Dept. & Dept of Biochemistry and Molecular Biology of Hospital of Sant Pau & Autonomous University. Barcelona. Full professor of Autonomous University. The author’s research interests include: Clinical biochemistry, cardiovascular biochemistry, Lipids, Lipoproteins, Biomarkers, Cardiac diseases. Published books/articles: 50/215
Esteban Ribera, senior consultant, Infectious Diseases Department, Hospital Universitari Vall d’Hebrón, Barcelona. The author’s research interests include HIV Clinical Trials, Clinical Pharmacology and Complication of HIV and antiretroviral therapy. Published books/articles: 45/>200.
Pere Domingo, professor of Medicine, Universitat de Lleida, Spain. The author’s research interests include HIV infecion, ART, co-morbidities, ART complications and Aging. Published books/articles: 108/426.
Josep Mallolas, professor of Medicine, Hospital Clinic de Barcelona. The author’s research interests include HIV, HIV-HCV, Pharmacokinetics of antiretrovirals and HIV co-morbidities. Published books/articles: 20/370.
Jordi Curto, Associate Lecturer University of Barcelona. Statistic in HIV and STD Unit, Hospital Universitari de Bellvitge. The author’s research interest includes: statistics, multivariate models, Survival Analysis, DDBB design. Published articles:14.
Josep M. Gatell, professor of Medicine, University of Barcelona. The author’s research interests include HIV antiretroviral treatment and HIV vaccine. Published books/articles:>50/>400.
Daniel Podzamczer, Section chief of HIV and STD Unit, Infectious Disease Service of Hospital de Bellvitge, Barcelona The author’s research interests include HIV clinical trials, HIV associated comorbidities, HIV reservoirs. Published books/articles: 51/272.
Maria Saumoy and Daniel Podzamczer designed the study and drafted the paper. Jordi Ordoñez-Llanos designed and conducted laboratory studies. Maria Saumoy, Juan M. Tiraboschi, Esteban Ribera, Pere Domingo, Josep Mallolas, and Josep M. Gatell included and collected data from participants. Jordi Curto performed data analyses. All authors reviewed and approved the final version of the manuscript.
Conflict of interest
The following authors have received research funding, consultancy fees or lecture sponsorships from or served on advisory boards for the following companies: J O-L: Abbott Diagnostics, Alere Diagnostics, Astute Diagnostics, Critical Diagnostics, Roche Diagnostics, Siemens Medical Solutions, Stat Diagnostics and ThermoFischer; E R: Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, Merck Sharp and Dohme, Tibotec and ViiV Healthcare; P D: Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp and Dohme; JM: Abbvie, Merck Sharp and Dohme, ViiV and Janssen; JM G: Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Abbott, Merck Sharp and Dohme, Tibotec and Viiv; D P: Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp and Dohme, Tibotec and ViiV Healthcare.
M S, JM T and J C declare no conflict of interest.
Funding
This work has been supported by the Plan Nacional R + D + I and cofinanced by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER) [grant number RD12/0017/0013].
References
- Baril J-G, Angel JB, Gill MJ, et al. Dual therapy treatment strategies for the management of patients infected with hiv: a systematic review of current evidence in ARV-naive or ARV-experienced, virologically suppressed patients. Plos one. 2016;11:e0148231.10.1371/journal.pone.0148231
- Arribas JR, Girard P-M, Paton N, et al. Efficacy of protease inhibitor monotherapy vs triple therapy: meta-analysis of data from 2303 patients in 13 randomized trials. HIV Med. 2016;17:358–367.10.1111/hiv.12348
- Arribas JR, Girard P-M, Landman R, et al. Dual treatment with lopinavir–ritonavir plus lamivudine versus triple treatment with lopinavir–ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:785–792.10.1016/S1473-3099(15)00096-1
- Perez-Molina JA, Rubio R, Rivero A, et al. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:775–784.10.1016/S1473-3099(15)00097-3
- Santos JR, Saumoy M, Curran A, et al. the lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:403–408.10.1093/cid/civ296
- Hirayama S, Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin Chim Acta. 2012;414:215–224.10.1016/j.cca.2012.09.010
- Lp-PLA(2) Studies Collaboration, Thompson A, Gao P, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 2010; 375:1536-1544.
- Freedman DS, Otvod JD, Jeyarajah EJ, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham study. Clin Chem. 2004;50:1189–1200.10.1373/clinchem.2004.032763
- Saumoy M, Ordoñez-Llanos J, Martínez E, et al. Low-density lipoprotein size and lipoprotein-associated phospholipase A2 in HIV-infected patients switching to abacavir or tenofovir. Antivir Ther. 2011;16:459–468.10.3851/IMP1785
- Mangili A, Ahmad R, Wolfert RL, et al. Lipoprotein-associated phospholipase a2, a novel cardiovascular inflammatory marker, in HIV-infected patients. Clin Infect Dis. 2014;58:893–900.10.1093/cid/cit815
- Papakonstantinou VD, Chini M, Mangafas N, et al. In vivo effect of two first-line ART regimens on inflammatory mediators in male HIV patients. Lipids Health Dis. 2014;13:90–99.10.1186/1476-511X-13-90