Abstract
Objective: To evaluate the impact of Hepatitis B virus (HBV) coinfection on response to antiretroviral treatment in pregnant women with HIV.
Methods: Retrospective analysis of a large case series of pregnant women with HIV in Italy; outcome measures were CD4 changes, HIV viral load, and main pregnancy outcomes (preterm delivery, low birthweight, intrauterine growth restriction, mode of delivery, and major birth defects).
Results: Rate of HBV coinfection among 1462 pregnancies was 12.0%. Compared to the HBV-uninfected, HBV-coinfected women had a significantly lower median CD4 cell gain between first and third trimester (26.5 vs. 60 cells/mm3, p = 0.034), with similar rate of undetectable (<50 copies/ml) HIV-RNA at third trimester (70.5% vs. 65.2%, p = 0.229), and no differences in all the main maternal and infant outcomes. A multivariable linear regression analysis identified four variables significantly and independently associated with a lower CD4 response in pregnancy: HBV coinfection (–35 cells/mm3), being on antiretroviral treatment at conception (–59.7 cells/mm3), AIDS status (–59.8 cells/mm3) and higher first CD4 levels in pregnancy (–0.24 cells per unitary CD4 increase).
Conclusions: HBV coinfection had no adverse influence on the main pregnancy outcomes or on HIV viral load suppression in late pregnancy but was associated with a significantly reduced CD4 response in pregnancy. This effect might have clinical relevance, particularly in women with advanced immune deterioration.
Introduction
Hepatitis B virus (HBV) coinfection is relatively frequent among people with HIV and represents a significant cause of comorbidity, with additional adverse consequences on treatment tolerability and even survival.Citation1−3 Some studies have shown a worse response to antiretroviral treatment among HIV patients coinfected with HBV,Citation1,4,5 although others have failed to demonstrate a negative impact of HBV coinfection on immunological or virological markers of response to antiretroviral therapy.Citation2,3,6,7 The issue remains controversial, and there is minimal information on the influence of HBV coinfection on immunological and virological response to antiretroviral treatment during pregnancy. In order to further explore this issue, we used data from a national cohort of pregnant women with HIV, comparing CD4 response, HIV viral load, and main pregnancy outcomes among HIV-infected pregnant women with and without HBV coinfection.
Methods
Data from the Italian National Program on Surveillance on Antiretroviral Treatment in Pregnancy were used.Citation8 This is a national observational study of pregnant women with HIV established in Italy in 2001, reflecting current clinical care. Only HIV-positive pregnant women are included, and no specific guidance is given in terms of antiretroviral treatment, which is decided by the treating physician. Laboratory and clinical data are collected from hospital records of Obstetrics, Infectious Diseases, and Paediatrics departments, following the women’s informed consent. Information and measurements are collected at routine visits performed during pregnancy (with no restrictions in gestational age at entry into prenatal care), at delivery, during postpartum, and during a follow-up of mothers and newborns for up to 18 months. The present analysis involved all pregnancies with known HBV serostatus enrolled in the cohort who had antiretroviral treatment in pregnancy and live births between 2001 and 2016. Ethics approval was obtained from the Ethics Committee of the I.N.M.I. Lazzaro Spallanzani in Rome (Ref. deliberation 578/2001, 28 September 2001). HBV and HCV coinfection were diagnosed according to existing diagnostic guidelines for clinical practice during the study period. HBV infection was usually defined by presence in serum of HBsAg or HBV-DNA plus variable combinations of other virological markers (HBeAg, anti-HBcAg), according to diagnostic guidelines. HCV coinfection was defined by the presence of anti-HCV antibodies or HCV-RNA. For both HBV and HCV, detailed information on viral load levels was not available in the database. None of the pregnant women received HCV treatment. We compared CD4 gain in pregnancy between first and third trimester, rate of undetectable viral load at third trimester and main pregnancy outcomes between women with and without HBV coinfection, assessing in a multivariable analysis potential predictive factors for different CD4 gain in pregnancy identified by univariate analyses (p values <0.05). Preterm delivery was defined as delivery before 37 completed weeks of gestation, and low birthweight by values below 2500 g. Cesarean section was considered elective if performed before the rupture of membranes and the onset of labor, and nonelective if performed after the rupture of membranes, or onset of labor, or both. Major birth defects were defined according to the Antiretroviral Pregnancy Registry criteria,Citation9 and gender- and gestational age-adjusted Z-scores for birthweight were calculated according to national reference standards.Citation10 Quantitative data were compared by the Mann–Whitney U-test and proportions by the chi-square test, with odds ratios (OR) and 95% confidence intervals (CI) calculated. P values below 0.05 were considered significant, using for all analyses the SPSS software, version 22.0 (IBM Corp, Released 2013, Armonk, NY, USA).
Results
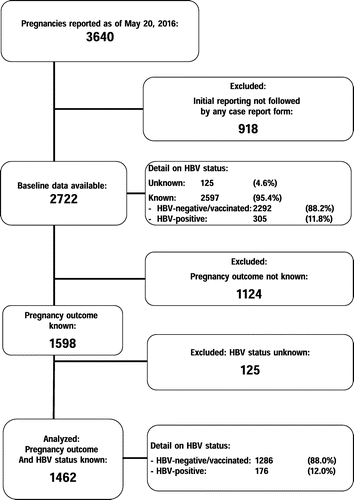
The flowchart of patient inclusion is shown in Figure . As of 20 May 2016, among 1462 pregnancies with available information on HBV serostatus, 176 were HBV-coinfected (12.0%). Among the remaining 1286 cases without HBV infection, 72 (5.6%) had been vaccinated against HBV. Women with and without HBV coinfection were similar for age, route of infection, CD4 cell count at first visit in pregnancy, CDC-HIV disease stage, antiretroviral treatment experience, treatment status at conception, time since HIV diagnosis and rate of HIV diagnosis during current pregnancy. Women with HBV coinfection were, however, significantly more likely to be of African origin and HCV-coinfected (Table ). The rate of use of HBV-active drugs in pregnancy was substantially identical in the two groups: at conception the rates of use in women with and without HBV were 33.5 and 34.9% for lamivudine (p = 0.716), 18.2 and 17.5% for tenofovir (p = 0.823), and 11.9 and 11.7%, respectively, for emtricitabine (p = 0.917). At delivery, the corresponding rates were 71.0% and 71.1% for lamivudine (p = 0.989), 26.1% and 22.1% for tenofovir (p = 0.228), and 18.2% and 16.6% for emtricitabine (p = 0.590).
Table 1 Population characteristics and main clinical and laboratory outcomes.
In terms of response to antiretroviral treatment in pregnancy, women with HBV had a significantly lower median CD4 cell gain between first and third trimester (26.5 vs. 60 cells/mm3, p = 0.034), but similar rate of undetectable (<50 copies/ml) HIV-RNA at third trimester (70.5% vs. 65.2%, p = 0.229). No differences between the two groups were also found for all the main maternal and infant outcomes (Table ). In order to adjust for potential confounders, the CD4 gain between first and third trimester was further evaluated in univariate and multivariate analyses. Univariate analyses showed no significant differences in CD4 gain by African ethnicity, HCV status, and antiretroviral treatment received in pregnancy (by presence of NRTI, NNRTI, PI in the regimen: all p values ≥ 0.2). Conversely, CD4 gain in pregnancy was significantly associated (p < 0.01) with age, HBV coinfection, duration of ARV in pregnancy, first CD4 count in pregnancy, CDC-HIV disease stage (AIDS vs. non-AIDS) and antiretroviral treatment status at conception (data not shown). The above variables were then entered as independent (predictive) variables in a multivariable linear regression model (900 cases) that used CD4 gain in pregnancy as the dependent (outcome) variable. In this analysis, four variables were independently associated with a significantly lower CD4 response in pregnancy: HBV coinfection (–35 cells/mm3), being on antiretroviral treatment at conception (–59.7 cells/mm3), AIDS status (–59.8 cells/mm3) and higher first CD4 cell counts in pregnancy (–0.24 cells per unitary CD4 increase). No significant independent effect was found for age and duration of antiretroviral treatment in pregnancy (Table ).
Table 2 Predictors of CD4 cell gain between first and third trimester in a multivariable linear regression model.
Discussion
We assessed for the first time in a large group of pregnant women with HIV the impact of HBV coinfection on response to antiretroviral treatment and on several maternal and neonatal outcomes, showing no adverse influence of HBV coinfection on the main pregnancy outcomes and on the rate of undetectable viral load in late pregnancy. Dual infection with HIV and HBV, however, was associated with a significantly lower CD4 response during pregnancy, a difference that persisted after adjusting for potential cofactors (age, ARV status at conception, HIV CDC stage disease, CD4, and duration of ARV in pregnancy). This effect could be quantified in multivariable analyses in 35 cells/mm3 and is consistent with the findings of other studies in the general population of people with HIV, and particularly with those from the Swiss HIV Cohort Study, that showed a significantly impaired CD4 recovery during antiretroviral treatment despite similar virological efficacy.Citation5 A possible explanation for these findings is represented by some effect of HBV replication on CD4 survival, possibly through changes induced in apoptotic pathways or in levels of regulatory cytokines.Citation11 We were unable to further investigate immunological and virological aspects, because no information was available on HBV viral load levels, HBV-specific antigens and antibodies, and possibly involved cytokines. In the absence of HBV-DNA information, we cannot link the observed reduced CD4 response to ongoing HBV replication, and further studies are needed to confirm this hypothesis. Many of the women were also receiving antiretroviral drugs which are expected to reduce or suppress HBV replication, such as lamivudine, tenofovir, and emtricitabine, and the observed effect might therefore also be due to other causes. We also found no effect of HCV coinfection but could not assess the level of HCV replication. We also did not collect specifically information on grade of severity of liver disease and were therefore unable to evaluate the independent influence of infrequent conditions, such as severe fibrosis or cirrhosis, on immunological response. In terms of risk of HIV vertical transmission, which is mainly linked to HIV viral load levels,Citation12 the similar HIV-RNA levels observed in pregnant women with and without HBV infection are reassuring, suggesting no additional risk of HIV transmission attributable to HBV coinfection. In terms of risk of opportunistic infections, the clinical significance of the reduced CD4 response due to HBV coinfection is uncertain. The study by Wandeler et al.,Citation5 based on a 3-year observation time suggests that the immune impairment induced by HBV is sustained even in the presence of ART. The observed effect, although not particularly large, could be relevant in the presence of very low CD4, and suggests the need to monitor with particular care the response to treatment in HBV-coinfected women who enter pregnancy in a condition of advanced immune deterioration, that requires a rapid CD4 cell recovery.
The Italian Group on Surveillance of Antiretroviral Treatment in Pregnancy
Project coordinators: M. Floridia, M. Ravizza, E. Tamburrini.
Participants: M. Ravizza, E. Tamburrini, F. Mori, P. Ortolani, E.R. dalle Nogare, F. Di Lorenzo, G. Sterrantino, M. Meli, S. Polemi, J. Nocentini, M. Baldini, G. Montorzi, M. Mazzetti, P. Rogasi, B. Borchi, F. Vichi, B. Del Pin, E. Pinter, E. Anzalone, R. Marocco, C. Mastroianni, V.S. Mercurio, A. Carocci, E. Grilli, A. Maccabruni, M. Zaramella, B. Mariani, G. Natalini Raponi, G. Guaraldi, G. Nardini, C. Stentarelli, B. Beghetto, A.M. Degli Antoni, A. Molinari, M.P. Crisalli, A. Donisi, M. Piepoli, V. Cerri, G. Zuccotti, V. Giacomet, S. Coletto, F. Di Nello, C. Madia, G. Placido, A. Vivarelli, P. Milini, F. Savalli, V. Portelli, F. Sabbatini, D. Francisci, G. Angeli, P. Grossi, L. Rizzi, M. Bernardon, G. Maso, C. Belcaro, E. Rizzante, A. Meloni, M. Dedoni, C. Cuboni, F. Ortu, P. Piano, A. Citernesi, I. Bordoni Vicini, K. Luzi, A. Spinillo, M. Roccio, A. Vimercati, A. Miccolis, A. De Gennaro, B. Guerra, F. Cervi, G. Simonazzi, E. Margarito, M.G. Capretti, C. Marsico, G. Faldella, M. Sansone, P. Martinelli, A. Agangi, A. Capone, G.M. Maruotti, C. Tibaldi, L. Trentini, T. Todros, G. Masuelli, V. Frisina, I. Cetin, T. Brambilla, V. Savasi, C. Personeni, C. Giaquinto, M. Fiscon, E. Rubino, A. Bucceri, R. Matrone, G. Scaravelli, O. Genovese, C. Cafforio, C. Pinnetti, G. Liuzzi, V. Tozzi, P. Massetti, A.M. Casadei, A.F. Cavaliere, M. Cellini, G. Castelli Gattinara, A.M. Marconi, S. Dalzero, V. Sacchi, M. Ierardi, C. Polizzi, A. Mattei, M.F. Pirillo, R. Amici, C.M. Galluzzo, S. Donnini, S. Baroncelli, M. Floridia.
Pharmacokinetics: P. Villani, M. Cusato.
Advisory Board: A. Cerioli, M. De Martino, F. Parazzini, E. Tamburrini, S. Vella.
SIGO-HIV Group National Coordinators: P. Martinelli, M. Ravizza.
Contributors
MF designed the study, drafted and finalised the manuscript and was responsible for statistical analysis; ET, SD, and MR were responsible for network coordination, clinical activities, acquisition of data and critical revision of the manuscript; GM, AS, GS, GG, AdA, PM, and VP substantially contributed to clinical activities, acquisition of data and to critical revision of the manuscript. All the authors gave approval to the final version to be published.
Funding
This work was supported by Agenzia Italiana del Farmaco, Ministero della Salute [grant number H85E08000200005].
Ethics approval
Ethics approval was obtained from the Ethics Committee of the I.N.M.I. Lazzaro Spallanzani in Rome (Ref. deliberation 578/2001, 28 September 2001).
Conflict of interest
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Cosimo Polizzi and Alessandra Mattei of the Istituto Superiore di Sanità in Rome, Italy, for providing technical secretarial for this study. No compensation was received for this contribution.
References
- Yang R, Gui X, Xiong Y, Gao SC, Yan Y. Impact of hepatitis B virus infection on HIV response to antiretroviral therapy in a Chinese antiretroviral therapy center. Int J Infect Dis. 2014; 28: 29–34.10.1016/j.ijid.2014.07.018
- Sarfo FS, Kasim A, Phillips R, Geretti AM, Chadwick DR. Long-term responses to first-line antiretroviral therapy in HIV and hepatitis B co-infection in Ghana. Journal of Infection. 2014; 69: 481–489.10.1016/j.jinf.2014.06.012
- Wang H, Li Y, Zhang C, et al. Immunological and virological responses to combined antiretroviral therapy in HIV/hepatitis B virus-coinfected patients from a multicenter cohort. AIDS. 2012; 26: 1755–1763.10.1097/QAD.0b013e328355ced2
- van Griensven J, Phirum L, Choun K, Thai K, De Wegghelere A, Lynen L. Hepatitis B and C Co-infection among HIV-infected adults while on antiretroviral treatment: long-term survival, CD4 cell count recovery and antiretroviral toxicity in Cambodia. PLoS One. 2014; 9(2): e88552.10.1371/journal.pone.0088552
- Wandeler G, Gsponer T, Bihl F, et al. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infectious Diseases. 2013; 208: 1454–1458.10.1093/infdis/jit351
- Okwuraiwe AP, Audu RA, Salu OB, et al. Immunological and virological response to haart in HIV-1 patients co-infected with hepatitis B and C viruses. West Afr J Med. 2012; 31: 124–128.
- Weimer LE, Fragola V, Floridia M, et al. Response to raltegravir-based salvage therapy in HIV-infected patients with hepatitis C virus or hepatitis B virus coinfection. J Antimicrob Chemother. 2013; 68: 193–199.10.1093/jac/dks341
- Floridia M, Ravizza M, Tamburrini E, et al. Diagnosis of HIV infection in pregnancy: data from a national cohort of pregnant women with HIV in Italy. Epidemiol Infect. 2006; 134: 1120–1127.10.1017/S0950268806006066
- Scheuerle A, Tilson H. Birth defect classification by organ system: a novel approach to heighten teratogenic signalling in a pregnancy registry. Pharmacoepidemiol Drug Saf. 2002; 11: 465–475.10.1002/(ISSN)1099-1557
- Bertino E, Spada E, Occhi L, et al. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. 2010; 51: 353–361.
- Martin CM, Welge JA, Shire NJ, Shata MT, Sherman KE, Blackard JT. Cytokine expression during chronic versus occult hepatitis B virus infection in HIV co-infected individuals. Cytokine. 2009; 47: 194–198.10.1016/j.cyto.2009.06.005
- Selvaraj S, Paintsil E. Virologic and host risk factors for mother-to-child transmission of HIV. Curr HIV Res. 2013; 11: 93–101.10.2174/1570162X11311020003