Abstract
Background: There is an international epidemic of hepatitis C virus (HCV) infection among HIV-infected men who have sex with men. We previously showed that adding telaprevir to pegylated interferon (IFN) and ribavirin (RBV) both shortened treatment and increased the cure rate of early HCV in these men. Whether shortening treatment of early HCV using IFN-free regimens would be similarly successful has not yet been demonstrated.
Methods: We performed a pilot study of treatment with sofosbuvir (SOF) + RBV for 12 weeks in early genotype 1 HCV infection in HIV-infected men. The primary endpoint was SVR 12.
Results: Twelve men were treated with 12 weeks SOF + RBV and 11 (92%) achieved SVR 12. Most (63%) were actively using recreational drugs, mostly methamphetamine. The one man who failed had laboratory results more characteristic of chronic than of early HCV infection. The overall safety profile was similar to that known for SOF + RBV.
Conclusions: The success of this short-duration IFN-free treatment in early HCV infection is proof in principle that enhanced treatment responsiveness is an inherent characteristic of early HCV infection and not a function of IFN treatment itself. Future studies should now be done with more potent regimens to try to further shorten therapy. In the mean time, in clinical practice early HCV infection should be treated immediately after detection to take advantage of short-duration treatments, as well as to decrease further HCV transmission among HIV-infected MSM.
Chronic infection with hepatitis C virus (HCV) was difficult to treat in the interferon (IFN) era. The side effects were many and onerous for the nearly year-long treatments, and the response rates were poor, especially for treatment of genotype 1 infections, the most common genotype in the Europe and the United States. In contrast, during early stages of HCV infection, commonly called “acute” infection, IFN-based regimens are at least twice as effective, with cure rates as high as 98% with half the length of treatment.Citation1 Excellent outcomes with shorter treatment were later demonstrated in HIV-infected men who have sex with men (MSM), an emerging risk group for HCV infection world-wide,Citation2–11 although the cure rates were lower, an average of about two thirds.Citation4,6,12–21 A decade after the first observation of sexual transmission of HCV among MSM, with the availability of the first direct-acting antivirals (DAA) against HCV, we demonstrated that adding telaprevir (TVR), a first-generation HCV protease inhibitor to pegylated IFN (peg-IFN) and ribavirin (RBV) for a 12-week course improved the cure rate for HIV-infected men with early HCV infection to 89% despite this much shorter treatment duration.Citation21,22 Our findings were overall replicated in a study in Holland treating HIV-infected MSM with early HCV infection using a 12-week regimen of boceprevir (BOC) + peg-IFN + RBV, although they found no improvement in cure rate compared to their experience with 24 weeks treatment using peg-IFN + RBV alone.Citation23
The release of sofosbuvir (SOF), a potent HCV nucleotide inhibitor, in the US in December 2013, marked the end of the IFN era. In traditionally difficult to treat populations, including those with genotype 1 HCV and HIV infection,Citation24,25 the 24-week regimen of SOF + RBV cured two thirds to three quarters of patients. Whether this IFN-free regimen could be used as short-course therapy to treat early HCV, however, was unknown. Based on our prior experience using IFN-based regimens in the treatment of early HCV infection we hypothesized that we could use 12 weeks of SOF + RBV, half the recommended treatment duration for chronic HCV, to treat early genotype 1 HCV infection in HIV-infected men with an efficacy at least comparable to that of the 24 week treatment in chronic HCV infection.
Methods
HIV-infected MSM suspected to have newly acquired HCV infection were referred to a practice (DSF) within the Mount Sinai Medical Center through a network of providers of HIV healthcare in the New York City area (New York Acute Hepatitis C Surveillance Network) established to study newly-acquired HCV infection among HIV-infected MSM.Citation7,21,26–29 Written informed consent was obtained with approval of the Mount Sinai/Icahn School of Medicine Institutional Review Board in accordance with the Helsinki Declaration of 1975, as revised in 2000, and the men were then screened for eligibility for the SOF treatment protocol. The eligibility criteria were: (1) new ALT elevation of any magnitude, with causes other than HCV excluded, plus, (a) for primary infection, documented HCV viremia and recent antibody (Ab) seroconversion; or (b) for re-infection, evidence of treatment-induced or spontaneous clearance of at least 12 weeks duration after the prior infection before detection of new viremia; and (2) genotype 1 HCV viremia. The exclusions were: (1) non-genotype 1 HCV; and (2) judgment of the principal investigator (DSF) that the patient was no longer in the enhanced treatment responsiveness period of early HCV infection. The clinical onset of HCV infection was defined as the first documented ALT elevation, HCV Ab seroconversion, or HCV viremia, whichever came first. We define acute HCV as the period between infection and seroconversion, and early HCV as the period encompassing enhanced treatment responsiveness until onset of chronic HCV infection, without a restrictive time period.
To ensure we wouldn’t treat men who would spontaneously clear their infections, we observed men for 12 weeks who met the following criteria for having a reasonable chance of spontaneous clearance: (1) the first measured HCV viral load (VL) was < 4 log10 IU/mL; or (2) ≥ 1 log10 IU/mL fluctuations in VL during the early pre-treatment observation period. We balanced the decision to delay treatment against our experience that some men have a short period of enhanced treatment responsiveness and that earlier treatment would be necessary in these cases.
The regimen was SOF 400 mg plus RBV (standard weight-based dosing) taken together in a single daily dose. Treatment duration was 12 weeks. Sustained virological response (SVR) 12, defined as HCV VL of < 15 IU/mL at least 12 weeks after completing treatment, was the primary study endpoint.
There were no requirements for receiving antiretroviral (ARV) medication, suppression of HIV viremia, or stability of ARV medication. Changes in ARV medications were allowed at any time prior to or during SOF + RBV treatment. The only excluded ARV was tipranavir. Suppression of HIV viremia was defined as HIV VL < 50 copies/mL. There were no specific exclusions for active cancers or opportunistic infections, or for active drug or alcohol use.
The HCV antibody test we used was the 3rd generation HCV EIA version 2.0 (Abbott Laboratories). The HCV VL test was COBAS AmpliPrep/COBAS TaqMan HCV Test (Roche Diagnostics), lower limit of quantification (LLOQ) 15 IU/mL. The HCV genotype was determined by sequencing portions of both the core and NS5B regions of the HCV genome (ARUP Laboratories). The HIV VL test was COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0 (Roche Diagnostics), LLOQ 20 copies/mL. IFNL3 (formerly IL28B) SNP-RS12979860 C and T alleles were determined using an allele-specific primer extension assay measured with the Luminex LX 200 instrument (Molecular Pathology Laboratory, Mount Sinai Medical Center).
Results
Baseline demographic and HIV characteristics
We enrolled 13 HIV-infected MSM with newly acquired HCV infection at the Mount Sinai Medical Center between October 2013 and August 2014. One spontaneously cleared HCV during the observation period and is not discussed further.
The median age was 43 years. Seven (63%) were white, two were black, two were Hispanic, and one was Asian. Seven (63%) had the favorable CC IFNL3 genotype. The median duration of diagnosed HIV infection was six years. Two (18%) were not receiving ARV medications at the time of HCV diagnosis and four (36%) did not have suppression of HIV viremia. The median CD4 count was 545 cells/μL; none had a CD4 count < 200 cells/μL. (Table ) Seven (63%) were actively using recreational drugs, mostly methamphetamine, including injection use in five (37%); all denied sharing of injection equipment.
Table 1 Baseline demographic characteristics of 12 HIV-infected men treated for early hepatitis C Infection with sofosbuvir plus ribavirin
HCV-related characteristics
All 12 men had HCV infection detected due to new elevations of ALT that led to specific HCV testing. None were symptomatic at the time of HCV diagnosis and none developed jaundice during their clinical course; the median peak total bilirubin was 1.1 mg/dL. HCV Ab was negative at first testing in five (42%); all seroconverted before treatment was started. The median peak ALT was 448 U/mL, ranging down to 103 U/mL (Patient 7, discussed below). The median peak HCV VL was 6.2 log10 IU/mL, ranging up to 8.0 log10 IU/mL. We documented wide (>1 log10 IU/mL) and rapid fluctuations in HCV VL during the observation period, which are characteristics of early HCV infection,Citation30 in 9 (75%) of men, with a median flux of 2.6 log10 IU/mL. The median treatment baseline HCV VL was 4.5 log10 IU/mL, although in two (17%) men it was very high (>7 log10 IU/mL). None of the men had an undetectable HCV VL at the time of initiation of treatment. (Table )
Table 2 Hepatitis C characteristics and outcomes of 12 HIV-infected men treated with sofosbuvir plus ribavirin for early hepatitis C infection
Treatment
Twelve men initiated and successfully completed 12 weeks of treatment with SOF + RBV. All had HCV VL < 15 log10 IU/mL at end of treatment and at week 4 post-treatment. Eleven men had HCV VL < 15 log10 IU/mL at 12 weeks after completing treatment, for an SVR 12 rate of 92%.
The time from diagnosis to treatment varied widely, from a relatively short period of one month to a very long period of nine months after diagnosis. While we attempted to treat quickly those with laboratory testing suggesting they were progressing toward chronic infection, such as HCV VL progressively increasing toward levels typical for chronic HCV (i.e. >6 log10 IU/mL), we were not always successful in accomplishing that goal. Figure shows this trend toward a shorter time to treatment in those with a higher VL (r2 = 0.2, p = 0.15). The two main reasons for delay in treatment were late referrals and long delays in obtaining approval from both public and private insurance companies, or through charitable foundations.
Figure 1. Correlation between HCV viral load at the time of treatment and time to treatment after HCV diagnosis
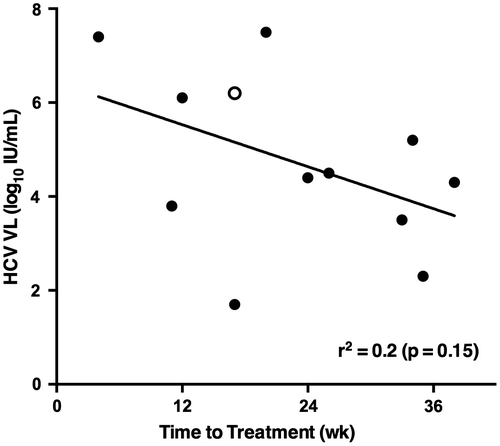
Only one man, Patient 7, failed treatment. (Figure , open circle) He had been completely adherent to treatment with both SOF and RBV, but had recrudescent viremia (genotype 1a HCV), which was detected at the week 8 (but not at week 4) post-treatment visit. We determined on epidemiological grounds that he failed treatment rather than having been re-infected, as he had stopped having sex completely after the diagnosis of HCV re-infection was made, and he never had parenteral exposures. Of note, he had laboratory results most characteristic of chronic rather than of early HCV infection (no fluctuation of HCV VL over 12 weeks observation; and treatment baseline HCV VL 6.1 log10 IU/mL and ALT of 53 U/mL) and he had a previous null response to treatment with peg-IFN + RBV for his primary HCV infection.
Safety
The side effects of fatigue and irritability that were experience by this cohort were similar to those previously described for SOF + RBV in HIV-infected patients.Citation25 There were no cases of significant anemia (hemoglobin < 10 g/dL) and none had RBV dose reduction. There were no HIV-related events.
Discussion
In this study we have shown that treatment of early HCV infection with 12 weeks of SOF + RBV is at least as effective as 24 weeks of this regimen for chronic HCV in HIV-infected patientsCitation25 (SVR 92% compared to 76%, respectively). Additionally, our results were achieved despite a high proportion of men actively using recreational drugs, mostly methamphetamine, a group frequently singled out as not ready or suited for treatment. These findings extend those of our previous studyCitation21,22 that showed treatment of early HCV with 12 weeks TVR + peg-IFN + RBV was at least as effective as 48 weeks of the TVR-containing regimen in HIV-infected patients with chronic HCV.Citation31
While the results of our study are promising, two other studies using this regimen were not as successful as ours. The multi-center ACTG “SWIFT-C” studyCitation32 treated for 12 weeks, curing just 8 (53%) of 15 men with genotype 1 HCV. Although our study and the “SWIFT-C” study used the same treatment duration, there were a number of factors that could have contributed to the difference in results. The “SWIFT-C” study, performed at multiple sites nationally in the United States, required a delay of between 12 and 24 weeks after detection of HCV infection before treatment to allow for spontaneous clearance, resulting in median time to treatment of 20 weeks, and a treatment baseline VL of 6.4 log10 IU/mL,Citation32 which is equivalent to that of chronic HCV in HIV-infected patients.Citation33 Further, the “SWIFT-C” study used twice daily RBV dosing and found RBV levels to be much lower than previously measured in other clinical trials; and low levels RBV correlated with treatment failure.Citation34 This observation does not in itself explain the poor treatment outcome, however, as low RBV levels could result in treatment failure with either early or chronic infection. In fact, given the high treatment baseline HCV VL in “SWIFT-C,” we hypothesize that many of those patients were, in fact, closer to chronic HCV, that is, no longer in the early treatment responsiveness phase of their HCV infection. This timing, in combination with low RBV levels, possibly due to poor adherence with the second dose, resulted in the high failure rate of the short-course treatment.Citation35 Similarly, the “DARE-C II” study in Australia treated patients with just 6 weeks SOF + RBV starting a median of 37 weeks after detection of HCV, curing just 3 (23%) of 13 with genotype 1 HCV.Citation36 Although the median treatment baseline HCV VL was 5.4 logs, those who failed treatment had VL in the chronic range. Taken together these two studies in contrast without study suggest that short- and very-short-course SOF + RBV is very sensitive to waiting too long after HCV infection, when the HCV VL increases from the low levels seen in early HCV due to endogenous partial control of viremia, into the range of chronic infection.
Other limitations of our study should be noted. The study was performed in a single clinical practice that is expert in diagnosing and treating early HCV infection, so our high SVR rate might not be generalizable to other clinical settings. Just over half of the men had the favorable IFNL3 CC genotype, although this is the usual proportion in treatment cohorts of early HCV infection.Citation21 Additionally, the study was small in size, was not randomized, and although achieved a numerically superior result, was not powered to detect statistical superiority over historical treatment cohorts. Further, the regimen of SOF + RBV has subsequently been superseded by more potent combination DAA regimens. Our results remain important, however, as proof in principle that the enhanced treatment responsiveness during early HCV infection is not limited to IFN-based treatment regimens, but rather is an inherent characteristic of early HCV infection. That is, early HCV can be effectively treated with short-course DAA regimens without using IFN. We need now to design studies to determine with which and for how long these newer DAA regimens should be used for the treatment of early HCV infection. We and others are studying the combination of SOF/ledipasvir (LDV) in early HCV infection of HIV-infected MSM, with promising preliminary results of a 4-weekCitation37 and a 6-weekCitation38 regimen presented as abstracts.
Regardless of the regimen chosen, however, we advocate for immediate treatment of patients with early HCV in clinical practice, rather than withholding treatment for 12 to 16 weeks of observation after diagnosis to “allow for spontaneous clearance,” as suggested in the current AASLD/IDSA guidelines.Citation39 This long observation period rarely (i.e. <10 to 15%) results in spontaneous clearance (reviewed in reference [21]), but instead can result in losing the opportunity of cure with a short course of DAA therapy. In the IFN era, delaying treatment to allow this small proportion to spontaneously clear was considered justifiable due to the toxicity of IFN, although some argued otherwise.Citation40 But with the safety of DAA regimens, the current recommendations cannot be justified on the basis of safety, or of cost. Ultimately, the cost of having to use a longer treatment course for the over 85% of HIV-infected men who do not have spontaneous clearance will outweigh the cost of possibly treating fewer than 10 to 15% of these men unnecessarily. In addition, waiting so long for spontaneous clearance results in other costly problems such as further HCV transmissions during the longer period of viremia, loss to follow-up, and other issues.Citation41 Studies of treatment as prevention in this high-transmission group are clearly necessary to better quantify the potential benefits. Unfortunately, even when we try to treat patients immediately, onerous insurance regulations, both government and commercial, significantly delay treatment and even deny it outright.Citation42 These government and commercial insurance entities have been able to justify these regulations, in part, due to lack of strong recommendations by specialty societies such as the Infectious Diseases Society of America (IDSA) and the American Association for the Study of Liver Diseases (AASLD) to quickly treat early HCV infection. Changing these guidelines would be an important step in changing these restrictive regulations and facilitating treatment of early HCV infection.
Finally, we offer this analogy with HIV infection. When ARV medications were more toxic and less effective, we deferred treatment until later in the disease course. Now that ARV medications are safer and more effective, however, we treat immediately after detection of HIV infection to both improve the health of the patient,Citation43 as well as the health of future sex partners by decreasing HIV transmissions. We believe that much of the same reasoning applies to HCV infection and suggest that the recommendations about when to treat early HCV infection, at least in HIV-infected men, be changed to reflect this understanding.
In summary, we achieved a high (92%) SVR rate with just 12 weeks of SOF + RBV in treatment of early genotype 1 HCV infection, which serves as proof in principle that enhanced treatment responsiveness during early HCV infection is not a function of IFN treatment, but rather an inherent characteristic of early HCV infection. Our high SVR rate in men actively using recreational drugs shows that active substance use cannot be justified as an excuse to deny treatment with DAA. With the availability of more potent combination regimens, we need to determine which of these newer regimens to use, and for how long. Treating early HCV should not be relegated to clinical trials only, however, and in clinical practice we should treat acute and early HCV in HIV-infected men immediately to take advantage of short-course treatment for most, and not wait until patients lapse into chronic infection. By allowing these lapses, we miss both the opportunity to use treatment as prevention of sexually transmitted HCV infection among MSM,Citation44 and to save up to half the cost of these very expensive treatments, far outweighing any savings possible by delaying treatment.
Funding
This work was supported by the Icahn School of Medicine at Mount Sinai.
Statement of interests
DTD reports serving as a consultant or scientific advisor for Abbvie, BMS, Gilead Sciences, Inc., Intercept Pharmaceuticals, Inc., Merck & Co., Inc., and Janssen Therapeutics. He has received grants or research support from Abbvie, BMS, Gilead Sciences, Inc, Merck & Co., Inc., and Janssen Therapeutics. DSF is on the advisory board, is a speaker for, and received honoraria from the Chronic Liver Disease Foundation; is a speaker for and has received honoraria from the National Committee for Quality Assurance; and owns common stock in Gilead Sciences, Inc. No other authors report any conflicts of interest.
Notes
* Presented in part: American Association for the Study of Liver Disease, Poster 1090, 15 November 2015, Boston, MA.
References
- Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345(20):1452–1457.10.1056/NEJMoa011232
- Ghosn J, Pierre-Francois S, Thibault V, et al. Acute hepatitis C in HIV-infected men who have sex with men. HIV Med. 2004;5(4):303–306.10.1111/hiv.2004.5.issue-4
- Götz HM, van Doornum G, Niesters HG, et al. A cluster of acute hepatitis C virus infection among men who have sex with men–results from contact tracing and public health implications. AIDS. 2005;19(9):969–974.10.1097/01.aids.0000171412.61360.f8
- Gilleece YC, Browne RE, Asboe D, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. J Acquir Immune Defic Syndr. 2005;40(1):41–46.10.1097/01.qai.0000174930.64145.a9
- Vogel M, Bieniek B, Jessen H, et al. Treatment of acute hepatitis C infection in HIV-infected patients: a retrospective analysis of eleven cases. J Viral Hepat. 2005;12(2):207–211.10.1111/jvh.2005.12.issue-2
- Luetkemeyer A, Hare CB, Stansell J, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41(1):31–36.10.1097/01.qai.0000191281.77954.27
- Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198(5):683–686.10.1086/592362
- Bottieau E, Apers L, Van Esbroeck M, et al. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001–2009. Euro Surveill. 2010;15(39):19673.
- Barfod TS, Omland LH, Katzenstein TL. Incidence and characteristics of sexually transmitted acute hepatitis C virus infection among HIV-positive men who have sex with men in Copenhagen, Denmark during four years (2006–2009): a retrospective cohort study. Scand J Infect Dis. 2011;43(2):145–148.10.3109/00365548.2010.524660
- Sun HY, Chang SY, Yang ZY, et al. Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors. J Clin Microbiol. 2012;50(3):781–787.10.1128/JCM.06014-11
- Ishikane M, Watanabe K, Tsukada K, et al. Acute hepatitis C in HIV-1 infected Japanese cohort: single center retrospective cohort study. PLoS ONE. 2014;9(6):e100517.10.1371/journal.pone.0100517
- Dominguez S, Ghosn J, Valantin MA, et al. Efficacy of early treatment of acute hepatitis C infection with pegylated interferon and ribavirin in HIV-infected patients. AIDS. 2006;20(8):1157–1161.10.1097/01.aids.0000226956.02719.fd
- Vogel M, Nattermann J, Baumgarten A, et al. Pegylated interferon-alfa for the treatment of sexually transmitted acute hepatitis C in HIV-infected individuals. Antivir Ther. 2006;11(8):1097–1101.
- Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2009;48(5):650–658.10.1086/597768
- Piroth L, Larsen C, Binquet C, et al. Treatment of acute hepatitis C in human immunodeficiency virus-infected patients: the HEPAIG study. Hepatology. 2010;52(6):1915–1921.10.1002/hep.23959
- Lambers FA, Brinkman K, Schinkel J, et al. Treatment of acute hepatitis C virus infection in HIV-infected MSM: the effect of treatment duration. AIDS. 2011;25(10):1333–1336.10.1097/QAD.0b013e3283480144
- Obermeier M, Ingiliz P, Weitner L, et al. Acute hepatitis C in persons infected with the human immunodeficiency virus (HIV): the “real-life setting” proves the concept. Eur J Med Res. 2011;16(5):237–242.10.1186/2047-783X-16-5-237
- Thomson EC, Fleming VM, Main J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011;60(6):837–845.10.1136/gut.2010.217166
- Garg S, Taylor LE, Grasso C, et al. Prevalent and incident hepatitis C virus infection among HIV-infected men who have sex with men engaged in primary care in a Boston community health center. Clin Infect Dis. 2013;56(10):1480–1487.10.1093/cid/cit054
- Webster DP, Wojcikiewicz T, Keller M, et al. Spontaneous clearance and treatment of acute hepatitis C infection in HIV-positive men with 48 weeks of interferon-alpha and ribavirin. Int J STD AIDS. 2013;24(3):179–183.10.1177/0956462412472317
- Fierer DS, Dieterich DT, Mullen MP, et al. Telaprevir in the treatment of acute hepatitis C virus infection in HIV-infected men. Clin Infect Dis. 2014;58(6):873–879.10.1093/cid/cit799
- Fierer DS, Dieterich DT, Mullen MP, et al. Telaprevir in the treatment of acute HCV infection in HIV-infected Men: final results. American Association for the Study of Liver Disease. Poster 1112. Boston, MA, 15 February 2015.
- Hullegie SJ, Claassen MA, van den Berk GE, et al. Boceprevir, peginterferon and ribavirin for acute hepatitis C in HIV infected patients. J Hepatol. 2016;64(4):807–812.10.1016/j.jhep.2015.12.004
- Osinusi A, Meissner EG, Lee YJ, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics. JAMA. 2013;310(8):804–811.10.1001/jama.2013.109309
- Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312(4):353–361.10.1001/jama.2014.7734
- Fierer DS, Factor SH, Uriel AJ, et al. Sexual transmission of hepatitis C virus (HCV) among HIV-infected men who have sex with men (MSM), New York City, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60(28):945–950.
- Fierer DS, Mullen MP, Dieterich DT, et al. Early-onset liver fibrosis due to primary hepatitis C virus infection is higher over time in HIV-infected men. Clin Infect Dis. 2012;55(6):887–888.10.1093/cid/cis538
- Fierer DS, Dieterich DT, Fiel MI, et al. Rapid progression to decompensated cirrhosis, liver transplant, and death in HIV-infected men after primary hepatitis C virus infection. Clin Infect Dis. 2013;56(7):1038–1043.10.1093/cid/cis1206
- Turner SS, Gianella S, Yip MJ-S, et al. Shedding of hepatitis C virus in semen of HIV-infected men. Open Forum Infect Dis. 2016;3(2). ofw057.
- Heller T, Rehermann B. Acute hepatitis C: a multifaceted disease. Semin Liver Dis. 2005;25(1):7–17.10.1055/s-2005-864778
- Sulkowski MS, Sherman KE, Dieterich DT, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159(2):86–96.
- Naggie S, Marks KM, Hughes M, et al. Sofosbuvir plus ribavirin without interferon for treatment of acute hepatitis C virus infection in HIV-1 infected individuals (SWIFT-C). American Association for the Study of Liver Disease. Abstract 1094. Boston, MA, 15 November 2015.
- Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon Alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351(5):451–459.
- MacBrayne CE, Hughes M, Hollabaugh KM, et al. Lower ribavirin exposures in HIV+ patients that relapsed to acute HCV treatment. Conference on Retroviruses and Opportunistic Infections. Abstract 99. Boston, MA, 15 February 2016.
- Taylor LE, Fierer DS. Treatment of early hepatitis C infection in HIV-infected men – when to treat, whom, and with what? Curr Treat Options Infect Dis. e-pub. 10 October 2016.
- Martinello M, Gane EJ, Hellard M, et al. Sofosbuvir and ribavirin for 6 weeks is not effective among people with recent hepatitis C virus infection: the DARE-C II study. Hepatology. epub 17 September 2016.
- Basu P, Shah NJ, John N, et al. Sofosbuvir and ledipasvir versus sofosbuvir and simeprevir combination therapy in the management of acute hepatitis C: a randomized open label prospective clinical pilot study. SLAM C study. Interim data. American Association for the Study of Liver Disease (AASLD). Abstract 1074. Boston, MA, 15 November 2015.
- Rockstroh JK, Bhagani S, Hyland RH, et al. Ledipasvir/sofosbuvir for 6 weeks in HIV-infected patients with acute HCV infection. Conference on Retroviruses and Opportunistic Infections. Abstract 154LB. Boston, MA, 25 February, 2016.
- AASLD-IDSA. Management of acute HCV infection. Recommended treatment for Patients with acute HCV infection. Accessed 24 July 2016. http://hcvguidelines.org/full-report/management-acute-hcv-infection.
- Calleri G, Cariti G, Gaiottino F, et al. A short course of pegylated interferon-alfa in acute HCV hepatitis. J Viral Hepat. 2007;14(2):116–121.10.1111/jvh.2007.14.issue-2
- Kaplan-Lewis E, Fierer DS. Acute HCV in HIV-Infected MSM: modes of acquisition, liver Fibrosis, and treatment. Curr HIV/AIDS Rep. 2015;12(3):317–325.10.1007/s11904-015-0279-3
- Barua S, Greenwald R, Grebely J, et al. Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C Virus infection in the United States. Ann Intern Med. 2015;163(3):215–223.10.7326/M15-0406
- HHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Updated 28 January 2016. Accessed 1 May 2016. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0.
- Martin NK, Thornton A, Hickman M, et al. Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis. 2016;62(9):1072–1080.10.1093/cid/ciw075
