Abstract
Background: Nucleos(t)ide reverse transcriptase inhibitor (NRTI)-sparing regimens may potentially minimize antiretroviral (ART) toxicities, but demonstrate mixed efficacy and toxicity results. The impact of an integrase strand transfer inhibitor (INSTI) and protease inhibitor (PI) regimen on HIV viral dynamics and T cell kinetics remains underdescribed.
Objective: To compare the effect of raltegravir + ritonavir boosted lopinavir (RAL + LPV/r) to efavirenz/tenofovir disoproxil fumarate/emtricitabine (EFV/TDF/FTC) on HIV kinetics and T cell dynamics.
Methods: Fifty participants naïve to ART underwent HIV viral kinetic sampling evaluated using biexponential mixed effects modeling. A subset of 28 subjects (with complete viral suppression) underwent flow cytometry and evaluation of soluble markers of inflammation at weeks 0, 4, and 48 of ART.
Results: RAL + LPV/r compared to EFV/TDF/FTC resulted in a prolonged first phase viral decay rate (18 vs. 13 days p < 0.01). From weeks 0 to 4, RAL + LPV/r was associated with a trend toward greater decreases in activated CD4+ T cells (−3.81 vs. −1.18 p = 0.09) and less decreases in activated effector memory CD4+ T cells (−0.63 vs. −2.69 p-0.07). These trends did not persist to week 48. No differences were noted at any time point for soluble markers of immune activation.
Conclusions: The prolonged first phase viral decay observed with RAL + LPV/r in persons starting ART did not result in differences in viral suppression at week 48. We also observed trends in declines in certain cellular markers of immune activation but it remains unclear if this could translate to long-term immunologic benefits in persons on an INSTI + PI.
Introduction
Currently recommended combined antiretroviral therapy (cART) regimens for use in persons with HIV infection naïve to cART all include nucleos(t)ide reverse transcriptase inhibitors (NRTIs).Citation1 However, the use of some NRTIs is associated with adverse effectsCitation2–5 and this drug class is frequently subject to transmitted drug resistance.Citation6, 7 Early NRTI-sparing studies were pursued due to use of didanosine (ddI), stavudine (D4T), and zidovudine (ZDV), which are no longer recommended by guidelines due to clinical and long-term toxicities. Several studies in cART experienced patients that undergo a switch from an NRTI-containing regimen to an NRTI-sparing regimen suggest the removal of specific NRTI agents from a cART regimen improves mitochondrial toxicity and lipoatrophy (d4T, ddI, ZDV),Citation8, 9 bone and renal diseases (tenofovir disoproxil fumarate [TDF]),Citation10 and cardiovascular risk (ABC)Citation11, 12 adding support to the use of NRTI-sparing regimens. Riddler et al. (ACTG A5143) reported that participants naïve to cART who initiated the NRTI-sparing regimen of lopinavir-ritonavir (LPV/r) + efavirenz (EFV) had similar virologic efficacy when compared to EFV + TDF and emtricitabine (FTC), but did have greater clinical and lipid toxicity and greater levels of drug resistance at virologic failure.Citation13 More recent studies of NRTI-sparing regimens have included newer antiretrovirals and have mostly demonstrated similar virologic responsesCitation14–19 but several studies have found inferior virologic response in subjects with low CD4 or HIV RNA > 100,000 copies/mL.Citation19 Also some combinations (raltegravir [RAL] + atazanavir [ATV]) may still be suboptimal to current standards of care because of the emergence of drug resistance mutations.Citation15
While the virologic efficacy of NRTI-sparing regimens remains debated, research has revealed both potential benefits and risks to treating naïve HIV-infected persons. Participants in ACTG A5142 enrolled in the NRTI-sparing arm (LPV/r + EFV) were less likely to demonstrate lipoatrophy than NRTI regimens with d4T and ZDV, but similar to TDF-containing regimens.Citation20 This study evaluated older NRTIs and in the current era, issues such as lipoatrophy may be less relevant. However, issues such as decreases in bone mineral density are relevant as our HIV population ages; a more recent study did demonstrate that DRV/r + RAL had significantly less reduction in bone mineral density,Citation18 compared to DRV/r + FTC/TDF.Citation21, 22
We recently reported that an NRTI-sparing regimen of RAL + LPV/r compared to EFV/TDF/FTC had similar virologic outcomes in HIV-infected persons naïve to antiretroviral therapy (California Collaborative Trials Group 589 or CCTG 589).Citation23 This paper further evaluates the impact of an INSTI + PI regimen on HIV viral kinetics, T cell subset dynamics, and soluble markers of inflammation which may impact the infectious period prior to complete suppression as well as chronic inflammation and subsequent development of HIV-associated non-AIDS diseases.Citation24–26
Methods
Patient population and study design
This manuscript reports the viral kinetic results of CCTG 589 (NCT00752856) and the results of a planned immunologic sub-study. CCTG 589 was a 1:1 randomized open-label 48-week pilot study comparing EFV/TDF/FTC, a fixed dose combination of a non-nucleoside reverse transcriptase inhibitor (NNRTI), and NRTIs to RAL + LPV/r, a twice daily integrase strand transfer inhibitor (INSTI) and protease inhibitor (PI) in HIV-infected (plasma HIV-1 RNA ≥ 5000 copies/mL and a CD4 cell count ≥ 50 cells/mmCitation3), treatment-naïve subjects. All study participants underwent informed consent prior to entry of the study and received a random study number to ensure patient anonymity. Study procedures were subject to approval by local IRBs. Eligibility criteria have previously been described.Citation23 Study participants underwent intensive monitoring of viral decay dynamics with plasma HIV-1 RNA measurements at baseline and days 2, 7, 10, and 14 followed by viral loads at weeks 4, 8, 12, 16, 24, 36, and 48.
The immunologic sub-study evaluates the participants of CCTG 589 that achieved virologic suppression by week 24 and maintained suppression to week 48 (Supplemental Figure 1).
Evaluations of cellular immune activation
Fresh whole blood was collected from participants at study entry (week 0), weeks 4 and 48 of study, and processed using density gradient centrifugation to obtain viable peripheral blood mononuclear cells (PBMCs). PBMCs were washed and aliquoted into tubes at concentration of 1 million PBMCs/200 uL prior to incubation with Aqua live/dead (Invitrogen, Grand Island, NY) and conjugated antibodies (Becton Dickinson and Co., Franklin Lakes, New Jersey) to CD3 (APC-Cy7), CD4 (PerCP-Cy5.5), CD8 (Pac-Blue), CD45RA (PE), CD27 (APC), CCR5 (FITC), CCR7 (PE-Cy7), HLA-DR (FITC), CD38 (PE-Cy7), CCR6 (PE-Cy7), and CD28 (PE-Cy7).Citation27 Conjugated antibodies to intracellular FoxP3 (FITC), intracellular IgG1 (FITC), and intracellular Ki67 (FITC) were used with fixation and permeability buffers (eBioscience, San Diego, CA), per manufacturer instructions. Samples were run on the BDFACSCanto (BD Biosciences, San Jose, CA) and data analyzed with FlowJo software (Tree Star Inc, Ashland, OR).
Evaluations of soluble markers of the immune system
Blood plasma samples were collected from participants at weeks 0 and 48 and stored at −80 °C until the time of analysis. Interleukin-6, soluble CD163 (sCD163), and soluble CD14 (sCD14) were evaluated using Quantikine ELISA Kits (R&D Systems, Minneapolis MN).
Statistical analysis
To study the viral kinetics, the first 8 weeks of HIV-1 RNA data were used. Response profiles that were inconsistent with monotonic viral level decay were truncated at the first signs of rebound (defined as an increase of >0.3 log10 copies/ml from the previous observation). The biexponential model requires a monotonic viral decay pattern. Thus, data points were removed for three subjects: one in the EFV/TDF/FTC group and two in the RAL + LPV/r group. All exclusions occurred at or after week 4.
A parametric non-linear mixed effects model was used to fit the viral dynamic model to the remaining data. The model takes a bi-exponential form for HIV-1 RNA copies/mL and is fitted to data on a log10 scale to normalize the error distribution. Estimation of the model uses a Newton–Raphson algorithm with an embedded multiple imputation to randomly impute for HIV-1 RNA levels censored below 50 copies/mL. Empirical Bayes estimates of the first and second phase decay rates from this model were compared between treatment groups with non-parametric Wilcoxon tests. In the event of between-group differences, group-specific biexponential mixed effect models were fitted.
To study the T cell dynamics, baseline characteristics of this subset population were summarized by treatment groups and overall. For each of CD4 and CD8 T cell subset outcomes, mixed model repeated measures analysis was performed. The model included change from baseline in each outcome at weeks 4 and 48 as the dependent variable, treatment, visit, treatment-by-visit interaction, and baseline value as fixed effects. Visit was treated as a categorical variable and an unstructured variance–covariance error matrix was applied. Differences in least-square means between the treatment groups were reported. A p-value of <0.05 was considered statistically significant. As there were no statistically significant findings in planned immunologic analyses, no adjustments were made for multiple comparisons. For exploratory outcomes, all values with a p-value of <0.1 were reported. As these outcomes were exploratory, we did not adjust for multiple comparisons. Statistical analyses were performed in R (http://cran.r-project.org), version 2.14.0.
Results
Study participants
The demographics of study participants in CCTG 589 have been previously described.Citation23 CCTG 589 screened 65 subjects over one year and 51 met entry criteria and were randomized to either RAL + LPV/r (n = 26) or EFV/TDF/FTC (n = 25). Fifty underwent intensive viral kinetics (Supplemental Figure 1). We previously demonstrated that use of RAL + LPV/r compared to EFV/TDF/FTC had lower viral suppression rates at week 4 (54% vs. 12% p = 0.003), but no differences in viral suppression between the two groups was observed at weeks 8 or 48.Citation23
Differences in viral kinetics
To better characterize the virologic response to RAL + LPV/r compared to EFV/TDF/FTC, we evaluated viral kinetics using biexponential modeling. Use of RAL + LPV/r resulted in a slower first phase viral decay rate median = 0.47, (IQR: 0.42–0.52) compared to EFV/TDF/FTC median = 0.55, (IQR: 0.52–0.58) (p < 0.001). In spite of this slower decay rate, RAL + LPV/r prolonged phase 1 viral decline median = 18 days, (IQR: 16–22) vs. median = 13 days, (IQR: 12–13; p < 0.001) resulting in lower viral loads at the time of transition from Phase 1 to Phase 2 viral decay median = 1.96 log10 copies/mL (IQR: 1.83, 2.37) vs. median = 2.82 log10 copies/mL (IQR: 2.46, 2.97) (Figure with data in Table ). The second phase viral decay rates were similar between RAL + LPV/r and EFV/TDF/FTC (Table ).
Figure 1 Viral kinetics RAL + LPV/r compared to EFV/TDF/FTC.
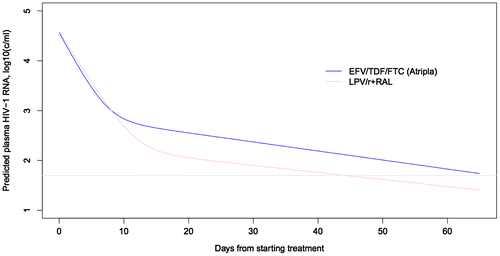
Table 1 Comparison of empirical Bayes parameter estimates between arms
Description of immunologic sub-study population who achieved and maintained viral suppression
At weeks 24 and 48, a total of 28 (62.2%) participants maintained virologic suppression and were included in an apriori immunologic sub-study (Supplemental Figure 1). Of the persons who achieved and maintained virologic suppression, those who initiated EFV/TDF/FTC and persons who initiated the NRTI-sparing regimen RAL + LPV/r did not differ by age, gender, ethnicity, or race. Despite randomization, at baseline, persons who initiated RAL + LPV/r were more likely to report heterosexual sex and intravenous drug use (IDU) as routes of transmission of HIV (p = 0.014) (Table ).
Table 2 Baseline characteristics of immunologic sub-study participants
Cellular markers of proliferation and immune activation
In previous trials, use of RAL + LPV/r compared to EFV/TDF/FTC does not significantly impact CD4 + T cell counts.Citation23 To build on this work and better understand if an INSTI + PI regimen impacts specific T cell subset dynamics, we performed analyses of activated and proliferating CD4+ and CD8+ T cells with a specific focus on mature T cell subsets (central memory, effector memory, and effector cells). At study entry, participants had similar percentages of activated central memory (CD4+CD45RA-CD27+CD38+) and effector memory (CD4+CD45RA-CD27-CD38+) CD4+ T cells and activated central memory (CD8+CD45RA-CD27+CD38+) and effector (CD8+CD45RA-CD27-CD38+) CD8+ T cells.
Analyses of CD4+ T cell dynamics reveal persons taking RAL + LPV/r had a trend for a greater decrease in activated (CD4+CD38+) CD4+ T cells mean change −3.81 (95% CI: −6.12, −1.51) compared to persons taking EFV/TDF/FTC −1.18 (95% CI: −3.17,0.80) (p = 0.092) from weeks 0 to 4, but this effect did not persist on evaluations of T cell dynamics of weeks 0 to 48 (Table ). Conversely, we noted a trend for less decreases in activated effector memory (CD4+CD45RA-CD27-CD38+) CD4+ T cells –0.63 (95% CI: −2.31, 1.06) in the RAL + LPV/r arm compared to EFV/TDF/FTC −2.69 (95% CI: −4.15, −1.23) (p = 0.07) from weeks 0 to 4 without any difference in rate of change from weeks 0 to 48 (Table ). There were no statistically significant differences or trends noted in total percentages of activated or proliferating lymphocytes between the two arms at weeks 4 or 48.
Table 3 Estimated mean change from baseline in activated and proliferating T cell dynamics
Analyses of CD8+ T cell dynamics did not reveal significant differences between the two arms (Table ).
Exploratory analyses of CD4+ T cell subsets
To evaluate the impact of an NRTI-sparing regimen on other CD4+ and CD8+ immunologic parameters, exploratory analyses of T cell subsets were performed. No significant differences were observed between the two arms among the proportions of CD4+ T cell subsets at any time but significant differences did exist in the T cell subset dynamics. Persons taking RAL + LPV/r had significantly greater increases in the percentage of CD38-HLA-DR+ central memory CD4+ T cells (CD4+CD45RA-CD27+CD38-HLA-DR+) at week 4 mean change = 2.86 (95% CI: 1.40, 4.32) vs. 0.55 (95% CI: −0.71, 1.81); p = 0.02) (Supplementary Table 1).
Evaluations of T cell dynamics from weeks 0 to 48 revealed participants in the RAL + LPV/r arm had greater increases in the proportion of proliferating naïve (CD4+CD45RA+cKi67+) CD4+ T cells 7.63 (95% CI: 4.39, 10.86) vs. −0.83 (95% CI: −3.2, 1.83) (p < 0.001). Treatment with RAL + LPV/r also demonstrated increases in natural T regulatory cells (CD4bright FoxP3+CD45RA+) with a mean slope 2.5 (95% CI: −2.54, 7.59) while participants on EFV/TDF/FTC decreased −8.03 (95% CI: −12.4, −3.7) (p = 0.003). Induced T regulatory cells (CD4bright FoxP3+CD45RA-) changed in proportion with natural T regulatory cells, with participants on RAL + LPV/r showing a decrease in this subset of −0.51 (95% CI: −4.25, 3.2) compared to persons on EFV/TDF/FTC with 9.3 (95% CI: 6.08,12.55) (p = 0.004) (Supplementary Table 1).
Exploratory analyses of CD8+ T cell subsets
There were also observed differences in other CD8+ T cell dynamics. From weeks 0 to 4, persons on RAL + LPV/r had significantly greater increases in CD38-HLA-DR+ effector memory CD8+ T cells (CD8+CD45RA+CD27-CD38-HLADR+) 3.23 (95% CI: 2.04, 4.42) than EFV/TDF/FTC 0.996 (95% CI: −0.3, 2.02) (p = 0.006) but this was not sustained to week 48 (Supplementary Table 1).
Soluble markers of immune activation
To evaluate if differences in rate of viral load suppression impacted other markers of inflammation, we also evaluated levels of IL-6, CD163, and sCD14 at baseline and at week 48. There were no significant differences between persons on RAL + LPV/r and EFV/TDF/FTC in baseline levels of these markers. No differences were noted in these markers between the two groups over time (Data not shown).
Discussion
Biexponential modeling of HIV viral kinetics revealed that starting persons on RAL + LPV/r resulted in a slower but prolonged first phase viral decay compared to EFV/TDF/FTC that ultimately resulted in lower HIV viral loads at time of transition from phase 1 to phase 2. Although we evaluated viral kinetics in a novel combination (INSTI + boosted PI), this finding is consistent with what has been observed with INSTI + NRTIs regimens that demonstrate longer first phase decay compared to NNRTI + NRTIs-based regimens and PI + NRTIs or PI + NNRTI.Citation28–31 In this study, we observed an even longer and slower phase 1 decay with INSTI + PI compared to historical data on INSTI + NRTIs but the median HIV VL at transition to phase 2 was similar.Citation30 Yet, it remains unknown if this prolonged phase 1 decay and subsequent early viral suppression can decrease risk of onward HIV transmission in HIV-infected persons who continue to participate in condomless sex shortly after ART initiation.Citation32, 33 It has been proposed that the rate of viral decay is a function of the “fastest acting drug;” thus, the longer first phase viral decay is likely related to the INSTI rather than combination of INSTI + PI.Citation34 Of note, the recently available tenofovir alafenamide fumarate (TAF) demonstrates a more rapid first phase viral decay than TDFCitation35 and combinations with INSTIs may prove to be particularly potent.
We also observed that the use of RAL + LPV/r compared to EFV/TDF/FTC resulted in trends toward more rapid decrease of total activated CD4+ T cells at week 4, but not at week 48. This likely reflects early decreases in HIV VL and subsequent decreases in activated CD4+ T cells or more rapid clearance of productively infected activated CD4+ T cells due to the prolonged phase 1 HIV viral kinetics of the INSTI + PI-based regimen. In exploratory analyses, RAL + LPV/r compared to EFV/TDF/FTC also altered the dynamics of other T cell subsets demonstrating both early and late changes. However, no correction for multiple comparisons was applied to this portion of the analysis and it is unclear if our findings are of early or clinical significance.
Overall, the T cell dynamics observed in persons on RAL + LPV/r compared to EFV/TDF/FTC suggest this regimen may promote decreased cellular immune activation likely due to its impact on viral load decay. However, we cannot differentiate if these differences were due to INSTI,Citation36 INSTI + PI combination, or NRTI sparing. Early decreases in activated CD4+ T cells during HIV treatment may be clinically relevant because it could: (1) minimize productive infection that is fueled by activated CD4+ T cellsCitation37–40 and (2) minimize the latent reservoir, by limiting the amount of infected activated CD4+ T cells that are returning to quiescence (particularly in persons starting ART in acute HIV).Citation41 However, this study did not pursue those evaluations and cannot definitively state that RAL + LPV/r offered any immunologic benefit over EFV/TDF/FTC. Additionally, the differences in T cell dynamics we observed were not reflected in soluble markers of inflammation. The main limitation of this study is the small number of participants that may have limited the statistical power for biologic markers of interest. Additionally, the parent study was a randomized controlled clinical trial, but this retrospective study only evaluated persons who achieved and maintained virologic suppression, possibly introducing selection biases. Thirdly, in our attempts to evaluate an NRTI-sparing regimen with previous documented virologic efficacy, the two study arms did contain ART drugs with very different mechanisms of action making it difficult to definitively assert that our observations were due to sparing of NRTI, INSTI alone, or the combination of INSTI + PI.
No single NRTI-sparing regimen has demonstrated consistent efficacy in ART naïve persons infected with HIV. While there may be long-term benefits to specific NRTI-sparing regimens beyond lipodystrophy,Citation20 in select populations,Citation42 we did not observe any clinical relevant virologic or immunologic differences between naïve persons taking RAL + LPV/r or EFV/TDF/FTC.
Funding
This publication was supported by the California HIV Research Program grant MC08-SD-700. Support also came in part by the National Institutes of Health [grant number R01 HD083042], [grant number R21 MH100974], and [grant number R24 AI106039]. Additionally, this publication was supported by the University of California, San Diego Center for AIDS Research (CFAR), an NIH-funded program [grant number P30 AI036214], which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure statement
In accordance with Taylor & Francis policy and our ethical obligation as researchers, Drs. Goicoechea, Jain, Kemper, and Ms. Sun have no conflicts of interest to report. Dr. Karris receives funding to the institution from GS-US-311-1717 (Gilead Sciences) and has served as on an advisory board for Gilead Sciences. Dr. Dube receives grant support from BMS, Merck, Gilead, Serono, and ViiV and has served as a consultant to Serono. Dr. Haubrich is currently employed by Gilead Sciences. These companies may be affected by the research reported in the enclosed paper. We have disclosed those interests fully to Taylor & Francis.
Notes on contributors
Maile Y. Karris is an assistant professor at UCSD who is a physician scientist interested in clinical and translational research.
Sonia Jain is the lead biostatician for several collaborative projects with specific interest in Bayesian biostatistics.
Tyler R.C. Day worked at UCSD Transitional virology core and assisted with some of the studies; he is currently in medical school at Washington University in Saint Louis.
Josué Pérez-Santiago received his PhD in bioinformatics and systems biology at UCSD.
Miguel Goicoechea is an Infectious Diseases and HIV specialist at Scripps Healthcare in San Diego.
Michael P. Dubé is the associate director of the 5P21 Rand Schrader Health and Research Center at LA County-USC Medical Center who specializes in the metabolic complications of HIV therapies.
Xiaoying Sun is a senior biostatistician at UCSD.
Celsa Spina is the director of UCSD’s Center For AIDS Research Flow Cytometry core and focuses on HIV immunology specifically as it relates to latency.
Eric S. Daar is the chief of the Division of HIV Medicine at Harbor-UCLA Medical Center and processor of medicine at the David Geffen School of Medicine at UCLA.
Richard H. Haubrich is an HIV clinical trials expert, who is currently employed at Gilead Sciences.
Sheldon Morris is the current director for the California Collaborative Treatment Group and specializes in the prevention of sexually transmitted infections including HIV.
California collaborative treatment group (CCTG) 589 protocol team
Members
In addition to the authors, other members of the CCTG 589 protocol team included the following: Vi Q. Bowman, Gunter Rieg (Kaiser Permanente); Stefan Schneider (Living Hope Clinical Foundation); Ashwaq Hermes (Abbott Laboratories); Shubha Kerkar (Desert Regional Medical Center), Carol Kemper (Santa Clara Valley Medical Center); Catherine Diamond (University of California Irvine); M. Witt, J. Tilles, R. Larsen (David Geffen School of Medicine at UCLA Harbor-UCLA Medical Center); and R. Thomas, F. Wang, and E. Seefried (University of California, San Diego).
Supplementary material
Supplemental data for this article can be accessed here http://dx.doi.org/10.1080/15284336.2017.1282578.
YHCT_1282578_Supplementary_Material.zip
Download Zip (60.3 KB)Acknowledgments
We thank all of the patients for their participation in the study.
References
- Adolescents PoAGfAa. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. San Diego, CA: Department of Health and Human Services. Accessed June 2/2/2016.
- Cotter AG, Powderly WG. Endocrine complications of human immunodeficiency virus infection: hypogonadism, bone disease and tenofovir-related toxicity. Best Pract Res Clin Endocrinol Metab. 2011;25:501–515.10.1016/j.beem.2010.11.003
- Margolis AM, Heverling H, Pham PA, Stolbach A. A review of the toxicity of HIV medications. J Med Toxicol. 2013;10:26–29.
- McComsey GA, O’Riordan M, Choi J, et al. Mitochondrial function, inflammation, fat and bone in HIV lipoatrophy: randomized study of uridine supplementation or switch to tenofovir. Antivir Ther. 2012;17:347–353.
- Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25:1289–1298.10.1097/QAD.0b013e328347fa16
- Readhead AC, Gordon DE, Wang Z, et al. Transmitted antiretroviral drug resistance in New York State, 2006–2008: results from a new surveillance system. PLoS ONE. 2012;7:e40533.10.1371/journal.pone.0040533
- MacVeigh MS, Kosmetatos MK, McDonald JE, Reeder JL, Parrish DA, Young TP. Prevalence of drug-resistant HIV type 1 at the time of initiation of antiretroviral therapy in Portland, Oregon. AIDS Res Hum Retroviruses. 2013;29:337–342.
- Negredo E, Miró Òscar, Rodríguez‐Santiago B, et al. Improvement of mitochondrial toxicity in patients receiving a nucleoside reverse‐transcriptase inhibitor–sparing strategy: results from the multicenter study with nevirapine and kaletra (multineka). Clin Infect Dis. 2009;49:892–900.10.1086/599189
- Valantin MA, Lanoy E, Bentata M, et al. Recovery of fat following a switch to nucleoside reverse transcriptase inhibitor-sparing therapy in patients with lipoatrophy: results from the 96-week randomized ANRS 108 NoNuke Trial. HIV Med. 2008;9:625–635.10.1111/hiv.2008.9.issue-8
- Casado JL. Renal and bone toxicity with the use of tenofovir: understanding at the end. AIDS Rev. 2016;18:59–68.
- Sabin CA, Reiss P, Ryom L, et al. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14:61.
- Marcus JL, Neugebauer RS, Leyden WA, et al. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr. 2016;71:413–419.10.1097/QAI.0000000000000881
- Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106.10.1056/NEJMoa074609
- Reynes J, Lawal A, Pulido F, et al. Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/ emtricitabine in antiretroviral-naïve subjects: the progress study, 48-week results. HIV Clin Trials. 2011;12:255–267.10.1310/hct1205-255
- Kozal MJ, Lupo S, DeJesus E, et al. A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in antiretroviral treatment-naïve HIV-infected patients: SPARTAN study results. HIV Clin Trials. 2012;13:119–130.10.1310/hct1303-119
- Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS. 2011;25:2113–2122.10.1097/QAD.0b013e32834bbaa9
- Boyd MA, Kumarasamy N, Moore CL, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet. 2013;381:2091–2099.
- Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2013;29:256–265.
- Raffi F, Babiker AG, Richert L, et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet. 2014;384:1942–1951.10.1016/S0140-6736(14)61170-3
- Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–1118.10.1097/QAD.0b013e32832b4377
- Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–831.10.1097/QAD.0b013e32835192ae
- Bernardino JI, Mocroft A, Mallon PW, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2015;2:e464–e473.10.1016/S2352-3018(15)00181-2
- Karris MY, Jain S, Bowman VQ, et al. Nucleoside-sparing regimens with raltegravir and a boosted protease inhibitor: an unsettled issue. J Acquir Immune Defic Syndr. 2016;72:e48–50.
- Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126.10.1093/cid/cir627
- Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123.
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155.10.1146/annurev-med-042909-093756
- Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the national institute of allergy and infectious diseases, division of AIDS. Cytometry. 1993;14:702–715.10.1002/(ISSN)1097-0320
- Andrade A, Guedj J, Rosenkranz SL, et al. Early HIV RNA decay during raltegravir-containing regimens exhibits two distinct subphases (1a and 1b). AIDS. 2015;29:2419–2426.10.1097/QAD.0000000000000843
- Murray JM, Emery S, Kelleher AD, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–2321.10.1097/QAD.0b013e3282f12377
- Andrade A, Rosenkranz SL, Cillo AR, et al. Three distinct phases of HIV-1 RNA decay in treatment-naive patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J Infect Dis. 2013;208:884–891.10.1093/infdis/jit272
- Haubrich RH, Riddler SA, Ribaudo H, et al. Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, nonnucleoside reverse transcriptase inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS. 2011;25:2269–2278.10.1097/QAD.0b013e32834d0c20
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505.10.1056/NEJMoa1105243
- Mujugira A, Celum C, Coombs RW, et al. HIV transmission risk persists during the first 6 months of antiretroviral therapy. J Acquir Immune Defic Syndr. 2016;72:579–584.
- Donahue DA, Sloan RD, Kuhl BD, Bar-Magen T, Schader SM, Wainberg MA. Stage-dependent inhibition of HIV-1 replication by antiretroviral drugs in cell culture. Antimicrob Agents Chemother. 2010;54:1047–1054.10.1128/AAC.01537-09
- Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449–455.10.1097/QAI.0b013e3182965d45
- Funderburg NT, Andrade A, Chan ES, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naive patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One. 2013;8:e83514.
- Lees O, Ramzaoui S, Gilbert D, et al. The impaired in vitro production of interleukin-2 in HIV infection is negatively correlated to the number of circulating CD4+ DR+ T cells and is reversed by allowing T cells to rest in culture: arguments for in vivo CD4+ T cell activation. Clin Immunol Immunopathol. 1993;67:185–191.10.1006/clin.1993.1063
- Spina C, Prince H, Richman D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785.10.1172/JCI119342
- Tang S, Patterson B, Levy JA. Highly purified quiescent human peripheral blood CD4+ T cells are infectible by human immunodeficiency virus but do not release virus after activation. J Virol. 1995;69:5659–5665.
- Zack J. The role of the cell cycle in HIV-1 infection. Adv Exp Med Biol. 1995;374:27–31.10.1007/978-1-4615-1995-9
- Siliciano RF, Greene WC. HIV latency. Cold Spring Harb Perspect Med. 2011;1:a007096.
- Battegay M, Elzi L. Does HIV antiretroviral therapy still need its backbone? Lancet. 2014;384:1908–1910.10.1016/S0140-6736(14)61226-5
