Abstract
Background: FTC/TAF was shown to be noninferior to FTC/TDF with advantages in markers of renal and bone safety.
Objective: To evaluate the efficacy and safety of switching to FTC/TAF from FTC/TDF by third agent (boosted protease inhibitor [PI] vs. unboosted third agent).
Methods: We conducted a 48-week subgroup analysis based on third agent from a randomized, double blind study in virologically suppressed adults on a FTC/TDF-containing regimen who switched to FTC/TAF vs. continued FTC/TDF while remaining on the same third agent.
Results: We randomized (1:1) 663 participants to either switch to FTC/TAF (N = 333) or continue FTC/TDF (N = 330), each with baseline third agent stratifying by class of third agent in the prior treatment regimen (boosted PI 46%, unboosted third agent 54%). At week 48, significant differences in renal biomarkers and bone mineral density were observed favoring FTC/TAF over FTC/TDF (p < 0.05 for all), with similar improvements in the FTC/TAF arm in those who received boosted PI vs. unboosted third agents. At week 48, virologic success rates were similar between treatment groups for those who received a boosted PI (FTC/TAF 92%, FTC/TDF 93%) and for those who received an unboosted third agent (97% vs. 93%).
Conclusions: In virologically suppressed patients switching to FTC/TAF from FTC/TDF, high rates of virologic suppression were maintained, while renal and bone safety parameters improved, regardless of whether participants were receiving a boosted PI or an unboosted third agent. FTC/TAF offers safety advantages over FTC/TDF and can be an important option as an NRTI backbone given with a variety of third agents.
Introduction
While tenofovir disoproxil fumarate (TDF) is a potent and generally well-tolerated nucleotide analog, it has been associated with renal and bone toxicity.Citation1–6 As a prodrug, TDF is metabolized to tenofovir (TFV), which, in turn, is metabolized intracellularly to its active metabolite, TFV diphosphate (TFV-DP). Higher circulating plasma levels of TFV have been correlated with both renal and bone adverse effects of TDF.Citation7 Tenofovir alafenamide (TAF) is also an oral prodrug of TFV, but is much more stable in plasma. These characteristics result in a substantial reduction (90%) in circulating TFV exposure, while achieving higher intracellular levels of TFV-DP.Citation8
To date, the efficacy and safety of TAF have been mostly evaluated in the context of the coformulation of elvitegravir (E), cobicistat (C), emtricitabine (FTC, F), and TAF (E/C/F/TAF). Multiple clinical trials of E/C/F/TAF have consistently demonstrated the advantages of TAF over TDF for renal and bone safety.Citation9–12 However, the beneficial effect of switching to TAF from TDF may vary by the third agent. For example, the effect may be greater when the pre-switch plasma TFV exposures are higher (i.e. upon coadministration of TDF with boosted protease inhibitor [PI] vs. unboosted third agent). Similarly, the third agent also affects plasma TAF exposures, although this has been taken into account in the TAF dose selection (i.e. 10 mg with boosted PI and 25 mg with unboosted third agent).
We sought to evaluate the efficacy and safety of FTC/TAF by the class of coadministered third agent (boosted PI vs. unboosted third agent) by conducting a subgroup analysis of the 48-week data from a large, double-blind, multicenter trial (ClinicalTrials.gov number NCT02121795). The overall primary 48-week results were previously reported Citation13 and demonstrated that switching to FTC/TAF was noninferior to continued use of FTC/TDF while remaining on the same third agent in maintaining viral suppression and led to improvements in markers of bone and renal safety.
Methods
Study design and participants
The design and inclusion criteria of the trial have been previously described in the primary 48-week report.Citation13 Briefly, HIV-infected adults (aged ≥18 years) who were virologically suppressed (HIV-1 RNA <50 copies/mL) for at least 6 months on FTC/TDF-containing regimens and had creatinine clearance (CrCl) of >50 mL/min (calculated by the Cockcroft-Gault [CG] equation) were randomized (1:1) to either switch to FTC/TAF or to continue FTC/TDF while remaining on the same third agent with a double-blind, double-dummy design. Participants on boosted PIs (atazanavir [ATV] + ritonavir [RTV], darunavir [DRV] + RTV, or lopinavir/RTV [LPV/r]) who were randomized to the FTC/TAF group received FTC/TAF 200/10 mg; those on unboosted third agents (efavirenz [EFV], rilpivirine [RPV], nevirapine [NVP], raltegravir [RAL], dolutegravir [DTG], or maraviroc [MVC]) received FTC/TAF 200/25 mg. Randomization was stratified by third agent (boosted PI vs. unboosted third agent) at screening.
Randomized participants were seen at screening, baseline, and at weeks 2, 4, 8, 12, 16, 24, 36, 48. Laboratory tests included hematological analysis, serum chemistry tests, fasting lipid parameters, CD4 counts, measures of renal function (CrCl, urine protein to creatinine ratio, urine albumin to creatinine ratio, retinol binding protein to creatinine ratio, and β2-microglobulin to creatinine ratio (Covance Laboratories, Indianapolis, IN, USA), and measurement of HIV RNA concentration (Roche TaqMan 2.0; Roche Diagnostics, Rotkreuz, Switzerland). We defined virological failure as either having virological rebound confirmed within 3–6 weeks or being viremic at the study endpoint or at the time of study drug discontinuation with plasma HIV-1 RNA of 50 copies/mL or higher. Confirmatory (or last available) samples with HIV-1 RNA of 400 copies/mL or higher were sent for HIV-1 genotype and phenotype analysis (PhenoSenseGT for protease and reverse transcriptase genes, GenSeq Integrase and Phenosense Integrase for the integrase gene; Monogram Biosciences, South San Francisco, CA, USA). Dual energy X-ray absorptiometry (DEXA) of the hip and lumbar spine was conducted at baseline and weeks 24 and 48 and processed by BioClinica (Newton, PA, USA).
The study was undertaken in accordance with the Declaration of Helsinki and was approved by central or site-specific review boards or ethics committees. All patients gave written informed consent.
Statistical analysis
Non-inferiority of TAF compared with TDF when each was combined with FTC and given with baseline third agent was assessed by examining the proportion of participants in each arm with plasma HIV-1 RNA less than 50 copies/mL at week 48 as defined by the US Food and Drug Administration (FDA) snapshot algorithm.Citation14,15 An noninferiority margin of 10% was pre-specified with a one-sided 95% CI (alpha level 0.025). We summarized safety data in the safety analysis set with descriptive statistics. To compare between the two treatment groups, we used analysis of variance (ANOVA) for % change in BMD and 2-sided Wilcoxon rank sum tests for renal biomarkers (SAS; version 9.2). Adverse events (AEs) were coded with the Medical Dictionary for Regulatory Activities (Version 18.0).
Results
Of the 780 participants screened, 668 were randomized and 663 received at least one dose of study drug (FTC/TAF, N = 333, FTC/TDF, N = 330). Baseline characteristics, as previously reported in the primary 48-week report,Citation13 were similar between treatment groups; the median (Q1, Q3) age was 49 (22, 79) years, 15% were female, and 21% identified themselves as Black or of African descent. The median baseline CD4 count was 646 (491, 835) cells/μL, with approximately three quarters (74.2%) of subjects having a baseline CD4 count ≥500 cells/μL. The percentages of participants taking boosted PIs or unboosted third agents were similar between the two treatment groups (boosted PI: FTC/TAF 47%, FTC/TDF 45%; unboosted third agent: FTC/TAF 53%, FTC/TDF 55%). Overall, the median time of FTC/TDF use prior to dosing was 5.1 years. Most subjects (91%) had no proteinuria (Grade 0 by dipstick) on urinalysis, and baseline CrCl was similar between the two treatment groups.
At week 48, switching to an FTC/TAF-containing regimen was noninferior to staying on a baseline FTC/TDF-containing regimen in maintaining HIV-1 RNA <50 copies/mL for participants received either a boosted PI (91.6% vs. 92.7%; difference −1.1%, 95% CI −7.1 to 4.9%) or an unboosted third agent (96.6% vs. 93.3%; difference 3.3%, 95% CI −1.2 to 7.9%). Mean changes in CD4 cell counts were small and similar between groups, regardless of third agent: boosted PI with FTC/TAF + 21 cells/μL, FTC/TDF + 7 cells/μL; unboosted third agent with FTC/TAF + 20 cells/μL, FTC/TDF + 19 cells/μL. One participant in the FTC/TAF group whose third agent was DRV + RTV experienced virologic failure at week 36 with emergent M184V reverse transcriptase mutation; the patient subsequently discontinued study drug.
Both regimens, regardless of third agent, were well tolerated through week 48. AEs leading to study drug discontinuation were uncommon (boosted PI: FTC/TAF 4%, FTC/TDF 1%; unboosted third agent: FTC/TAF 1%, FTC/TDF 1%). The type and frequency of treatment-emergent AEs were similar. Serious AEs were also uncommon (boosted PI: FTC/TAF 5%, FTC/TDF 6%; unboosted third agent: FTC/TAF 3%, FTC/TDF 6%). No treatment-related deaths occurred; one participant in the FTC/TAF group died due to lymphoma and elevated lipase.
We noted decreases from baseline in serum creatinine at week 48 and corresponding increases in CrCl for participants who switched to FTC/TAF as compared with minimal changes from baseline among those who remained on an FTC/TDF regimen, with differences favoring FTC/TAF vs. FTC/TDF regardless of third agent; p values for all between-group differences were <0.05 (Table ). Generally, measures of proteinuria (urine protein and urine albumin to creatinine ratio) and tubular proteinuria (urine retinol binding protein and urine β2-microglobulin to creatinine ratio) decreased in the FTC/TAF group but increased in the FTC/TDF group, with differences favoring FTC/TAF vs. FTC/TDF regardless of third agent; p values for all between-group differences were <0.05. No participants in the FTC/TAF group discontinued study drug due to renal AEs. One participant in the FTC/TDF group, who had underlying hypertension, had an increase in serum creatinine and discontinued study drug due to this renal AE. No cases of proximal tubulopathy or Fanconi syndrome were reported in either group.
Table 1 Quantitative measures of proteinuria and lipid changes by third agent
We also noted small increases from baseline in fasting lipids at week 48 in the FTC/TAF group as compared with minimal changes among those who remained on an FTC/TDF regimen regardless of third agent; however, the total cholesterol to HDL ratio did not differ between treatment groups (Table ).
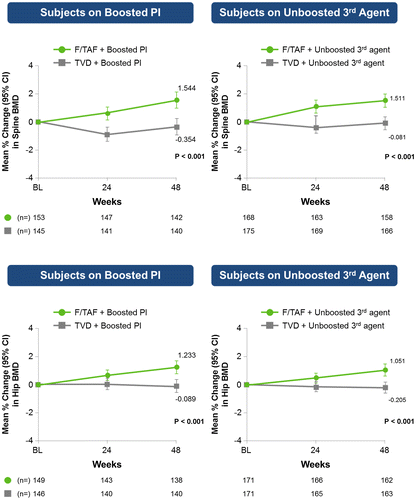
Regardless of third agent, BMD increased in the FTC/TAF group while remaining stable or decreasing in the FTC/TDF group (Figure ). One participant (0.3%) in the FTC/TAF group and 2 (0.6%) in the FTC/TDF group had traumatic fractures, considered unrelated to study drugs.
The incidence of laboratory abnormalities was similar in each third agent subgroups for both treatment groups.
Discussion
Regardless of third agent (boosted PI or unboosted third agent), FTC/TAF was noninferior to FTC/TDF in maintaining virologic suppression at week 48. Overall safety was similar for FTC/TAF administered with a boosted PI or an unboosted third agent, and renal and bone parameters consistently improved in those who switched.
Multiple clinical trials in treatment-naïve and virologically suppressed patients demonstrated the renal and bone safety advantages of FTC/TAF over FTC/TDF, mostly in combination with elvitegravir/cobicistat (i.e. E/C/F/TAF).Citation9–12 E/C/F/TAF, which contains TAF 10 mg, achieves TAF exposures that are comparable to TAF 25 mg, as cobicistat increases TAF exposure via Pgp/BCRP inhibition.Citation16
In the current study, we examined the two doses of FTC/TAF (200/25 and 200/10 mg), each to be prescribed depending on the third agent, or specifically, on whether the third agent requires boosting. By performing efficacy and safety analyses by third agent, we explored if there were any differences in the safety advantages of TAF over TDF by the use of boosted vs. unboosted third agents.
Across assessments of multiple markers of renal safety, the direction of change and magnitude of difference between treatment groups demonstrated an improved renal safety profile with TAF compared with TDF when given with a boosted PI or an unboosted third agent. Both urine protein and urine albumin to creatinine ratios decreased from baseline in those who received FTC/TAF. Evaluation of tubular proteinuria (urine retinol binding protein and urine β2-microglobulin to creatinine ratios), as recommended for monitoring TFV nephrotoxicity,Citation17 also demonstrated a reduced effect of FTC/TAF on tubular function. These data suggest that the 90% lower plasma TFV exposure observed with FTC/TAF may reduce TFV-mediated renal side effects in clinical practice. Although the study population may not have been at high risk of TDF-associated renal events as they were tolerating treatment prior to study entry, the renal findings from our study are consistent with those from other trials, including those in naïve patients, and provide evidence of an improved renal safety profile of TAF over TDF regardless of third agent.
Across assessments of BMD, the direction of change and magnitude of difference between treatment groups demonstrated an improved bone safety profile with TAF compared with TDF when either was given with a boosted PI or an unboosted third agent. Our study confirmed the previous finding of an increase in BMD after switching to FTC/TAF-containing regimen (E/C/F/TAF) from FTC/TDF-containing regimen.Citation11 An increase in bone density is desirable in the aging, HIV-infected population where bone health may be a concern.
In conclusion, FTC/TAF demonstrated high efficacy in HIV-infected virologically suppressed patients along with renal and bone safety advantages regardless of the class of coadministered third agent. FTC/TAF offers an important option as an NRTI backbone for use with a spectrum of third agents in the treatment of HIV-infected patients, with safety advantages over FTC/TDF.
Financial disclosures and conflicts
FAP has received funding to attend conferences or educational meetings, honoraria and/or research funding from Abbvie, Gilead Sciences, Bristol-Myers Squibb, Janssen-Cilag, GlaxoSmithKline/ViiV Healthcare and Merck. YY reports personal fees from Abbott, Bristol-Myers Squibb, Gilead, MSD, Roche, Tibotec, ViiV Healthcare. AL has received honoraria and given expert testimony for ViiV Healthcare, Bristol-Myers Squibb, Janssen Pharmaceuticals, Gilead, Abbvie, and Merck Sharp & Dohme. JR has received funding to attend conferences or educational meetings, honoraria, and/or research funding from Gilead Sciences, Janssen-Cilag, GlaxoSmithKline/ViiV Healthcare, and Merck. FM has served as a consultant on advisory boards for Bristol-Myers Squibb, Gilead, ViiV, Merck Sharp and Dome, Roche, Tibotec; he has received lecture fees from Abbott, Bristol-Myers Squibb, Gilead, ViiV, Merck Sharp, and Dome, and has received research and educational grants from Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Jansen-Cilag. MY, MA, CT-M, AC, and MSR are employees of Gilead Sciences and own Gilead stock.
Funding
This work was supported by Gilead Sciences.
Geolocation information
Country of origin: United States.
Notes
* Previously presented at HIV Glasgow Drug Therapy Congress (Oct 23–26, 2016; Glasgow, UK)
References
- Hall AM, Hendry BM, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57:773–780.10.1053/j.ajkd.2011.01.022
- Gupta SK. Tenofovir-associated fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDs. 2008;22:99–103.10.1089/apc.2007.0052
- Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55:49–57.10.1097/QAI.0b013e3181dd911e
- Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir‐lamivudine versus tenofovir‐emtricitabine in HIV‐infected adults: 48‐week results from the ASSERT study. Clin Infect Dis. 2010;51:963–972.10.1086/652982
- Schafer JJ, Manlangit K, Squires KE. Bone health and human immunodeficiency virus infection. Pharmacotherapy. 2013;33:665–682.10.1002/phar.2013.33.issue-6
- McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801.10.1093/infdis/jir188
- Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52:3144–3160.10.1128/AAC.00350-08
- Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-Day Monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449–455.10.1097/QAI.0b013e3182965d45
- Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615.10.1016/S0140-6736(15)60616-X
- Wohl D, Oka S, Clumeck N, et al. Brief report: a randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. J Acquir Immune Defic Syndr. 2016;72:58–64.10.1097/QAI.0000000000000940
- Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16:43–52.10.1016/S1473-3099(15)00348-5
- Pozniak A, Arribas JR, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48 week results from a single-arm, multi-center, open-label, phase 3 study. J Acquir Immune Defic Syndr. 2016;71:530–537.10.1097/QAI.0000000000000908
- Gallant JE, Daar ES, Raffi F, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3:e158–e165.10.1016/S2352-3018(16)00024-2
- Smith F, Hammerstorm T, Soon G, et al. A meta-analysis to assess the FDA DAVP’s TLOVR algorithm in HIV submissions. Drug Inf J. 2011;45:291–300.
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), Guidance for Industry Human Immunodeficiency Virus-1 Infection: Developing Antiretroviral Drugs for Treatment. Draft Guidance. 2013;1–43. Available at chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM355128.pdf. Accessed April 15, 2016.
- Gilead Sciences International Ltd. DESCOVY (emtricitabine/tenofovir alafenamide 200/10 mg). Summary of Product Characteristics. Accessed January 16, 2016.
- Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e96–e138.10.1093/cid/ciu617