Abstract
Background: The HIV Care Cascade model can be used to measure how clinical services align with United Nations’ (UN) HIV treatment targets. Previous models have highlighted sequential losses at each step of the Cascade with a significant proportion being not retained in care (NRIC).
Objective: We aimed to assess the feasibility of meeting the UN targets and assess factors associated with, and calculate the true proportion of those, NRIC.
Methods: All people living with HIV who were linked to our service, one of three specialist HIV care providers in Dublin Ireland, from its establishment in 1993 to 1 December 2014, were included in the cohort and were categorized as linked to care, retained in care (RIC), on antiretroviral therapy (on ART), virally suppressed (HIV RNA <40copies/ml), and NRIC. An analysis of those NRIC was performed to categorize their current status through direct/indirect contact.
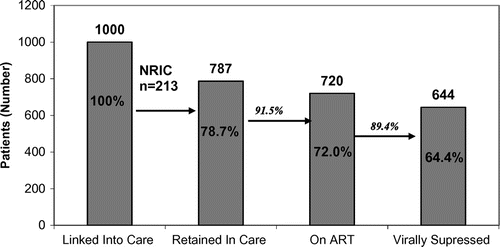
Results: Of 1000 patients linked to care, 78.7% (n = 787) were RIC, of whom 91.5% (n = 720) were on ART, with 89.9% (n = 644) virally suppressed. Those RIC were more likely older (p = 0.006) and non-IVDU (p < 0.001). Of 213 (21.3%) NRIC, 56 (26.3%) emigrated, 27 (12.7%) transferred care, 15 (7.0%) stopped attending but were contactable, 38 (17.8%) died, and 77 (36.1%) were lost to follow-up. After revision, 10.5% of the cohort was confirmed as NRIC, with 6 of 15 defined as “stopped attending” re-linked to care following direct contact.
Conclusions: Our HIV Care Cascade model demonstrates that the true numbers of patients NRIC may be significantly lower than previously estimated and once RIC, treatment goals approaching the United Nations Programme on HIV and AIDS targets are possible with 91.5% on treatment and almost 90% of those on treatment virally suppressed. That 40% reengaged following direct contact suggests benefit through regular monitoring and direct contact based on the HIV Care Cascade model.
Background
With effective antiretroviral therapy (ART), people living with HIV (PLWH) who are engaged in care can expect to live longer and healthier lives than in the pre-ART era. In addition to personal health benefits from effective treatment regardless of CD4+ T-cell count.Citation1, there is also a potential public health benefit, with those individuals on effective ART with suppressed plasma HIV RNA unlikely to contribute to onward sexual transmission of HIV.Citation2–4 However, despite significant progress in our understanding of use of treatment as prevention, there remains a sustained global HIV epidemic, with numbers of new infections remaining static or increasing, even in resource-rich regions with full ART access.Citation5,6
Building on the concept of treatment as prevention, the Joint United Nations Programme on HIV and AIDS (UNAIDS) in 2014 issued new targets for service providers to focus efforts in an attempt to bring the HIV epidemic under control. Commonly referred to as “90–90–90,” these propose three targets: that 90% of the population living with HIV are aware of their diagnosis, that 90% of this diagnosed population are treated, and that 90% of those on ART are virally suppressed, with this strategy, if successfully implemented, expected to have a significant impact on the HIV epidemic by 2030.Citation7
Against this backdrop, the HIV Care Cascade model, which examines population engagement with HIV care and treatment, is becoming increasingly utilized by HIV service providers to explore how closely individual clinic, country, or regional services align with the “90–90–90” targets. Although these models vary considerably in their design and definitions, many present the cascade as five sequential steps, the first estimating of all PLWH in a given area, the second those diagnosed and linked to care, the third PLWH retained in care, the fourth those on ART, and the fifth those on ART with suppressed plasma HIV RNA. Previous models, particularly those from North America, have shown that although there are significant losses of subjects at each sequential step in the cascade, often the biggest loss occurs between the steps of those linked to and those retained in care, with one US study suggesting that approximately 50% of PLWH are not actively engaged in care despite 79% being aware of their diagnosisCitation8; however, few have examined in detail the reasons for losses of subjects between steps in the cascade.
Through the use of a HIV Care Cascade model, we explored how closely a service based within a European tertiary referral service aligned with the “90–90–90” targets and aimed to elicit reasons underlying significant gaps or losses identified in the HIV Care Cascade model.
Methods
Study design
We conducted an analysis of data from the Mater Misericordiae University Hospital (MMUH) Infectious Diseases Cohort, a prospective cohort of subjects attending for ambulatory care at the MMUH, a tertiary teaching hospital located in Dublin’s north inner city which is one of the three specialist centers providing HIV services in Dublin, Ireland and one of the seven on the island of Ireland. All patients diagnosed with HIV linked to our clinic from its establishment in 1993 to the 1st December 2014 and attended on at least one occasion were included in the analysis. Incarcerated individuals were included as prison authorities facilitate clinic attendances when possible to minimize disruption to HIV care. Data regarding ART dispensation were collected from the MMUH Infectious diseases pharmacy through which all ART was dispensed. Laboratory test results (CD4 count and HIV viral load) were collected from the electronic patient record and appointment records of those accessing clinic using private health insurance were obtained and recorded in the database.
Ethics
The local research ethics committee approved the study protocol and subjects enrolled in the cohort provided written, informed consent for use of their routine clinical data for research purposes.
Analysis
Categories within our HIV Care Cascade model were defined as follows:
| • | “Linked to care” – a subject with a diagnosis of HIV with at least one attendance to the clinical service. | ||||
| • | “Retained in care” (RIC) – those “linked to care” with two documented clinic attendances and/or laboratory tests (CD4+ T cell count and/or HIV viral load) at least three months apart within the 12 months preceding the analysis. | ||||
| • | “On ART” – those retained in care with at least two ART dispensations at least three months apart within the preceding year. | ||||
| • | “Virally suppressed” – defined as those on HAART with most recent HIV RNA readings less than 40 copies/ml in the preceding 12 months. | ||||
| • | “Not Retained in Care” (NRIC) – those “linked to care” but without a clinic visit or routine diagnostic test within the previous 12 months. | ||||
An in-depth analysis of those NRIC was conducted examining age, ethnicity, and transmission risk. Attempts were made to contact these patients directly by telephone to assess current status and if not contactable, indirectly through local doctor and listed next of kin. The hospital electronic patient record was consulted in conjunction with the patient’s local doctor to determine those deceased and assess cause of death. Following this, their current status was classified as “emigrated,” “transfer-of-care,” “deceased,” “stopped attending but contactable,” and “lost to follow up” (LTFU), the latter being those who were un-contactable with no further information available from local doctor or next of kin.
Data are presented as mean (standard deviation (SD)) or numbers (percentages (%)) as appropriate, with between-group differences compared using χ2test for categorical variables and T-test for continuous variables.
Results
At the time of analysis, 1000 PLWH had been linked to care. Both genders were well represented (59.3% male) with the majority of subjects of either Caucasian (54.4%) or African (37.3%) origin and all three main transmission risk groups represented: heterosexual sex (50%), people who inject drugs (PWID, 22.2%), and men who have sex with men (MSM, 21.0%), respectively.
Of the 1000 patients linked to care, 787 (78.7%) were classified as retained in care and 213 (21.3%) classified as not retained in care (NRIC). Compared to those retained in care, those NRIC were more likely to be younger (38.3 (9.0) vs. 41.0 (9.5) years, p < 0.001) and of African origin (p = 0.045, see Table ). However, there was no significant difference in transmission risk between those retained and not retained in care.
Table 1 Description of and comparisons within the HIV Care Cascade
Of the 787 retained in care, 720 (91.5%) were on ART of whom 644 (81.8%) were on ART with an undetectable HIV RNA (<40 copies/ml) (Figure ). Those on ART who were virally suppressed were significantly older (mean (SD) age 42.0 (9.5) vs. 39.0 (8.7) years, p = 0.006) and were less likely to be PWID (80.6% PWID virally suppressed on ART vs. 92.0% non-PWID virally suppressed on ART; p < 0.001). There was no difference noted between other transmission risk groups or genders.
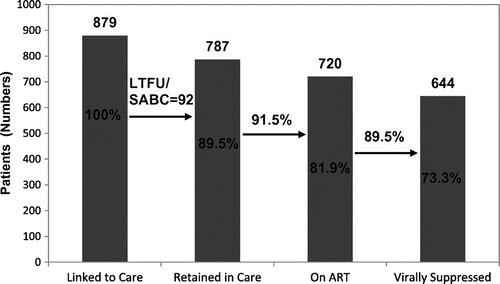
Figure 1 Proportions of patients by stage of HIV cascade of care model
A further analysis of those NRIC (n = 213) revealed that 56 (26.3%) emigrated, 27 (12.7%) transferred care to another institution, 15 (7.0%) stopped attending and had disengaged from HIV care, 38 (17.8%) had died, and 77 (36.1%) were LTFU. Of the 38 patients who had died, non-AIDS-related conditions accounted for 17 (44.7%) deaths, AIDS defining illnesses for 7 (18.4%) deaths, and unnatural death/misadventure the cause of death in a further 7 (18.4%) cases. The cause of death could not be ascertained during the analysis in seven cases due to incomplete data or death having occurred at another institution.
Of the 15 who had stopped attending and disengaged from HIV care but were contactable, almost half (46,6%) were of African origin while 7 of the remaining 8 patients were PWID. Although prison authorities facilitate attendances to HIV clinic, two people cited imprisonment as a reason for disengagement. As a result of direct contact, 6 of the 15 in this group were successfully re-linked to care. Recalculating the cascade, excluding those who had died, emigrated, or transferred care, (total remaining population 879) the percentage of those linked to and who are retained in care rose to 89.5% (787/879) (Figure ).
Discussion
Our HIV Care Cascade model demonstrates that once retained in care, treatment goals approaching the UNAIDS targets are possible with 91.5% on treatment and almost 90% of those on treatment virally suppressed, although, like other studies, we observed a significant drop in numbers between those linked to care and retained in care, a significant obstacle in achieving the second “90” goal of the UNAIDS 90–90–90 strategy. However, further analyses of this group demonstrated that the true numbers of patients lost to follow-up may be significantly lower than previously estimated from similar analyses, with only 10.5% subjects confirmed as truly lost to follow-up.
As previous models have shown, a substantial proportion of PLWH fail to remain engaged in care, with estimates of 45– 65% in US studies, with lack of engagement considered a significant barrier to the use of treatment-as-prevention to control the HIV epidemic.Citation8,9 The proportion of patients NRIC in our analysis (21.3%), although smaller than US studies, is broadly similar to previously reported Canadian models at approximately 20%Citation10 and higher than those quoted for other high-income European countries such as the UK and Denmark at 10.8 and 7.4%, respectively.Citation11 However, our in-depth analysis of those “not retained in care” in our cohort goes some way in explaining the difference between our cohort and those of neighboring countries, with a much smaller number truly disengaged with care. Only 92 (9.2%) of the whole cohort intentionally disengaged from care or were truly lost to follow-up, the remainder having emigrated, transferred care, or died.
Although there is no certainty that those who emigrated or transferred care to another institution actually remained in care once leaving our service, even if assuming that 9.2% of these individuals also disengaged from care, the overall rate of those not engaged in care would only rise to 9.96% of the total population linked to care, suggesting that there is not a large proportion of patients actively disengaging from care. Although national health care databases and registries have the ability to assess ongoing engagement with care in subjects transferring between health care institutions, this can be complex to accurately estimate and most will be unable to determine emigration status of specific individuals which may lead to overestimates of those truly not retained in care in some capacity (26.3% of those NRIC in our analysis).
Excluding those who had died, transferred care, or emigrated, the proportion retained in care rises to 89.5% (787/879), with the proportion of those retained in care who are on ART at 81.9% (720/879), which, although less than the target set by the UNAIDS 90–90–90 goals, is likely underestimated as some will have been prescribed ART elsewhere. In addition, the numbers on ART will probably rise as more patients are commenced on ART with changes in treatment guidelines arising from results of recent trials suggesting a health benefit for ART at any CD4+ T-cell count.Citation12
Interestingly, 6 of the 15 patients (40%) who had stopped attending HIV services re-engaged with care as a direct result of being contacted for the purpose of the study. This unexpected benefit from performing our additional in-depth analysis suggests potential patient and public health benefits through regular monitoring of the HIV Care Cascade model, including direct contact with those who have stopped attending. Various initiatives to enhance engagement and retention, such as financial incentives, have had mixed resultsCitation13 and the results from this analysis suggest that simpler approaches through directly engaging with those who stop attending may have significant benefits. However, these approaches would need to be validated in different health care settings.
One of the most significant challenges in controlling the HIV epidemic, and one which we were unable to address in this study, is to both accurately assess and increase the proportion of PLWH who are aware of their diagnosis. It is currently estimated that 20–30% of PLWH in EU countries are unaware of their diagnosis and as such remain at risk of developing AIDS-related conditions as well as contributing to onward HIV transmission, with those who have newly acquired HIV or in the advanced stages of infection through to disproportionately contribute to onward HIV transmission.Citation14–17 In Ireland, the number of new cases of HIV continues to rise with 491 new cases being diagnosed in 2015, representing a 30% year-on-year increase in new diagnosisCitation18, a trend being replicated in many EU countries. Although such rises in newly diagnosed HIV may partly reflect increased testing and surveillance for HIV, combined with our analysis demonstrating targets of those retained in care on effective therapy being close to the UNAIDS 90–90–90 target, these data suggest that greater focus is required within European centers on increasing HIV testing and other prevention strategies if meaningful progress is to be made in controlling the HIV epidemic.
Our study has limitations. In addition to lacking information on the true population prevalence of HIV infection, our single-site study may not reflect cascade of care models throughout Ireland, although there is a consistent, hospital-based approach to HIV management in Ireland that would support these data being broadly representative of the other six treatment centers. This cohort accounts for 13.6% of people diagnosed with HIV in Ireland since the early 1980s until the time of study,Citation19 and its demographics are similar to that of the overall HIV cohort attending ambulatory care in Ireland,Citation20 which supports the view that, in the absence of national data, this study may be reflective of what is occurring on a national level . The use of laboratory tests as surrogate markers for retention in care has also been suggested to overestimate retention in the newly diagnosed HIV cohort. However, whether a similar over estimation occurs in a cohort of more established patients such as our own, who may not have as frequent laboratory tests as those newly diagnosed, is not clear.Citation21 In addition, although we were able to establish estimates of those who had emigrated or transferred care, we were unable to accurately determine in all cases if those individuals were subsequently retained in care and can only assume similar rates of disengagement to those observed within our cohort. As the date at which disengagement occurred or after what time period in care was not recorded on an individual basis for those NRIC, the rate of loss to follow-up could not be determined.
Despite these limitations, our approach of in-depth analysis of the HIV Care Cascade model provides useful insights, not only into HIV service provision, but also suggests areas where focus on public health activities may have a greater impact. Specifically, our data suggest that, as numbers NRIC may be significantly lower than previously estimated and with good proportions on successful treatment, the goals set by the UNAIDS 90–90–90 strategy, although not achieved to date, appear at least in part feasible; however, greater focus on testing and prevention activities for those either unaware of their HIV status or at risk of HIV acquisition will be required. Until such time, our analysis highlights the benefit of regularly analyzing the HIV Care Cascade within clinical care, focusing on in-depth analysis of those NRIC to obtain a true representation of the cascade but also as a tool to optimize the numbers retained in care.
Notes on contributors
P. McGettrick is a graduate of University College Dublin School of Medicine, and is currently undertaking Higher Specialist Training in Infectious Disease and General Medicine with the Royal College of Physicians of Ireland. Dr. McGettrick has collaborated with the HIV Molecular Research Group, UCD School of Medicine, on a number of projects and has a particular interest in HIV medicine and translational research, an area in which he is continuing to work.
B. Ghavami-Kia graduated from University College Dublin in 2009 to carry out his basic specialist training in the Mater Misericordiae Univeristy Hospital working in neurology and infectious diseases and subsequently taking a role as tutor and lecturer in University College Dublin and working with the HIV Molecular Research Group. He is currently on the CIT training scheme in Newcastle-Upon-Tyne to obtain dual specialisation in infectious diseases and microbiology. Special areas of particular interest to him are neuroinfectious diseases, which he hopes to partake in further research activities related to the same with a view to subspecialising in this area.
W. Tinago has worked with the HIV Molecular Research Group, School of Medicine, UCD since 2011 as the lead medical statistician. His work includes the design and analysis of HIV epidemiological studies and clinical trials.
A. Macken has worked as data manager with the HIV Molecular Research Group (HMRG) UCD School of Medicine, based at the Mater Misericordiae University Hospital since 2011. Prior to this he worked in a variety of clinical data management roles.
J. O’Halloran completed her medical training in National University of Ireland Galway in 2006 and subsequently entered the Higher Specialist training in Infectious Diseases and General medicine. She has worked extensively with and completed her PHD with, the HIV Molecular research Group, UCD School of Medicine Dublin. She is currently undertaking a fellowship in infectious diseases in the Washington University School of Medicine, St. Louis Missouri.
J. S. Lambert is an infectious diseases specialist with special interest in HIV, Hepatitis and infectious causes of chronic fatigue. He has published widely on vaccines and HIV and hepatitis, and has conducted clinical trials in the USA, Ireland and South Africa primarily focused on HIV in children, in the mother to child transmission of HIV, and adolescent HIV transition. He has over 100 peer-reviewed publications and 10 book chapters to his name. Lambert has received a 1.8 million euro European Union research grant for HCV in vulnerable populations, focused on community implementation of the new Direct Acting Antivirals in People who inject drugs and homeless populations.
G. Sheehan is a 1979 University College Cork graduate who trained in Internal Medicine, Infectious Diseases (ID), and Intensive Care Medicine (ICM) at the University of Manitoba, Canada. He was a consultant in ID and ICM at the University of Alberta Hospital, Edmonton, Canada from 1987 to 1993. In 1993, he was appointed as the first ID consultant physician in the Republic of Ireland to both the Mater Misericordiae University and Beaumont Hospitals and set up and developed HIV services for North Dublin, including the rapid roll-out of anti-retroviral therapy from 1996. He set up the National Isolation Unit (NIU) at the Mater which opened in 2008 and continues to be involved in teaching and research at the Mater Misericordae Hospital and University College Dublin.
P.W.G. Mallon is an infectious diseases specialist at the Mater Misericordiae University Hospital, Dublin. Mallon graduated in medicine from Queen’s University Belfast, Northern Ireland and underwent training in infectious diseases in Sydney, Australia, where he completed a PhD examining antiretroviral toxicities. Mallon heads the HIV Molecular Research Group, which focuses on translational research into toxicities of antiretroviral therapy, including cardiovascular disease, adipose tissue toxicity and bone disease. Mallon is a Regional Representative for the European AIDS Clinical Society (EACS), is Deptuy Chair of the EACS Comorbidities Guidelines Panel and is Deputy Director of the Wellcome Trust/HRB Irish Clinicial Academic Training Program. He has published more than one hundred peer-reviewed manuscripts and sits on the editorial board of AIDS, HIV Medicine, and AIDS Research and Therapy.
References
- Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc. 2015;18(1):20052. doi:10.7448/IAS.18.1.20052
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with early antiretroviral therapy. New Engl J Med. 2011;365(6):493–505.10.1056/NEJMoa1105243
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57.10.1016/S0140-6736(08)61697-9
- Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–539.10.1016/S0140-6736(10)60936-1
- ECDC. HIV/AIDS surveillance in Europe 2014. Stolkholm: European Centre for Disease Prevention and Control. WHO Regional Office for Europe, 2015.
- UNAIDS. Global AIDS update. Geneva: Joint United Nations Programme on HIV and AIDS, 2016.
- UNAIDS. 90–90–90; An ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS, 2014.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800.10.1093/cid/ciq243
- Yehia BR, Stephens-Shields AJ, Fleishman JA, Berry SA, Agwu AL, Metlay JP, et al. The HIV care continuum: changes over time in retention in care and viral suppression. PLOS One. 2015;10(6):e0129376.10.1371/journal.pone.0129376
- Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14(1):40–49.10.1016/S1473-3099(13)70254-8
- Alice R, editor. Large disparities in HIV treatment cascades between eight European and high-income countries: analysis of break points. Glasgow: International Congress of Drug Therapy in HIV Infection, 2014.
- Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. New Engl J Med. 2015;373(9):795–807.
- Pettifor A, MacPhail C, Nguyen N, Rosenberg M. Can money prevent the spread of HIV? A review of cash payments for HIV prevention. AIDS Behav. 2012;16(7):1729–1738.10.1007/s10461-012-0240-z
- Hamers FF, Phillips AN. Diagnosed and undiagnosed HIV-infected populations in Europe. HIV Med. 2008;9(s2):6–12.10.1111/j.1468-1293.2008.00584.x
- Pilcher CD, Tien HC, Eron JJ Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–1792.10.1086/jid.2004.189.issue-10
- Hollingsworth TD, Pilcher CD, Hecht FM, Deeks SG, Fraser C. High transmissibility during early HIV infection among men who have sex with men—San Francisco, California. J Infect Dis. 2015;211(11):1757–1760.10.1093/infdis/jiu831
- Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-Analysis of High-Risk Sexual Behavior in Persons Aware and Unaware They are Infected With HIV in the United States. JAIDS J Acquir Immune Defici Syndr. 2005;39(4):446–453.10.1097/01.qai.0000151079.33935.79
- HIV in Ireland 2015 (Provisional Data) Health Protection Surveillance Center, 2016 [cited 2016 May]. Available from: http://www.hpsc.ie/A-Z/HIVSTIs/HIVandAIDS/SurveillanceReports/.
- HIV in Ireland 2014. Health Protection Surveillance Center; 2015 [cited 2015 December]. Available from: http://www.hpsc.ie/A-Z/HIVSTIs/HIVandAIDS/SurveillanceReports/.
- Tuite H, Horgan M, Mallon PW, McConkey SJ, Mooka B, Mulcahy F, et al. Patients accessing ambulatory care for HIV-infection: epidemiology and prevalence assessment. Irish Med J. 2015;108(7):199–202.
- Halperin J, Bean MC, Richey LE. Laboratory markers slightly overestimate retention in HIV care among newly diagnosed individuals. AIDS Care. 2016;28(9):1188–1191.10.1080/09540121.2016.1164291