Abstract
Introduction: The HIV Prevention Trials Network (HPTN) 052 trial demonstrated that early antiretroviral therapy (ART) prevented 93% of HIV transmission events in serodiscordant couples. Some linked infections were observed shortly after ART initiation or after virologic failure.
Objective: To evaluate factors associated with time to viral suppression and virologic failure in participants who initiated ART in HPTN 052.
Methods: 1566 participants who had a viral load (VL) > 400 copies/mL at enrollment were included in the analyses. This included 832 in the early ART arm (CD4 350–550 cells/mm3 at ART initiation) and 734 in the delayed ART arm (204 with a CD4 < 250 cells/mm3 at ART initiation; 530 with any CD4 at ART initiation). Viral suppression was defined as two consecutive VLs ≤ 400 copies/mL after ART initiation; virologic failure was defined as two consecutive VLs > 1000 copies/mL > 24 weeks after ART initiation.
Results: Overall, 93% of participants achieved viral suppression by 12 months. The annual incidence of virologic failure was 3.6%. Virologic outcomes were similar in the two study arms. Longer time to viral suppression was associated with younger age, higher VL at ART initiation, and region (Africa vs. Asia). Virologic failure was strongly associated with younger age, lower educational level, and lack of suppression by three months; lower VL and higher CD4 at ART initiation were also associated with virologic failure.
Conclusions: Several clinical and demographic factors were identified that were associated with longer time to viral suppression and virologic failure. Recognition of these factors may help optimize ART for HIV treatment and prevention.
Introduction
The risk of HIV transmission is correlated with HIV viral load.Citation1,2 Effective antiretroviral therapy (ART) inhibits viral replication and reduces HIV viral load to low or undetectable levels. In the multi-national HIV Prevention Trials Network (HPTN) 052 clinical trial, early ART initiation significantly reduced the risk of sexual HIV transmission in serodiscordant couples and improved the health of index participants.Citation3,4
Results from HPTN 052 and other studies indicate that sexual transmission of HIV is very unlikely when the infected individual is virally suppressed. In HPTN 052, 78 partner infections were observed with 1763 couples followed for > 8500 person-years.Citation4 Genetic linkage analysis was performed to determine the likely source of the partner’s infection.Citation5,6 Over the course of the trial, eight linked infections were diagnosed after the index participant started ART.Citation6 Four of those infections occurred close to the time of index ART initiation (most likely before the index participant was virally suppressed) and four occurred after the index participant failed ART.Citation6,7 Linked partner infections were not observed when index participants were stably suppressed on ART. Partner infections have also been evaluated in observational studies of serodiscordant couples when the infected individual was on ART.Citation8–10 In a prospective cohort study with 168 person-years follow-up, three linked infections were observed within six months of ART initiation, before the index was virally suppressed.Citation11 In an observational study, no linked infections were observed during 1238 couple-years of follow up when index participants were virologically suppressed on ART.Citation12
In this report, we evaluated time to viral suppression and virologic failure among index participants who started ART in HPTN 052. These studies are relevant to use of ART for HIV prevention, since viremia increases the risk of HIV transmission. The analysis in this report includes a comparison of these outcomes in the two study arms of HPTN 052 (early vs. delayed ART), and evaluates demographic and clinical factors associated with time to viral suppression and virologic failure. These analyses provide new information on clinical outcomes in the setting of early ART initiation and use of ART for HIV prevention.
Methods
Study cohort
The HPTN 052 trial enrolled 1763 HIV serodiscordant couples (≥18 years of age) at 12 sites in low- or middle-income countries (Botswana, Kenya, Malawi, South Africa, Zimbabwe, India, Thailand, Brazil); two additional couples were enrolled in the United States of America (NCT00074581; pilot enrollment from 2005 to 2007, full enrollment from 2007 to 2010). The trial design and results have been reported previously.Citation3,4 Couples were randomized to one of two study arms. In the early ART arm, index participants initiated ART immediately after enrollment (CD4 cell count between 350 and 550 cells/mm3). In the delayed ART arm, ART was initiated after the CD4 cell count fell below 250 cells/mm3 on two consecutive study visits, or when the index participant developed an AIDS-defining illness. At enrollment, HIV-infected index participants reported no prior ARV drug use with the exception of short-term ARV drug use for prevention of mother-to-child transmission. However, retrospective ARV drug testing revealed that some participants were on ART at the time of study enrollment.Citation13 The most common ART regimen was a combination of efavirenz (EFV), lamivudine (3TC), and zidovudine (ZDV); other drugs used for treatment included atazanavir, atazanavir/ritonavir, emtricitabine, lopinavir/ritonavir (LPV/r), nevirapine, stavudine, and tenofovir disoproxil fumarate.Citation3 The analyses in this report include data from the start of the trial through May, 2015 (end of study). In May 2011, participants were informed of interim study results that demonstrated that early ART prevented 96% of linked HIV transmissions.Citation3 After that date, all study participants were counseled on the personal and public health benefits of early ART, and ART was offered to all index participants regardless of CD4 cell count. In this report, virologic outcomes were evaluated in participants in the early and delayed ART arms. Participants in the delayed ART arm were also stratified into two groups based on whether they started ART before or after release of interim study results. Participants who had a viral load <400 copies/mL at study enrollment were excluded from analysis.
Laboratory and statistical methods
HIV viral load and CD4 cell count assays were performed at study sites.Citation3 Prior to November 2006, viral load testing was performed quarterly after ART initiation. Follow-up visits were allowed to occur within a two-week window of the targeted visit dates. After November 2006, viral load testing was performed at an additional visit one month after ART initiation. The majority of participants were enrolled after this date and had a one-month visit. Additional study visits with viral load assessments were permitted at the discretion of site investigators for clinical management of participants on ART. Viral suppression was defined as the first of two consecutive viral load measurements ≤400 copies/mL after ART initiation. Virologic failure was defined as the first of two consecutive viral load measurements >1000 copies/mL after 24 weeks on ART. Potential ascertainment bias in determining the timing of viral suppression due to variation in the timing of viral load measurements (interval censorship) was examined. Characteristics of study participants in different groups (early ART arm; delayed ART arm; delayed ART arm with ART initiation before vs. after May 2011) were analyzed using the Chi-square test (for categorical variables) and the Wilcoxon rank sum test (for continuous variables). Cox regression and Kaplan-Meier methods were used to analyze the association of demographic and other factors with time to viral suppression and virologic failure. The multivariate Cox regression model was created using a backward model selection method; p < 0.05 was used to exclude variables in the backward selection model.
Ethical considerations
Institutional Review Boards/Ethics Committees at each participating institution approved the HPTN 052 trial. Written informed consent was obtained from all study participants.
Results
Study cohort
We evaluated viral suppression and virologic failure outcomes in 1566 index participants in HPTN 052 who had a viral load >400 copies/ml at study enrollment and initiated ART (Figure 1). Table shows demographic and clinical characteristics of study participants at the time of ART initiation. At ART initiation, the median CD4 cell count was 439 cells/mm3 in the early ART arm and 314 cells/mm3 in the delayed ART arm. At the time of ART initiation, index participants in the early ART arm were younger and had higher CD4 cell counts and lower viral loads; they were also more likely to receive EFV/3TC/ZDV than other regimens, have a lower educational status, and have >1 sexual partner compared to participants in the delayed ART arm. In the delayed ART arm, index participants who started ART before release of interim study results in May 2011 had lower CD4 cell counts and higher viral loads at ART initiation; those participants also had a different regional distribution and were more likely to be male compared to index participants in the delayed ART arm who started ART after May 2011 (Table ).
Table 1 Characteristics of participants at the time of ART initiation (N = 1566)
Analysis of viral suppression after ART initiation
Viral suppression was evaluated in 1566 index participants (7397 person-years of follow up on ART): 832 in the early ART arm and 734 in the delayed ART arm (Figure 1). In the delayed ART arm, 204 participants initiated ART before May 2011 and 530 initiated ART after May 2011 (Figure 1). Overall, 93% of the participants achieved viral suppression by 12 months after ART initiation. At 1, 3, 6, 9, and 12 months after ART initiation, the cumulative probabilities of viral suppression were 49, 83, 89, 91, and 92% in the early ART arm; 25, 72, 92, 95, and 96% in the delayed ART arm among participants who initiated ART before May 2011; and 43, 77, 87, 90, and 93% in the delayed ART arm among participants who initiated ART after May 2011.
In Kaplan-Meier analysis, there was no significant difference in time to viral suppression among participants in the following three groups: early ART arm; delayed ART arm with ART initiation before May 2011; delayed ART with ART initiation after May 2011 (p = 0.08, Figure A). Some differences were noted in the timing of viral load measurements in these three participant groups. More detailed analyses indicated that these differences were not likely to have impacted the analysis of time to viral suppression in these groups (Supplemental File 1). There was no difference in time to viral suppression between the two study arms (Table ). Time to viral suppression was longer among participants in the delayed ART arm who initiated ART before May 2011 than in the early ART arm, but this difference was not statistically significant in the multivariate model (Table ). In univariate analyses, the following variables were associated with a longer time to viral suppression: younger age, lower CD4 cell count at ART initiation, higher viral load at ART initiation, region, and regimen. However, only three of these factors were associated with longer time to viral suppression in the multivariate model: younger age (<25 years), higher viral load at ART initiation, and region (Africa, compared to Asia, Table ). Kaplan-Meier plots were used to assess the proportional hazards assumption for these three variables (Supplemental File 2A); the plots indicate that the proportional hazards assumption is appropriate for the Cox model.
Figure 1 Study cohort for virologic outcomes analysis.
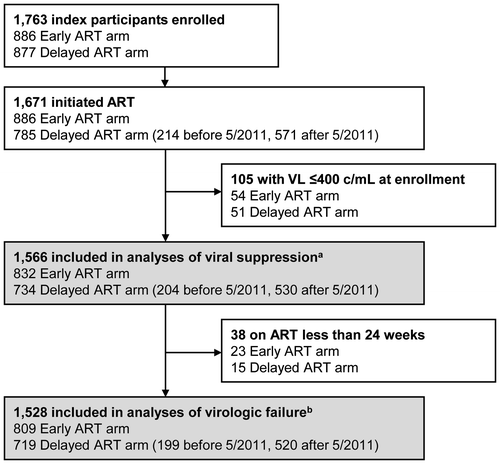
Figure 2 Kaplan-Meier estimates for virologic outcomes after ART initiation by study group.
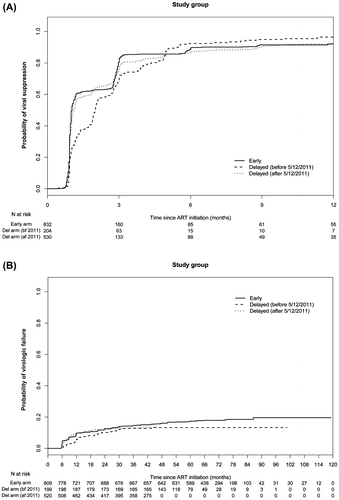
Table 2 Factors associated with time to viral suppression after ART initiation (censored at 12 months after ART initiation)
Analysis of virologic failure
Virologic failure was evaluated in 1528 index participants with 6662 person-years of follow up on ART (Figure 1). The annual incidence of virologic failure was 3.6% (95% confidence intervals [CI]: 3.1–4.1%) overall; 3.4% (95% CI: 2.9–4.0%) in the early ART arm, and 3.8% (95% CI: 3.1–4.7%) in the delayed ART arm (p = 0.37). In the delayed ART arm, the annual incidence of virologic failure was 2.9% (95% CI: 1.9–4.3%) among participants who initiated ART before May 2011 and 4.4% (95% CI: 3.4–5.6%) among participants who initiated ART after May 2011 (p = 0.08).
In Kaplan-Meier analysis, there was no significant difference in virologic failure among participants in the three study groups described above (p = 0.32, Figure B). In univariate analyses, there was also no significant difference in virologic failure in the early vs. delayed ART arms or in the early ART arm compared to either delayed ART group (before vs. after May 2011, Table ). Other factors were associated with a higher risk of virologic failure, including younger age (<25 years), female gender, higher CD4 cell count at ART initiation, lower education level, marital status (married), and lack of viral suppression by three months. All of these variables were independently associated with virologic failure in the multivariate model with the exception of gender. In addition, lower viral load at ART initiation was associated with virologic failure in the multivariate model (Table ). Kaplan-Meier plots were used to assess the proportional hazards assumption for variables that were associated with virologic failure in the multivariate model (see Supplemental File 2B); the plots indicate that the proportional hazards assumption is appropriate for the Cox model.
Table 3 Factors associated with virologic failure
Overall (in both study arms), only 16 (6.7%) of the 238 virologic failures occurred after four years on ART. To explore whether the findings were affected by late failure events, the analysis shown in Table was repeated using the same methods after censoring data from study visits more than four years after ART initiation. In the multivariate model that included censoring, the same variables were significantly associated with virologic failure with the exception of CD4 cell count. Furthermore, the hazard ratios for all variables analyzed were similar with and without censoring; the p-values for these variables were similar with and without censoring with one exception: the p-value for viral load at ART initiation was lower with censoring (p = 0.0035).
Discussion
Achieving and maintaining viral suppression after ART initiation directly benefits those on ART and has public health benefits by reducing HIV transmission.Citation4,14 The HPTN 052 trial identified two risk periods for HIV transmission when ART is used for HIV prevention: near the time of ART initiation (before the index was virally suppressed) and after virologic failure (when the index was viremic).Citation6 Overall, 93% of the participants included in this report were virally suppressed by 12 months after ART initiation. The time to viral suppression was similar between the two study arms (early ART arm vs. delayed ART arm, p = 0.06). We did observe a longer time to viral suppression for participants in the delayed ART arm who initiated ART before May 2011, compared to those in the early ART arm (p = 0.038, univariate analysis); this subset of participants in the delayed ART arm started ART at lower CD4 cell counts than the participants in the other two groups (early ART arm, delayed ART arm with ART initiation after May 2011). This difference was not observed in the multivariate model where the analysis was adjusted for other factors. There was also no significant difference when time to viral suppression was compared across all three participant groups (p = 0.095). CD4 cell count at ART initiation was highly associated with time to viral suppression in the univariate model, but was not significantly associated with time to viral suppression in the multivariate model that adjusted for other variables.
In this cohort, three factors were independently associated with longer time to viral suppression: higher viral load at ART initiation, younger age (<25 years), and geographic region (Africa compared to Asia). Higher viral load at ART initiation was also associated with a longer time to viral suppression in studies where ART was initiated at lower CD4 cell counts.Citation15 Younger age has been associated with lack of viral suppressionCitation16,17 and poor ART adherenceCitation18,19 in previous studies. In HPTN 052, self-reported ART adherence was higher among older participants in the early ART arm, but this association was not statistically significant in multivariate analysis.Citation20 The regional differences in time to viral suppression observed in HPTN 052 may reflect differences in HIV subtype or other factors, such as adherence. Regional differences in adherence were observed in the early ART arm in HPTN 052.Citation20 Previous studies have reported more rapid viral suppression in individuals with certain HIV subtypes.Citation21,22
We did not find an association between ART regimen (EFV/3TC/ZDV vs. other) and time to viral suppression in HPTN 052. Other studies have demonstrated that the rate of viral load decline varies with different ARV drug regimens.Citation23,24 For example, shorter time to viral suppression has been observed using EFV-based ART compared to LPV/r-based ARTCitation25 and with regimens that include an HIV integrase strand transfer inhibitor (INSTI).Citation26,27 In HPTN 052, 72% of the index participants on ART were on EFV/3TC/ZDV; the remaining participants were on a variety of different ART regimens;Citation3 none received an INSTI-based regimen.
Among the HPTN 052 participants in this report, virologic failure was uncommon, with an annual incidence of 3.6%. Three factors were strongly associated with virologic failure in multivariate analyses: younger age (<25 years, compared to ≥40 years), a lower level of education, and lack of viral suppression by three months; lower viral load and higher CD4 cell count at ART initiation were also associated with virologic failure. An association between younger age and virologic failure was observed in a previous study.Citation28 An association between lower educational level and virologic failure was not observed in previous studies performed in the US and Southern Africa Citation29,30; further studies are needed to determine whether the association observed in this study is due to lower adherence or other factors. We did observe a weak association between a higher CD4 cell count at ART initiation and virologic failure. A possible explanation for this finding is that participants who had higher CD4 cell counts at the time of ART initiation may have had lower adherence to ART because they were in better health. Of note, the incidence of virologic failure was similar in the two study arms in the interim analysis of the HPTN 052 trial (data through February 2011)Citation3 and in this extended analysis (data through May 2015). This may have reflected the fact that the interim analysis included a shorter follow-up period with fewer virologic failure events, and that in the extended analysis, the delayed ART arm included two distinct participant groups: (1) before May 2011, participants initiated ART when their CD4 cell count fell below 250 cells/mm3 or they developed an AIDS defined illness; (2) after May 2011, participants started ART at higher CD4 cell counts and were aware of the benefits of early ART. Interestingly, participants in the latter group had a higher annual incidence of virologic failure than those who started ART before May 2011 (2.9% vs. 4.4%, p = 0.08). This indicates that knowledge of the beneficial effects of early ART did not improve treatment outcomes.
Other studies have shown that HIV subtype is associated with virologic failure. HIV subtypes were not available for all participants in this study; however the prevalent HIV subtypes are different in the Americas (mostly B and F in Brazil), Africa (mostly C in sub-Saharan Africa). In the ACTG 5175 study (PEARLS), which was performed at many of the same study sites as HPTN 052, subtype C infection was associated with a higher rate of virologic failure compared to subtype B infection.Citation31 The frequency of ART failure was also higher in subtype C compared to subtype B in a cohort study from Sweden.Citation32
The findings from HPTN 052 and other studies provide strong support for universal HIV treatment where HIV-infected individuals are eligible to start ART early regardless of CD4 cell count,Citation4,33 which is now recommended for all HIV-infected individuals.Citation34 Community randomized trials are underway to determine the best way to deliver universal ART and assess the impact of universal ART on HIV incidence at a population level. Additional interventions (e.g. use of pre-exposure prophylaxis in couples at the start of ART until viral suppression is well-established, and use of INSTI-based ART regimens) may help reduce transmission risk. This study identified several clinical and demographic factors associated with time to viral suppression after ART initiation and virologic failure. Recognition of these factors may help to optimize ART for HIV treatment and prevention.
Supplementary material
Supplemental data for this article can be accessed here http://dx.doi.org/10.1080/15284336.2017.1311056.
Declaration of Interests
None of the authors has a financial or personal relationship with other people or organizations that could inappropriately influence (bias) their work, with the following exceptions.
JEG receives honoraria for advisory board membership from Bristol-Myers Squibb, Gilead Sciences, Theratechnologies, Merck & Co., ViiV Healthcare. JEG’s organization receives research support from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck & Co., Sangamo BioSciences, ViiV Healthcare. JJE receives honoraria for advisory board membership from Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck & Co., ViiV Healthcare and research support from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck & Co., Sangamo BioSciences, ViiV Healthcare through contracts to the University of North Carolina. MCH has received honoraria for advisory board membership from ViiV healthcare. KM received research support from Gilead Sciences, Inc. and ViiV Healthcare during the conduct of the study. MC receives honoraria for advisory board membership from Janssen Global Services, Roche Molecular Systems, and Merck Research.
Funding
This work was supported by the grants from the Division of AIDS of the U.S. National Institute of Allergy and Infectious Diseases (NIAID); and by the Office of AIDS Research of the U.S. National Institutes of Health (NIH) [UM1-AI068613 (Eshleman); UM1-AI068617 (Donnell); and UM1-AI068619 (Vermund/El-Sadr)]. The HPTN 052 trial also received funding through U01-AI018136. Study drugs used in HPTN 052 were donated by Abbott Laboratories, Boehringer-Ingelheim Pharmaceuticals, Inc, Bristol-Myers Squibb, Gilead Sciences, Inc., GlaxoSmithKline/ViiV Healthcare, and Merck & Co., Inc.
YHCT_1311056_Supplementary_Material.zip
Download Zip (250.3 KB)Acknowledgements
The authors thank the HPTN 052 study team and participants for providing the samples and data used in this study. We also thank the laboratory staff who helped with sample management and testing.
Notes
This paper was submitted with the permission of the Director of Kenya Medical Research Institute (KEMRI). This work was presented in part at the 8th IAS Conference on HIV Pathogenesis, Treatment & Prevention (July, 2015), Vancouver, Canada and at the Conference on Retroviruses and Opportunistic Infections (CROI) 2017 (February, 2017), Seattle, WA.
References
- Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS ONE. 2010;5:e12598.10.1371/journal.pone.0012598
- Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929.10.1056/NEJM200003303421303
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505.10.1056/NEJMoa1105243
- Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839.10.1056/NEJMoa1600693
- Eshleman SH, Hudelson SE, Redd AD, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV prevention trials network 052 trial. J Infect Dis. 2011;204:1918–1926.10.1093/infdis/jir651
- Eshleman SH, Hudelson SE, Redd AD, et al. Treatment as prevention: characterization of partner infections in the HIV prevention trials network 052 trial. J Acquir Immune Defic Syndr. 2016;74:112–116.
- Ping LH, Jabara CB, Rodrigo AG, et al. HIV-1 transmission during early antiretroviral therapy: evaluation of two HIV-1 transmission events in the HPTN 052 prevention study. PLoS ONE. 2013;8:e71557.10.1371/journal.pone.0071557
- Anglemyer A, Rutherford GW, Horvath T, et al. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev. 2013;4:CD009153.
- Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098.10.1016/S0140-6736(10)60705-2
- Lu W, Zeng G, Luo J, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan. China. J Acquir Immune Defic Syndr. 2010;55:232–238.
- Mujugira A, Celum C, Coombs RW, et al. HIV transmission risk persists during the first 6 months of antiretroviral therapy. J Acquir Immune Defic Syndr. 2016;72:579–584.
- Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316:171–181.10.1001/jama.2016.5148
- Fogel JM, Wang L, Parsons TL, et al. Undisclosed antiretroviral drug use in a multi-national clinical trial (HPTN 052). J Infect Dis. 2013;208:1624–1628.10.1093/infdis/jit390
- Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–290.10.1016/S1473-3099(13)70692-3
- Phillips AN, Staszewski S, Weber R, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001;286:2560–2567.10.1001/jama.286.20.2560
- Mujugira A, Celum C, Tappero JW, et al. Younger age predicts failure to achieve viral suppression and virologic rebound among HIV-1-infected persons in serodiscordant partnerships. AIDS Res Hum Retroviruses. 2016;32:148–154.10.1089/aid.2015.0296
- Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–1473.
- Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in Southern Africa. J Acquir Immune Defic Syndr. 2009;51:65–71.10.1097/QAI.0b013e318199072e
- Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDs. 2014;28:128–135.10.1089/apc.2013.0345
- Safren SA, Mayer KH, Ou SS, et al. Adherence to early antiretroviral therapy: results from HPTN 052, a Phase III, multinational randomized trial of ART to prevent HIV-1 sexual transmission in serodiscordant couples. J Acquir Immune Defic Syndr. 2015;69:234–240.10.1097/QAI.0000000000000593
- Paraskevis D, Touloumi G, Bakoyannis G, et al. Effect of HIV type 1 subtype on virological and immunological response to combination antiretroviral therapy: evidence for a more rapid viral suppression for subtype A than subtype B-infected greek individuals. AIDS Res Hum Retroviruses. 2013;29:461–469.10.1089/aid.2012.0143
- Geretti AM, Harrison L, Green H, et al. Effect of HIV‐1 subtype on virologic and immunologic response to starting highly active antiretroviral therapy. Clin Infect Dis. 2009;48:1296–1305.10.1086/599387
- Haubrich RH, Riddler SA, Ribaudo H, et al. Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, nonnucleoside reverse transcriptase inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS. 2011;25:2269–2278.10.1097/QAD.0b013e32834d0c20
- Kuritzkes DR, Ribaudo HJ, Squires KE, et al. Plasma HIV‐1 RNA dynamics in antiretroviral‐naive subjects receiving either triple‐nucleoside or efavirenz‐containing regimens: ACTG A5166s. J Infect Dis. 2007;195:1169–1176.10.1086/522475
- Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106.10.1056/NEJMoa074609
- Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133.10.1097/QAI.0b013e318157131c
- Murray JM, Emery S, Kelleher AD, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–2321.10.1097/QAD.0b013e3282f12377
- Rupérez M, Pou C, Maculuve S, et al. Determinants of virological failure and antiretroviral drug resistance in Mozambique. J Antimicrob Chemother. 2015;70:2639–2647.10.1093/jac/dkv143
- Beer L, Skarbinski J. Adherence to antiretroviral therapy among HIV-infected adults in the United States. AIDS Educ Prev. 2014;26:521–537.
- Labhardt ND, Bader J, Ramoeletsi M, et al. Clinical and socio-demographic predictors for virologic failure in rural Southern Africa: preliminary findings from CART-1. J Intl AIDS Soc. 2014;17:19666.
- Kantor R, Smeaton L, Vardhanabhuti S, et al. Pretreatment HIV drug resistance and HIV-1 subtype C are independently associated with virologic failure: results from the multinational PEARLS (ACTG A5175) clinical trial. Clin Infect Dis. 2015;60:1541–1549.
- Häggblom A, Svedhem V, Singh K, et al. Virological failure in patients with HIV-1 subtype C receiving antiretroviral therapy: an analysis of a prospective national cohort in Sweden. Lancet HIV. 2016;3:e166–e174.10.1016/S2352-3018(16)00023-0
- INSIGHT START Study Group, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807.
- World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed; 2016. Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed June 21, 2016.
