Abstract
Background: Darunavir 800 mg once daily (QD) is indicated for HIV-1–infected treatment-naïve and treatment-experienced (without darunavir resistance-associated mutations [RAMs]) individuals, and has been evaluated in phase 2/3 studies with durations between 48 and 192 weeks.
Objective: To summarize the development (or identification) of post-baseline resistance (RAMs and antiretroviral phenotypic susceptibility) among subjects receiving darunavir QD dosing.
Methods: Seven phase 2/3 studies with available genotypes/phenotypes for subjects treated with ritonavir- or cobicistat-boosted darunavir 800 mg QD regimens were assessed: ARTEMIS (NCT00258557; n = 343), GS-US-299-0102 (NCT01565850; n = 153), GS-US-216-0130 (NCT01440569; n = 313), ODIN (NCT00524368; n = 294), INROADS (NCT01199939; n = 54), MONET (NCT00458302; n = 256), and PROTEA (NCT01448707; n = 273). Genotypic analyses were conducted at baseline (except switch studies enrolling virologically suppressed subjects [MONET, PROTEA]). Criteria for post-baseline resistance testing and evaluation of the development (or identification [switch studies]) of RAMs (respective IAS-USA mutations) varied slightly across studies.
Results: Among 1686 subjects treated with darunavir 800 mg QD regimens, 184 had protocol-defined virologic failure; 182 had post-baseline genotypes analyzed. Overall, 4/1686 (0.2%) developed (or had identified [switch studies]) primary protease inhibitor and/or darunavir RAMs (ARTEMIS, n = 1; GS-US-216-0130, n = 1; ODIN, n = 1; MONET, n = 1). Only 1/1686 (<0.1%) subject lost darunavir phenotypic susceptibility (ODIN; possibly related to prior ritonavir-boosted lopinavir virologic failure). Among 1103 subjects using a nucleos(t)ide reverse transcriptase inhibitor (N[t]RTI) backbone, 10 (0.9%) developed ≥ 1 N(t)RTI RAM (8 had the emtricitabine RAM M184I/V).
Conclusions: Darunavir has a high genetic barrier to resistance. Across a diverse population of HIV-1–infected subjects treated with darunavir 800 mg QD regimens, the development of darunavir resistance was rare (<0.1%).
Introduction
Human immunodeficiency virus (HIV)-1 drug resistance testing can provide important information to guide antiretroviral (ARV) treatment strategy because resistance-associated mutations (RAMs) harbored by the virus may restrict treatment options.Citation1,2 To minimize the risk of developing RAMs, the use of ARV agents with a high genetic barrier to resistance, such as protease inhibitors (PIs), are desirable.Citation1,2 In the case of boosted darunavir, potent antiviral activity against wild-type and multidrug-resistant HIV-1 and a high genetic barrier to the development of resistance have been demonstrated.Citation3–5
Darunavir dosed once daily (QD) has been studied extensively across diverse populations in clinical trials, demonstrating excellent efficacy and safety.Citation6–12 These studies of varying duration have enrolled both treatment-naïve and treatment-experienced subjects and included a range of combination therapies and monotherapy. Boosted darunavir 800 mg, in combination with 2 nucleos(t)ide reverse transcriptase inhibitors (N[t]RTIs), is a recommended initial ARV treatment option in certain clinical situations for HIV-1 infection per guidelines from the United States Department of Health and Human Services (US DHHS; with ritonavir or cobicistat).Citation1 Boosted darunavir 800 mg, in combination with 2 N(t)RTIs, is also a recommended initial regimen per the European AIDS Clinical Society (EACS; with ritonavir or cobicistat).Citation2 Darunavir QD is indicated for HIV-1–infected individuals who are treatment-naïve or treatment-experienced without darunavir RAMs.Citation13 Eleven HIV-1 protease (PR) mutations associated with darunavir resistance were previously identified based on an analysis of a pooled dataset of studies in highly treatment-experienced subjects (subjects had received treatment with darunavir 600 mg and ritonavir 100 mg twice daily).Citation14,15 Consistent with darunavir having a high genetic barrier to the development of resistance, its virologic efficacy has been shown to be compromised only in the presence of 3 or more darunavir RAMs in the background of a high number (median of 14 or 15, respectivelyCitation14,15) of International Antiviral Society–USA (IAS-USA) PI RAMs.Citation16
The current report is an analysis of the development (or identification) of post-baseline resistance, including primary PI, darunavir, N(t)RTI, and non-nucleoside reverse transcriptase inhibitor (NNRTI) RAMs, as well as loss of ARV phenotypic susceptibility, among HIV-1–infected subjects in the darunavir 800 mg QD dosing arms from seven completed clinical studies at their respective final analysis time points.
Methods
Study designs
This analysis was based on data from seven company-sponsored, phase 2 and 3 studies that investigated darunavir 800 mg QD–containing ARV regimens and had available genotype and phenotype data (ARTEMIS [ClinicalTrials.gov Identifier: NCT00258557], GS-US-299-0102 [NCT01565850], GS-US-216-0130 [NCT01440569], ODIN [NCT00524368], INROADS [NCT01199939], MONET [NCT00458302], and PROTEA [NCT01448707]); detailed study designs have been published for each study.Citation6–12 Only the darunavir 800 mg QD treatment arms were included in this analysis. As shown in Figure , the studies differed in clinical phase, sample size, and final analysis time point. The subject populations also varied in regards to treatment experience (i.e. treatment-naïve or treatment-experienced) and, for treatment-experienced subjects, history of ARV use and viral suppression status (i.e. viral load of ≥50 or <50 HIV-1 RNA copies/mL at screening). Treatment varied in terms of boosting agent (ritonavir or cobicistat) and background regimen.
Figure 1 Study summaries of darunavir 800 mg QD–containing treatment arms
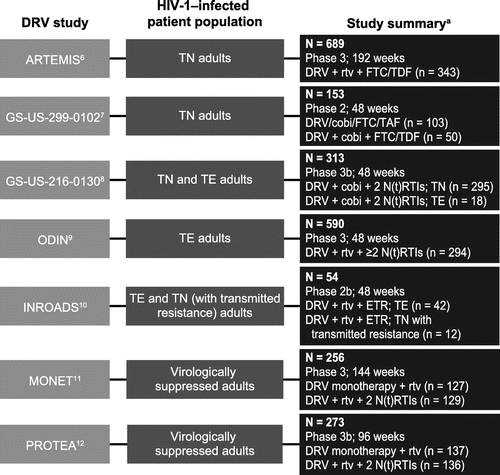
Three of the seven studies (ARTEMIS, GS-US-216-0130, and ODIN) were pivotal phase 3 studies that evaluated the safety and efficacy of darunavir QD treatment (with N[t]RTIs). ARTEMIS was a 192-week, phase 3 study of treatment-naïve adults; among the 689 enrolled subjects, 343 were included in this analysis (i.e. were treated with darunavir 800 mg QD) and had received ritonavir-boosted darunavir with emtricitabine and tenofovir disoproxil fumarate.Citation6 GS-US-299-0102 was a 48-week, phase 2 study of treatment-naïve adults; all 153 subjects were included in this analysis and had received cobicistat-boosted darunavir and emtricitabine, with either tenofovir disoproxil fumarate or tenofovir alafenamide.Citation7 GS-US-216-0130 was a 48-week, phase 3b study of both treatment-naïve and treatment-experienced adults; all 313 subjects received cobicistat-boosted darunavir and 2 N(t)RTIs.Citation8 ODIN was a 48-week, phase 3 study of treatment-experienced adults; of 590 enrolled subjects, 294 were included in this analysis and had received ritonavir-boosted darunavir and ≥2 N(t)RTIs.Citation9 INROADS was a 48-week, phase 2b study of treatment-experienced adults and treatment-naïve adults with transmitted resistance; all 54 subjects were included in this analysis and had received ritonavir-boosted darunavir and etravirine.Citation10 MONET was a 144-week, phase 3 study of virologically suppressed adults; all 256 enrolled subjects were included in this analysis and had received either ritonavir-boosted darunavir monotherapy or ritonavir-boosted darunavir and 2 N(t)RTIs.Citation11 PROTEA was a 96-week, phase 3b study of virologically suppressed adults; all 273 enrolled subjects were included in this analysis and had received either ritonavir-boosted darunavir monotherapy or ritonavir-boosted darunavir and 2 N(t)RTIs.Citation12
Key eligibility criteria related to resistance and viral load at screening varied across studies (Table ). Viral load inclusion criteria for the studies that enrolled virologically failing subjects ranged from >500 to ≥5000 copies/mL. Studies varied in regards to excluded RAMs and the requirement of genotypic or phenotypic ARV susceptibility. Three studies included eligibility criteria that limited history of previous virologic failures.
Table 1 Key resistance eligibility criteria at screening
Evaluation of resistance
Genotypic and phenotypic analyses were conducted at screening (GS-US-299-0102, GS-US-216-0130) or screening/baseline (ARTEMIS, ODIN, INROADS), except for switch studies that enrolled virologically suppressed subjects (MONET, PROTEA). The criteria for protocol-defined virologic failure (PDVF) varied slightly across studies (Table ). Resistance testing was performed on samples from subjects experiencing PDVF (except MONET and PROTEA, for which any sample that exceeded the viral load threshold was tested). When feasible, resistance testing was performed at the time of (confirmed [preferable] or unconfirmed) virologic failure and/or at later time points. Post-baseline virologic failure plasma samples were sequenced (population sequencing) and phenotyped at Virco BVBA (Mechelen, Belgium) using the Virco®TYPE HIV-1 and Antivirogram® assays, or at Monogram Biosciences (South San Francisco, CA, USA) using the GenoSure® MG and PhenoSense® GT (PR-reverse transcriptase [RT]) assays. The viral load cut-off for attempting resistance testing was as low as 50 copies/mL for some studies (ARTEMIS, ODIN, INROADS, and MONET) and 400 copies/mL for other studies (GS-US-299-0102, GS-US-216-0130, and PROTEA; Table ).
Table 2 Viral load criteria for post-baseline resistance testing
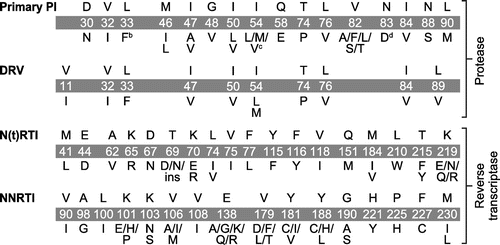
Evaluation of the development of RAMs (or identification of RAMs, in the case of switch studies) was based on PI, N(t)RTI, and NNRTI IAS-USA mutation lists defined in each study (Figure ). RAMs were considered to have developed if they were detected post-baseline but not at baseline or screening. Mutations that were identified among subjects with virologic failure in the switch studies could not be compared with screening/baseline sequences to evaluate whether they developed during the study due to being virologically suppressed at screening and, therefore, lacking genotypes. If available, loss of phenotypic susceptibility to ARVs was defined as fold change (FC) in the 50% effective concentration (EC50; in cell-based assays) below or equal to the lower clinical cut-off or biological cut-off at baseline, but above the cut-off value post-baseline.
Figure 2 RAM listsa
Results
Resistance at baseline
Across the seven studies, a total of 2328 subjects were enrolled and 1686 of these were treated with darunavir 800 mg QD–based regimens. Among non-virologically suppressed subjects at baseline, the majority in each study harbored HIV-1 subtype B (Table ). In general, baseline RAMs tended to be more common among treatment-experienced subjects (ODIN and INROADS) than treatment-naïve subjects (ARTEMIS, GS-US-299-0102, and most subjects in GS-US-216-0130). All three studies that enrolled treatment-experienced subjects excluded individuals with darunavir RAMs at screening, but notably, three subjects receiving darunavir QD–based regimens in ODIN had ≥1 darunavir RAM at baseline. For studies of treatment-experienced subjects, previous use of PIs, N(t)RTIs, and NNRTIs was common (Table).
Table 3 Baseline virus characteristics
Table 4 Previous ARV useTable Footnotea
Baseline phenotypic susceptibility data were available in two studies of treatment-experienced subjects. In ODIN, 100% (291/291) of subjects with available data were susceptible to darunavir; susceptibility to N(t)RTIs in the optimized background regimen was as follows: 18% (53/290) of subjects were susceptible to 1 N(t)RTI, 75% (218/290) to ≥2 N(t)RTIs, and 7% (19/290) were not susceptible to any N(t)RTIs used in their background regimen. In INROADS, 100% (54/54) of subjects were susceptible to both study drugs (darunavir and etravirine).
Development of resistance
Of the 1686 subjects included in this analysis, 184 had PDVF and 182 had post-baseline genotypes analyzed (Table ). Overall, only 4 of 1686 (0.2%) subjects developed (or, in the case of switch studies, had identified) primary PI and/or darunavir RAMs post-baseline (4 of 184 [2.2%] subjects with PDVF).
Table 5 Development (and identification) of post-baseline RAMs and loss of ARV phenotypic susceptibility across studies
Among the four subjects, one (ARTEMIS) developed V11I, a darunavir (and secondary PI) RAM, after treatment was discontinued due to noncompliance; no loss of phenotypic susceptibility to darunavir or any PI was observed.
A second subject (treatment-experienced, GS-US-216-0130) developed I84I/V, a primary PI and darunavir RAM; this subject did not lose phenotypic susceptibility to darunavir or any PI.
A third subject (ODIN), at Week 48, developed M46I, a primary PI RAM, as well as V32I, L76V, and I84V, all of which were primary PI and darunavir RAMs. This subject was a nonresponder (on-study at Week 12 and never achieved 2 consecutive viral loads <50 copies/mL) who did achieve a viral load of <50 copies/mL at 1 visit (Week 12). The subject lost phenotypic susceptibility to darunavir (FC of 24.4), amprenavir, atazanavir, indinavir, and nelfinavir, which was possibly related to a previous virologic failure with ritonavir-boosted lopinavir treatment (September 2005–March 2008) because all of the developing primary PI RAMs (listed above) and most of the developing secondary PI RAMs (L10I, K20R, I54V, and A71V) are also mutations associated with lopinavir resistance.Citation16 The subject had also failed an efavirenz-based regimen after the lopinavir treatment failure. At the baseline visit for ODIN, the subject already had 2 PI RAMs (the lopinavir RAMs L63P and V77I) and 4 N(t)RTI RAMs (M41L, M184V, L210W, and T215Y); however, ultra-deep sequencing (at 1% sensitivity, as previously describedCitation17) had not detected the developing mutations at the baseline visit.
A fourth subject (ritonavir-boosted darunavir monotherapy treatment arm of MONET) had L33F, a primary PI and DRV RAM, detected at Week 12 with a viral load of 63 copies/mL, but was resuppressed at subsequent visits until Week 144. The darunavir RAM did not confer phenotypic resistance to darunavir; moreover, it is possible that the mutation was already present at baseline (no sequence available due to suppressed viral load).
Among subjects treated with ritonavir-boosted darunavir monotherapy, only 1 of 264 (0.4%) had a post-baseline darunavir RAM. Overall, among all 1686 subjects treated with any regimen containing darunavir QD included in the analysis, only 1 (<0.1%) lost phenotypic susceptibility to darunavir.
Ten of the 1103 (0.9%) non-virologically suppressed subjects who used an N(t)RTI backbone developed N(t)RTI RAMs; 9 of these subjects also lost phenotypic susceptibility to ≥1 of the N(t)RTIs included in their regimen (Table ). Four subjects from ARTEMIS, all of whom used emtricitabine and tenofovir disoproxil fumarate, developed RAMs (M184I/V, n = 3; M184V and K70E, n = 1) and lost emtricitabine phenotypic susceptibility. Two subjects from GS-US-216-0130, both of whom used emtricitabine and tenofovir disoproxil fumarate (1 treatment-naïve and 1 treatment-experienced), developed M184V and lost emtricitabine phenotypic susceptibility. Four subjects from ODIN developed N(t)RTI RAMs: 1 subject (who used abacavir and lamivudine) developed M184V and lost abacavir and lamivudine phenotypic susceptibility; 1 subject (who used abacavir, lamivudine, and tenofovir disoproxil fumarate) developed V75I and M184V and lost abacavir and lamivudine phenotypic susceptibility; 1 subject (who used lamivudine, tenofovir disoproxil fumarate, and zidovudine) developed T215F without loss of phenotypic susceptibility to any of the background ARV agents; and 1 subject (who used lamivudine, tenofovir disoproxil fumarate, and zidovudine) developed T215Y and lost zidovudine phenotypic susceptibility.
Among the 54 subjects in INROADS who were treated with ritonavir-boosted darunavir and etravirine, 2 developed NNRTI RAMs (Table ). One subject developed E138K and M230L and lost phenotypic susceptibility to etravirine. The other subject developed L100L/I, E138E/G, and Y181Y/C, and had reduced susceptibility to etravirine at the endpoint (FC of 8.1).
Discussion
Across a diverse population of HIV-1–infected subjects taking a darunavir 800 mg QD–based regimen, only 4 of 1686 (0.2%) subjects developed (or had identified) post-baseline primary PI and/or darunavir RAMs, and only 1 subject lost darunavir phenotypic susceptibility. The extremely low prevalence of the development of darunavir RAMs and darunavir phenotypic resistance is consistent with the known high genetic barrier to resistance of darunavir. Notably, among treatment-naïve subjects treated with darunavir QD, none developed a primary PI RAM or lost phenotypic susceptibility to darunavir. Among those subjects who were treatment-experienced but PI-naïve, none developed phenotypic darunavir resistance. Importantly, the 1 treatment-experienced subject who lost darunavir phenotypic susceptibility had a prior history of virologic failure with ritonavir-boosted lopinavir treatment, which may have contributed cross-resistant PI RAMs.Citation14,15 The fact that many of this subject’s developing mutations were lopinavir RAMs suggests the possibility that archived resistance variants may have rapidly reemerged upon exposure to darunavir.
The development of resistance to N(t)RTIs in subjects’ backbone therapies was also rare in these studies. Ten of 1103 (0.9%) non-virologically suppressed subjects who used an N(t)RTI backbone developed N(t)RTI RAMs; the most common, the emtricitabine RAM M184I/V, was found in 8 subjects. Nine of these 10 subjects lost phenotypic susceptibility to ≥1 N(t)RTI in their background regimen.
Boosted-darunavir monotherapy is not recommended in guidelines from the US DHHS, and is an option only for exceptional persons who are not candidates for dual therapies per EACS guidelines, due to reduced efficacy compared with combination regimensCitation1,2; however, numerous studies have evaluated monotherapy as an ARV regimen simplification strategy in virologically suppressed individuals. Such studies allow for insights into the true genetic barrier to the development of resistance when subjects are treated with only boosted darunavir 800 mg QD. In the current analysis, among those receiving ritonavir-boosted darunavir monotherapy (in MONET and PROTEA), only 1 of 264 (0.4%) subjects was observed to have a darunavir RAM, (which, by itself, does not confer phenotypic resistance to darunavir).Citation14,15 Across six additional studies of ritonavir-boosted darunavir monotherapy (818 subjects in total), no post-baseline darunavir RAMs were detected and only 2 (0.2%) subjects had a post-baseline primary PI RAM.Citation18–24 Taken together, findings from studies of boosted-darunavir monotherapy further illustrate its high genetic barrier to resistance. This is in contrast to integrase inhibitor monotherapy, which has resulted in high rates of resistance development.Citation25,26
The rare development of darunavir resistance upon virologic failure, as seen in the current analysis, is consistent with findings based on a database analysis of US clinical samples.Citation5,27 Samples (from patients receiving varying ARV/PI regimens and with different treatment experiences and histories of resistance [no prior treatment information was available]) had been submitted for resistance testing (combined genotypic and phenotypic resistance testing) to Monogram Biosciences and were collected from 2006 through 2015.Citation5,27 The overall prevalence of darunavir RAMs among these submitted samples decreased over time; a corresponding decrease in darunavir phenotypic resistance was also observed.Citation5,27 The prevalence of darunavir phenotypic resistance remained lower than that of other PIs for each year during the analysis period.Citation5,27
Of note, there is another darunavir 800 mg QD–based regimen that is currently in clinical development. It is the first single-tablet regimen containing darunavir and combines darunavir 800 mg, cobicistat 150 mg, emtricitabine 200 mg, and tenofovir alafenamide 10 mg. This agent was evaluated in GS-US-299-0102 through 48 weeksCitation7 (included in the current analysis and showing no resistance to darunavir, emtricitabine, or tenofovir alafenamide) and is being evaluated in 2 ongoing phase 3 studies (AMBER [NCT02431247] and EMERALD [NCT02269917]Citation28); it was approved in Europe in September 2017 and submitted for regulatory approval in the United States in 2017. This potential regimen includes tenofovir alafenamide, a novel prodrug of tenofovir that provides comparable efficacy at one-tenth of the dose of tenofovir disoproxil fumarate, resulting in a ~90% lower tenofovir plasma concentration and fewer adverse effects, particularly renal and bone adverse effects.Citation7,29,30 Moreover, strategies to simplify ARV regimens and decrease pill burden, such as single-tablet regimens, may help improve treatment adherence, which may in turn lead to higher virologic suppression rates and further reduce the potential for development of drug resistance.Citation31–33
In summary, HIV-1 resistance to darunavir was rarely observed in the seven clinical studies of darunavir 800 mg QD–based treatment regimens that were included in this analysis; only 1 of 1686 (<0.1%) subjects lost darunavir phenotypic susceptibility. These findings are consistent with those from previous studiesCitation3–5 and demonstrate that darunavir dosed QD, which is indicated in treatment-naïve and treatment-experienced (without darunavir RAMs) patients, has a very high genetic barrier to the development of resistance.
Funding
This work was supported by Janssen Scientific Affairs, LLC. E.L. and S.D.M. are employees of Janssen Infectious Diseases BVBA. E.Y.W., D.L., S.S., and K.B. are employees of Janssen Scientific Affairs, LLC.
Contributors
E.L., E.Y.W., D.L., S.S., S.D.M., and K.B. contributed to the conception and design of the analysis, interpretation of the data, and drafting and revision of the article.
Acknowledgments
The authors would like to thank Anne Ghys of Janssen Infectious Diseases BVBA for her contributions. Medical writing support was provided by Courtney St. Amour, PhD, of MedErgy, and was funded by Janssen Scientific Affairs, LLC.
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed October 17, 2017.
- European AIDS Clinical Society. EACS Guidelines Version 9.0; October 2017.
- De Meyer S, Azijn H, Surleraux D, et al. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49(6):2314–2321.
- Dierynck I, De Wit M, Gustin E, et al. Binding kinetics of darunavir to human immunodeficiency virus type 1 protease explain the potent antiviral activity and high genetic barrier. J Virol. 2007;81(24):13845–13851.
- Lathouwers E, Gupta S, Haddad M, Paquet A, De Meyer S, Baugh B. Trends in darunavir resistance-associated mutations and phenotypic resistance in commercially tested United States clinical samples between 2006 and 2012. AIDS Res Hum Retroviruses. 2015;31(6):628–635.
- Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59.
- Mills A, Crofoot G Jr, McDonald C, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2015;69(4):439–445.
- Tashima K, Crofoot G, Tomaka FL, et al. Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a phase IIIb, open-label single-arm trial. AIDS Res Ther. 2014;11:39.
- Cahn P, Fourie J, Grinsztejn B, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25(7):929–939.
- Ruane PJ, Brinson C, Ramgopal M, et al. The Intelence aNd pRezista Once A Day Study (INROADS): a multicentre, single-arm, open-label study of etravirine and darunavir/ritonavir as dual therapy in HIV-1-infected early treatment-experienced subjects. HIV Med. 2015;16(5):288–296.
- Arribas JR, Clumeck N, Nelson M, Hill A, van Delft Y, Moecklinghoff C. The MONET trial: week 144 analysis of the efficacy of darunavir/ritonavir (DRV/r) monotherapy versus DRV/r plus two nucleoside reverse transcriptase inhibitors, for patients with viral load < 50 HIV-1 RNA copies/mL at baseline. HIV Med. 2012;13(7):398–405.
- Girard PM, Antinori A, Arribas JR, et al. Week 96 efficacy and safety of darunavir/ritonavir monotherapy vs. darunavir/ritonavir with two nucleoside reverse transcriptase inhibitors in the PROTEA trial. HIV Med. 2017;18(1):5–12.
- PREZISTA® (darunavir) [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
- De Meyer S, Vangeneugden T, van Baelen B, et al. Resistance profile of darunavir: combined 24-week results from the POWER trials. AIDS Res Hum Retroviruses. 2008;24(3):379–388.
- De Meyer S, Dierynck I, Lathouwers E, et al. Identification of mutations predictive of a diminished response to darunavir/ritonavir (refined profile): analysis of data from treatment-experienced subjects in POWER 1, 2, 3 and DUET-1 and DUET-2. Presented at: 6th European HIV Drug Resistance Workshop. Budapest; March 26–28, 2008. Abstract 54.
- Wensing AM, Calvez V, Gunthard HF, et al. 2017 Update of the drug resistance mutations in HIV-1. Top Antivir Med. 2017;24(4):132–141.
- Lathouwers E, De La Rosa G, Van de Casteele T, et al. Virological analysis of once-daily and twice-daily darunavir/ritonavir in the ODIN trial of treatment-experienced patients. Antivir Ther. 2013;18(3):289–300.
- Katlama C, Valantin MA, Algarte-Genin M, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24(15):2365–2374.
- Guaraldi G, Zona S, Cossarizza A, et al. Switching to darunavir/ritonavir monotherapy vs. triple-therapy on body fat redistribution and bone mass in HIV-infected adults: the Monarch randomized controlled trial. Int J STD AIDS. 2014;25(3):207–212.
- Arribas JR, Girard PM, Paton N, et al. Efficacy of protease inhibitor monotherapy vs. triple therapy: meta-analysis of data from 2303 patients in 13 randomized trials. HIV Med. 2016;17(5):358–367.
- Paton NI, Stohr W, Arenas-Pinto A, et al. Protease inhibitor monotherapy for long-term management of HIV infection: a randomised, controlled, open-label, non-inferiority trial. Lancet HIV. 2015;2(10):e417–426.
- Gianotti N, Galli L, Maserati R, et al. Monotherapy with darunavir/ritonavir or lopinavir/ritonavir versus standard antiretroviral therapy: a randomized clinical trial (2pm Study). New Microbiol. 2016;39(4):290–294.
- Gianotti N, Cozzi-Lepri A, Antinori A, et al. Refining criteria for selecting candidates for a safe lopinavir/ritonavir or darunavir/ritonavir monotherapy in HIV-infected virologically suppressed patients. PLoS One. 2017;12(2):e0171611.
- Santos JR, Llibre JM, Bravo I, et al. Short communication: efficacy and safety of treatment simplification to lopinavir/ritonavir or darunavir/ritonavir monotherapy: A randomized clinical trial. AIDS Res Hum Retroviruses. 2016;32(5):452–455.
- Wijting IEA, Rokx C, Boucher CAB, et al. Dolutegravir as maintenance monotherapy for HIV-1: a randomized clinical trial. Presented at: the Conference on Retroviruses and Opportunistic Infections (CROI). Seattle, WA; February 13–16, 2017. Abstract 451LB.
- Blanco JL, Oldenbuette C, Thomas R, et al. Pathways of resistance in subjects failing dolutegravir monotherapy. Presented at: the Conference on Retroviruses and Opportunistic Infections (CROI). Seattle, WA; February 13–16, 2017. Abstract 42.
- Brown K, Stewart L, Whitcomb JM, Yang D, Nettles RE, Lathouwers E. Prevalence of darunavir resistance in the United States (2010–2015). Presented at: 9th International AIDS Society (IAS) Conference. Paris; July 23–26, 2017. Poster TUPEB0372.
- Orkin C, Molina J-M, Negredo E, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2017. Epub ahead of print. doi: 10.1016/S2352-3018(17)30179-0
- Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–455.
- De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharmacol. 2016;119:1–7.
- World Health Organization. HIV Drug Resistance Surveillance Guidance: 2015 Update (Technical Update); 2015.
- Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets. 2011;11(2):167–174.
- Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65(5):587–596.
- Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2008. Top HIV Med. 2008;16(5):138–145.
- Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17(5):138–145.
- Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2010. Top HIV Med. 2010;18(5):156–163.
- Johnson VA, Calvez V, Gunthard HF, et al. 2011 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19(4):156–164.
