Abstract
Objectives
To evaluate serum-derived bovine immunoglobulin/protein isolate (SBI) for safety and impact on gastrointestinal (GI) symptoms in HIV patients with chronic idiopathic diarrhea.
Methods
A multi-center trial comprised of a double-blind, placebo (PBO)-controlled lead-in phase, (participants received PBO or SBI at 2.5 or 5.0 g BID for 4 weeks) followed by a 20-week, PBO-free phase (SBI at either 2.5 or 5.0 g BID). Participants included HIV-infected patients who were virologically suppressed with a history of chronic idiopathic diarrhea, defined as > 3 loose stools per day for ≥ 3 months without an identifiable cause. Safety was evaluated by monitoring adverse events (AEs) and clinical laboratory testing. Health status and changes in GI symptoms were assessed using validated questionnaires.
Results
SBI was well tolerated by the 103 participants with only 2 withdrawals due to AEs potentially associated with SBI. Mean number of daily unformed stools decreased from about 4 at baseline to less than 2 by week 4 for all study groups. Improvements in several other GI symptoms were also reported. Comparison of the PBO group to SBI groups showed no significant differences, although both SBI cohorts reported significantly improved health status scores. GI symptom improvements were maintained throughout the 20-week PBO-free phase.
Conclusions
Oral SBI is safe and well tolerated at the doses studied in HIV patients with chronic diarrhea. No conclusions could be drawn regarding impact on GI symptoms. Additional studies are ongoing to examine the biological and immunologic effects of SBI in virologically suppressed HIV-infected patients.
Introduction
Despite achieving viral suppression with ART, nearly 30% of HIV-infected adults continue to experience enteropathy, defined as chronic idiopathic diarrhea and malabsorption that preceded initiation of ART or was not impacted by a switch in ART to achieve virologic suppression.Citation1–3 HIV-associated enteropathy was a term first coined by Donald Kotler in 1984 to describe chronic HIV-associated diarrhea despite an extensive negative evaluation for enteric pathogens or related etiologies.Citation4 Indeed, HIV infection was initially called “Slim Disease” in East Africa due to the profound diarrheal and wasting illness associated with AIDS.Citation5 Extensive investigation of the impact of HIV infection on the gastrointestinal tract revealed early and profound structural and functional changes in intestinal epithelium as well as depletion of the gut-associated lymphoid tissue (GALT) immune system. Early after acquisition of HIV/SIV, nearly all CD4+ T-cells in the lamina propria are infected and depleted, and is associated with profound systemic inflammation due to destruction of gut barrier function.Citation1,6–10 While combined antiretroviral therapy has dramatically reduced opportunistic infections and increased survival, chronic idiopathic diarrhea remains a clinically relevant medical condition for people living with HIV. While the etiology for HIV-related chronic idiopathic diarrhea has not been definitively established, there are several lines of research in both the non-human primate model and clinical data that support the hypothesis that HIV dysbiosis and proinflammatory bacterial antigens underlies the pathophysiology.
Oral administration of plasma protein preparations containing high levels of immunoglobulins have been shown to reduce the severity of enteropathy in animalsCitation11 and attenuate gut inflammatory markers in animal models of colitis.Citation12,13 One mechanism put forward that might explain these benefits involves immunoglobulin binding of bacterial lipopolysaccharides (LPS, endotoxin), and other conserved microbial antigens,Citation14,15 which limits their translocation across the intestinal barrierCitation16 and ensuing inflammation and impaired gut barrier and immune function. Alternatively, the possible presence of bioactive proteins in plasma preparations may directly improve intestinal epithelial barrier function.Citation17 A more refined plasma protein isolate known as serum-derived bovine immunoglobulin/protein isolate (SBI) is the primary ingredient in a medical food that has been used successfully to manage patients with diarrhea-predominant irritable bowel syndrome (IBS-D) and inflammatory bowel disorder (IBD).Citation18–21 In a previous study involving eight patients with HIV-associated diarrhea, we found good tolerance to SBI for up to 48 weeks and improvement in stool frequency and consistency after 2–3 weeks. Improvements were also noted in CD4+ lymphocyte densities in small intestinal GALT and markers of intestinal repair following 8 weeks of SBI therapy.Citation22 The aims of the present study were to extend these findings by evaluating the safety and impact of oral SBI on GI symptoms in a larger population of virologically suppressed, HIV-infected subjects with chronic diarrhea and to assess how biomarkers of intestinal function and immune activation are altered by SBI. This manuscript will focus on the safety and efficacy of SBI in patients with chronic diarrhea. Our working hypothesis was that SBI is a safe and effective nutritional therapy for improving stool frequency and nutritional absorption in subjects with HIV-associated enteropathy. A separate communication will focus on the impact of SBI on peripheral biomarkers.
Methods
Study population
Adult HIV-infected male or non-pregnant female participants who were virologically suppressed on ART for at least 12 months prior to screening and reported a history of chronic idiopathic diarrhea were considered eligible for this study. Chronic diarrhea was defined as three or more unformed stools per day for at least 3 months with the following exclusions: (1) a positive stool test for pathogenic bacteria, ova or parasites during the 14-day screening period, (2) changes in antiretroviral therapeutic regimen during the 3-month period prior to screening, or (3) a history of a condition that required chronic therapy that might alter the gut flora or use of an antibiotic within 2 weeks prior to screening. Subjects were also excluded if lab results were significantly abnormal for alkaline phosphatase, AST, ALT, and total bilirubin. Subjects needed to be on stable cART for at least 1 year and virologically suppressed with “blips” permitted provided they remained suppressed thereafter. Additional exclusion criteria included active or a history of gastrointestinal disorders or pancreatitis, pregnancy, and history of drug or alcohol abuse that could have interfered with the subject’s ability to comply with the protocol. Institutional Review Boards at each site reviewed and approved the protocol [Clinical Trial Registry Number Identifier (Clinicaltrials.gov): NCT01828593] and all subjects provided written informed consent prior to any protocol-related procedures.
Design
This prospective, multicenter, outpatient study included a partial cross-over design comprised of two study phases: a double-blind placebo (PBO)-controlled phase and an open-label, PBO-free, dose-ranging extension phase (Figure ). Participants were randomized at study entry as follows: (1) PBO (2.5 or 5.0 g dextrose) BID for 4 weeks followed by SBI at 2.5 g BID (5 g daily dose) or SBI at 5.0 g BID (10 g daily dose) for another 20 weeks; (2) SBI 2.5 g BID for 24 weeks; or (3) SBI 5.0 g BID for 24 weeks. Those starting on PBO were divided evenly between 2.5 g (low-dose) and 5.0 g (high-dose) BID for the PBO-free phase. Participants received PBO or SBI dissolved in 120 mL of water.
Figure 1 Study design and schedule of outcome procedures
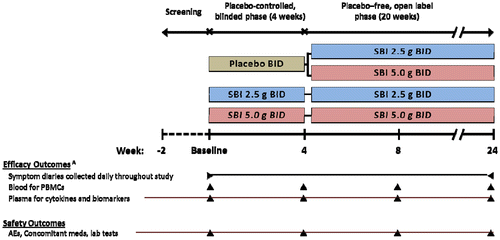
Participants reviewed instructions for study procedures including completion of daily symptom diaries regarding GI symptoms (baseline through week 24), at baseline and were encouraged to maintain their diet as per their usual routine. Dietary changes were not otherwise monitored. Symptoms relating to stool consistency were based on a validated pictorial scale ranging from 1 (normal, formed) to 6 (watery, puddle-like).Citation23 At the end of the PBO-controlled phase (week 4), study subjects submitted their responses to symptom questionnaires and were instructed on procedures for continuing to the open-label phase of the study. Health status measurements using the Medical Outcomes Study HIV Health Survey (MOS-HIV)Citation24 and Multidimensional Assessment of Fatigue (MAF) scoring questionnaireCitation25 were obtained from all study subjects at baseline and at the end of week 4, 8, and 24. All surveys and questionnaires were filled out by subject recall at the time of the next study visit .
Study outcomes
The primary efficacy endpoint was the change in the number of daily unformed stools, defined as a ranking of 4 or higher on the 6-point pictorial scale, from baseline to week 4. Secondary endpoints included change in other daily GI symptom scores, nutrient absorption (as measured by plasma concentrations of 25-hydroxyvitamin D [25(OH)D] and vitamin E), and change in health status (MOS-HIV)Citation24 and fatigue (MAF) scores.Citation26 The symptom score focused on bloating, pain, urgency, nocturnal bowel movements and incontinence. A subset of study subjects (N = 30) were evaluated for D-xylose absorption (4 h urine collection after consuming a solution containing D-xylose at a dose of 1 g/kg body weight) at baseline and weeks 4, 8, and 24 to assess duodenal absorptive function.Citation27
Safety assessment
Safety was assessed by conducting physical examinations, routine clinical laboratory testing, and through monitoring adverse events (AEs) following oral administration of SBI. Patient-reported concomitant medications taken during the study were recorded at each visit. Adverse events were recorded using the MedDRA coding dictionary and monitored for all subjects from the time of study initiation (informed consent) through the last administration of investigational product. Therapy emergent AEs (TEAEs) were defined as any event with a start date occurring on or after first dose date of investigational product and study investigators were solely responsible for determining the relationship of an AE to the investigational product.
Statistical methods
Sample size calculations were aimed at 2-group comparisons between SBI and PBO. Assuming alpha = 0.05 and a standard deviation for the frequency of abnormal bowel movements of 3/day, 26 subjects per group will provide at least 80% power to detect a difference in bowel movements on week 4 between SBI and PBO of at least 2.4/day. Type-I error was controlled using a hierarchical closed testing procedure across the 2 SBI groups compared to PBO. Subject demographics and baseline characteristics were summarized and compared across randomized treatment groups using Fisher’s-exact, Chi-squared tests and Wilcoxon, non-parametric tests as appropriate for the data type. Analysis of change from baseline results used all available double-blind data without imputation of missing data occurring during the two phases of the study. An end-of-treatment analysis combined data from participants that started on SBI at baseline (24 weeks on SBI) with data from participants that started on PBO at baseline and then converted to SBI during the open-label phase of the study (total of 20 weeks on SBI). A 2-sided significance test using an alpha of 0.05 was used to declare statistical differences between treatment groups. All statistical analyses were performed with SAS software version 9.3 (SAS Institute Inc., Cary, NC).
Results
Study population
Characteristics of the study participants at entry are summarized in Table . A total of 103 HIV-infected participants were enrolled and randomized at 10 study sites between April 2013 and March 2014. The median age of participants was 50 years; 69% were male, 61% were African-American, and the median number of years with HIV and HIV-associated diarrhea was 18.2 and 3.5, respectively. There were no statistically significant differences between groups for any of the demographic and baseline characteristics in the PBO-controlled phase. One subject who received high-dose SBI during the PBO-controlled phase of the study discontinued participation due to occurrence of an AE (Table ). A total of 12 subjects discontinued participation during the 20-week, open-label phase of the study (N = 6 for SBI 2.5 g BID group, N = 6 for SBI 5.0 g BID group). Reasons for early withdrawal included: requested by subject (N = 5), lost to follow up (N = 4), sponsor decision (N = 2), and noncompliance (N = 1). No statistically significant differences were found between study cohorts regarding reasons for premature discontinuations.
Table 1 Study population and cohort characteristics
Table 2 Disposition of participants
Safety
The safety population consisted of randomized subjects who received at least one dose of investigational product during the PBO-controlled phase of the study. All study participants tolerated SBI well with overall compliance estimated at approximately 96% (determined by monitoring unused packets at each study visit) during both the PBO-controlled and open-label phases of the study. A summary of subjects reporting TEAEs during each phase of the study is presented in Table . Twenty-one subjects reported AEs during the PBO-controlled phase of the study, which were evenly distributed across the PBO (N = 8), SBI 2.5 g BID (N = 7), and SBI 5.0 g BID (N = 6) cohorts (p > 0.05). Ten of these AEs were deemed by investigators to be potentially related to investigational product; PBO (N = 5), low-dose SBI (N = 4), and high-dose SBI (N = 1). The related AEs reported during the PBO-controlled phase of the study consisted of the following: flatulence (5), nausea (2), dyspepsia (2), headache (2), constipation (1), and rectal tenesmus (1). During the PBO-controlled phase of the study, one subject that received PBO reported two severe AEs (both diarrhea) and one subject in the high-dose SBI group withdrew from the study due to an AE (bacterial infection, night sweats), but none of these AEs were deemed by study investigators to be related to investigational product. All remaining AEs were judged as mild or moderate in intensity. No serious AEs (SAEs) were reported during the PBO-controlled phase of the study.
Table 3 Adverse events
Forty-four AEs were reported during the open-label phase of the study, but only four of these were considered by investigators to be related to SBI; two subjects each in the low-dose SBI group (abdominal pain, oral leukoplakia) and the high-dose SBI group (constipation and abnormal feces). Seven subjects reported 10 AEs that were severe in intensity during the open-label phase of the study [headache (1), influenza-like illness (1), anxiety (2), diarrhea (2), cerebral hemorrhage and subarachnoid hemorrhage (1), and toothache (1)]. None of these severe AEs were deemed related to product. Three subjects experienced a total of four SAEs (peripheral neuropathy, alcohol toxicity, cerebral hemorrhage, and subarachnoid hemorrhage due to car accident) during the open-label phase of the study, none of which were deemed related to investigational product. Only one subject withdrew from the study during the open-label phase due to an AE (high-dose SBI group due to nausea). No discernable pattern of AEs was evident with increasing dose of SBI.
Efficacy – symptom diary scores
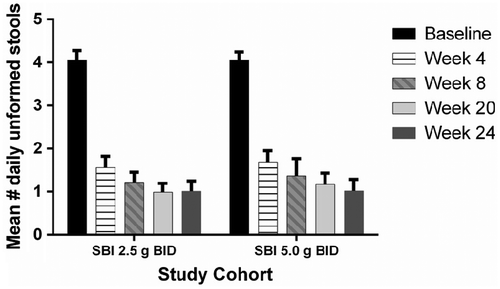
Participants in all three groups (PBO, SBI 2.5 g BID, SBI 5.0 g BID) reported similar improvements in GI symptoms from baseline to week 4. As shown in Figure , the mean number of daily unformed stools (scored as ≥4) decreased from baseline (score approximately 4.0) through week 4. The mean number of unformed stools at week 4 for each group was as follows: PBO, 1.5 ± 1.8 (p ≤ 0.0001); low-dose SBI, 1.9 ± 2.2 (p ≤ 0.0001); high-dose SBI, 1.9 ± 1.9 (p ≤ 0.0001). Mean score for daily stool consistency was about 5.0 at baseline for each of the study cohorts (Table ) and improved significantly by week 4 [PBO, 3.1 ± 1.4 (p ≤ 0.037); low-dose SBI, 3.4 ± 1.3 (p ≤ 0.016); high-dose SBI, 3.2 ± 1.5 (p ≤ 0.023)]. Comparison of the PBO group to either low-dose or high-dose SBI groups showed no significant differences in the mean number of daily unformed stools or stool consistency scores at week 4, demonstrating a strong placebo effect in this population. Study participants in each of the study cohorts also reported significant improvements in scores for other GI symptoms (Table ), including abdominal pain or discomfort (0 = none, 1 = mild, 2 = moderate, 3 = severe), urgency, fecal incontinence, and nocturnal bowel movements (each scored as 0 = no, 1 = yes). No statistically significant differences were observed between PBO and SBI groups with respect to improvements in any of these GI symptom scores through week 4. However, control of stool frequency, consistency, and symptoms was maintained throughout the PBO-free phase of the clinical trial. When compared to baseline scores, mean number of daily unformed stools continued to be significantly lower during the PBO-free open label phase of the study for both the low-dose SBI and high-dose SBI groups with no return of baseline symptoms in participants (Figure ).
Figure 2 Mean number of daily unformed stools during the PBO-controlled phase of the study
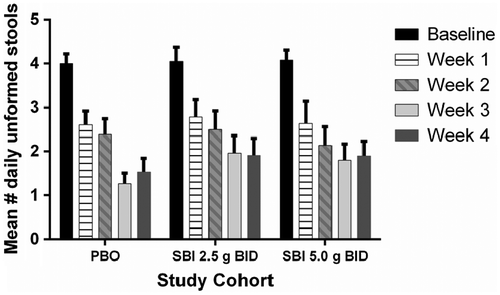
Table 4 Symptom questionnaire responses from baseline to week 4
Figure 3 Mean number of daily unformed stools from baseline through the end of study for participants that received SBI
Pairwise comparisons of PBO and SBI groups revealed no significant differences in MOS-HIV scores from baseline to week 4 during the PBO-controlled phase of the study. However, within group comparisons revealed improvements in scores for several health outcomes among patients given SBI but not subjects given PBO, as determined by various subscales of the MOS-HIV survey. Participants in the SBI 2.5 g BID group (N = 34) reported improvements from baseline in: cognitive functioning (week 4, p = 0.038; week 8, p ≤ 0.001; week 24, p ≤ 0.001); health status (week 8, p = 0.052); pain (week 8, p = 0.020); social functioning (week 4, p = 0.047). Participants in the long-term analysis, SBI 5.0 g BID group (N = 32) reported improvements in pain (week 4, p = 0.030; week 8, p = 0.011). No significant differences were found in MAF global fatigue scores following paired or within-group comparisons during the PBO-controlled or open-label phase of the study.
Nutrient absorption
Urinary D-xylose excretion (5 h) was within normal ranges for all subjects at baseline with a median level of 2.9 g (0.1 – 7.1). While within group D-xylose excretion comparing baseline to week 4 showed slight increases in excretion among participants in the PBO group (+0.19 ± 1.33; N = 8), slight decreases were observed in both the low-dose SBI group (−0.81 ± 1.24; N = 9; p = 0.039) and high-dose SBI (−0.80 ± 1.36; N = 10; p = 0.058), reflecting lower nutrient absorption. When compared to the PBO group, the decrease in D-xylose excretion by week 4 among the low-dose SBI group was significant (p = 0.039), while the decrease among participants in high-dose SBI group did not reach significance (p = 0.089]. No discernable changes in urinary D-xylose excretion were observed from baseline to week 8 (low-dose SBI: −0.03 ± 0.56; high-dose SBI:+0.08 ± 2.34) or week 24 (low-dose SBI: −0.07 ± 1.13; high-dose SBI + 0.35 ± 3.09) during the open-label phase of the study. No changes were observed in plasma concentrations of 25-hydroxyvitamin D [25(OH)D] and vitamin E during either the PBO-controlled or open-label phases of the study.
Discussion
Because there are limited treatment options available for HIV-infected patients with chronic diarrhea, it remains an important unmet medical need despite the effectiveness of cART on HIV-associated morbidity and mortality.Citation3 In fact, many patients will deny having diarrhea but will admit to multiple loose stools daily with significant urgency that disrupts their activities of daily living. Up to 30% of patients on successful cART will admit to disruptive GI symptoms according to recent publications.Citation2 Only one agent, a botanical known as crofelemer, has been approved by the FDA for symptomatic relief of non-infectious diarrhea in adult patients with HIV. Crofelemer acts locally within the gastrointestinal (GI) tract and has been shown to have limited efficacy in a phase III clinical trial.Citation28 Other options include various nutritional products and probiotics, all of which have shown equivocal results in clinical studies.
In this prospective and randomized clinical study, 103 virologically suppressed HIV patients with chronic diarrhea were evaluated for the safety and effectiveness of serum-derived bovine immunoglobulin/protein isolate (SBI) for decreasing the severity of diarrhea and improving related GI symptoms. Although both the placebo and SBI cohorts experienced a significant decline in bowel movement frequency and consistency, the improvements persisted for the duration of the treatment period of 24 weeks. Consistent with results from our previous study,Citation22 SBI was well tolerated by all study participants with excellent compliance during both phases of the 24-week study. More importantly, there were no differences in the incidence of AEs across study groups during the PBO-controlled phase of the study and only 4 related AEs were reported after consuming SBI for 20 weeks during the open label phase of the study. All related AEs were deemed mild or moderate in intensity and the most common were flatulence, dyspepsia, nausea, constipation, and headache. In addition, there were no unusual changes in blood analytes (e.g. sodium, chloride, BUN, hemoglobin, glucose) or nutritional markers (e.g. prealbumin, potassium, vitamin E, 25-hydroxyvitamin D) that could be reasonably attributed to investigational product during either the PBO-controlled or open-label phases of the study. Collectively, these results confirm and extend the findings from our previous pilot study by demonstrating the safety of oral SBI in HIV-infected patients on long-term suppressive ART.
Recent studies involving oral administration of SBI in humans have provided evidence of a benefit for GI symptoms. SBI is currently available as a medical foodCitation29 and intended for chronic diarrhea associated with conditions such as IBS-D, IBD, and HIV-enteropathy. Indeed, a number of clinical reports have described the effectiveness of SBI for managing the chronic loose and frequent stools found in these conditions.Citation18,19,21,30 Our results also showed an improvement in stool consistency leading to a reduction in the number of daily unformed stools in HIV-infected participants after 1 week of SBI treatment. Study participants also reported significant improvements in several other GI symptoms. These results are consistent with the reduction in daily bowel movements and improvements in stool consistency in our previous open-label study in patients with HIV-associated enteropathy.Citation22 Unfortunately, we were unable to show a difference in the change in GI symptoms before and after SBI therapy when comparing the SBI groups to the cohort that received PBO. Initial symptom reports were based on recall, which is inherently inaccurate, whereas symptom reports during the study were based on daily diary recordings. Participants may have over-reported stool frequency at enrollment to ensure eligibility for the study. In addition, despite being instructed to maintain their usual diets, participants may have begun to focus more on food selection and avoiding those foods, especially with a high fat content, that were known historically to exacerbate their symptoms . The strong PBO effect observed in our study may also suggest a neurological/psychological mechanism based on the well-recognized influence that the emotional and cognitive centers of the brain have on intestinal homeostasis via the gut-brain axis.Citation31 The act of taking a medication, hope that the medication was working, or more frequent clinical interactions may have contributed to this PBO effect. If one were to assume that it is unlikely that a PBO effect would last as long as 24 weeks, it is interesting to speculate that SBI was responsible for maintaining the improvement in GI symptoms through the end of treatment/study. However, future studies with longer PBO-controlled periods or a lead-in observation period as employed for clinical trials studying depression will be needed to confirm this finding. Nonetheless, only participants in the SBI groups, but not the PBO group, reported improvements in several outcomes associated with the Medical Outcomes Health Survey (MOS-HIV). Improvements were observed in cognitive functioning, overall health status, and pain during the PBO-free phase.
Our study had several important limitations that may explain the observed placebo effect. First, there was no lead-in double-blind phase that would exclude placebo responders. Despite being instructed to maintain their usual diets, it is possible that participants began to focus more on food selection and avoiding those foods, especially with a high fat content, that are known historically to exacerbate their symptoms. A longer placebo phase may have buffered that effect although there did appear to be attenuation of reported symptoms. Recall bias may have impacted descriptions of the initial duration of illness. Finally, we included subjects that spanned a wide range in both peripheral CD4+ T-cell count (189–1754 cells/μL) and time on ART (1.0–23.73 years). Intestinal homeostasis can be impacted by either of these variables, which could in turn influence the response to SBI by individual participants. Future studies will need to address these pitfalls of clinical investigations directed at symptomatic endpoints.
In conclusion, our results demonstrate that oral administration of SBI is safe at levels as high as 10 g per day in virologically suppressed, HIV-infected patients. The safety of SBI in otherwise healthy humans has already been documented in both clinical studies and the AE profile of SBI based upon hundreds of thousands of ingested doses of the commercial medical food product.Citation29 Our results also demonstrated a reduction in the severity of diarrhea and other GI symptoms following oral administration of SBI in HIV-infected participants. In addition, our earlier pilot clinical trial revealed several important insights into how SBI impacts stool microbiota, intestinal mucosal immunity, and systemic inflammation that are currently under intense investigation for the current clinical trial.Citation32 Additional analyses are underway to evaluate the impact of SBI on gut microbiota and plasma biomarkers of gastrointestinal and immunologic function.
Declaration of interest
Audrey Shaw, Bryon Petschow, Christopher Detzel, and Eric Weaver were all employees of Entera Health, Inc at the time the study was conducted. Authors declare no other conflicts of interest.
Contributors
DMA, EMW, and ALS conceived and designed the study; DMA, CJF, MS and AL directed the recruitment and management of study participants; ALS and EMW supervised and managed the collection and analysis of data; JEH conducted statistical analysis of the data; DMA, ALS, EMW, BWP, CJD and NSU analyzed and interpreted the data; BWP wrote the manuscript for publication; ALS, DMA, NSU, and CJD provided critical review of the manuscript.
Acknowledgments
This clinical trial was supported by Entera Health, Inc. (Ankeny, IA). We would like to thank Dr. Rodger MacArthur for his role in protocol development and Dr. Stephen Brown, Dr. Antonio Terrelonge, Dr. Patrick Clay, Dr. Mkaya Mwamburi, Dr. Kent Kamradt and Dr. Vandana Jain for their participation in enrolling subjects in this study.
We are deeply indebted to the generosity and willingness of the volunteers without whom this research would be impossible. We would like to thank the nurses and support personnel at the University of California CTSC Clinical Research Center, the Veterans Administration Hospital and University of California Gastroenterology Unit for their dedication and assistance in this research effort. We thank faculty and nursing staff of the Center for AIDS Research, Education and Services clinic for help in study subject recruitment.
Study concept and design: DMA, ALS, EMW, JEH.
Study recruitment and supervision: DMA, MS, AET, SB, AL, CJF.
Acquisition of data: DMA, MS, ALS.
Analysis and interpretation of data: DMA, BWP, EMW, MS, CJD, JEH, ALS.
Drafting and Critical revision of manuscript: BWP, DMA, CJD, MS, EMW, ALS, CJF.
Notes
Data from this study was presented in part at: the AAI Annual Meeting, New Orleans, LA 8–12 May 2015; the 8th IAS Conference on HIV Pathogenesis, Vancouver, BC 19–22 July 2015; and CROI 2016, Boston, MA 22–25 February.
References
- Brenchley JM. Mucosal immunity in human and simian immunodeficiency lentivirus infections. Mucosal Immunol. 2013;6(4):657–665.10.1038/mi.2013.15
- Siddiqui U, Bini EJ, Chandarana K, et al. Prevalence and impact of diarrhea on health-related quality of life in HIV-infected patients in the era of highly active antiretroviral therapy. J Clin Gastroenterol. 2007;41(5):484–490.10.1097/01.mcg.0000225694.46874.fc
- MacArthur RD, DuPont HL. Etiology and pharmacologic management of noninfectious diarrhea in HIV-infected individuals in the highly active antiretroviral therapy era. Clin Infect Dis. 2012;55(6):860–867.10.1093/cid/cis544
- Kotler DP, Gaetz HP, Lange M, et al. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101(4):421–428.10.7326/0003-4819-101-4-421
- Serwadda D, Mugerwa RD, Sewankambo NK, et al. Slim disease: a new disease in uganda and its association with HTLV-III infection. Lancet. 1985;2(8460):849–852.10.1016/S0140-6736(85)90122-9
- Griffin GE. Malabsorption, malnutrition and HIV disease. Baillieres Clin Gastroenterol. 1990;4(2):361–373.10.1016/0950-3528(90)90006-3
- Jarry A, Cortez A, Rene E, , et al. Infected cells and immune cells in the gastrointestinal tract of AIDS patients. An immunohistochemical study of 127 cases. Histopathology. 1990;16(2):133–140.
- Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–666.10.1038/nrmicro2848
- Ullrich R, Zeitz M, Heise W, et al. Small Intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med. 1989;111(1):15–21.10.7326/0003-4819-111-1-15
- Smith PD. Role of cytokines in infectious and noninfectious enteropathy in patients with AIDS. Immunol Res. 1991;10(3–4):447–451.10.1007/BF02919740
- Kuchibhatla R, Petschow BW, Odle J, et al. Nutritional impact of dietary plasma proteins in animals undergoing experimental challenge and implications for patients with inflammatory bowel disorders: a meta-analysis. Adv Nutr. 2015;6(5):541–551.10.3945/an.114.007930
- Perez-Bosque A, Miro L, Maijo M, et al. Dietary intervention with serum-derived bovine immunoglobulins protects barrier function in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2015;308(12):G1012–G1018.10.1152/ajpgi.00378.2014
- Henderson AL, Brand MW, Darling RJ, et al. Attenuation of colitis by serum-derived bovine immunoglobulin/protein isolate in a defined microbiota mouse model. Dig Dis Sci. 2015;60(11):3293–3303.10.1007/s10620-015-3726-5
- Horgan A, Maas KJ, Henderson A, et al. Serum-derived bovine immunoglobulin/protein isolate binds to pathogen-associated molecular patterns. FASEB J. 2014;128(Suppl 1):836.6.
- Weaver EM, Klein GL, DeVries BK, et al. Endotoxin neutralization activity (ENA) of bovine plasma and bovine Immunoglobulin (IgG)-rich fractions as compared to human plasma. FASEB J. 1079;2013(27):1058.
- Detzel CJ, Horgan A, Henderson AL, et al. Bovine immunoglobulin/protein isolate binds pro-inflammatory bacterial compounds and prevents immune activation in an intestinal co-culture model. PLoS One. 2015;10(4):e0120278.10.1371/journal.pone.0120278
- Li Y, Ostergaard MV, Jiang P, et al. Whey protein processing influences formula-induced gut maturation in preterm pigs. J Nutr. 2013;143(12):1934–1942.10.3945/jn.113.182931
- Wilson D, Evans MD, Weaver E, et al. Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome. Clin Med Insights: Gastroenterol. 2013;6:49–60.
- Good L, Rosario R, Panas R. New therapeutic option for irritable bowel syndrome: serum-derived bovine immunoglobulin. World J Gastroenterol. 2015;21(11):3361–3366.10.3748/wjg.v21.i11.3361
- Weinstock LB, Jasion VS. Serum-derived bovine immunoglobulin/protein isolate therapy for patients with refractory irritable bowel syndrome. Open J Gastroenterol. 2014;4:329–334.10.4236/ojgas.2014.410047
- Shafran I, Burgunder P, Wei D, et al. Management of inflammatory bowel disease with oral serum-derived bovine immunoglobulin. Therap Adv Gastroenterol. 2015;8(6):331–339.10.1177/1756283X15593693
- Asmuth DM, Ma ZM, Albanese A, et al. Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS. 2013;27:2207–2217.10.1097/QAD.0b013e328362e54c
- Mertz HR, Beck CK, Dixon W, et al. Validation of a new measure of diarrhea. Dig Dis Sci. 1995;40(9):1873–1882.10.1007/BF02208649
- Wu AW, Rubin HR, Mathews WC, et al. A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care. 1991;29(8):786–798.10.1097/00005650-199108000-00011
- Belza BL, Henke CJ, Yelin EH, et al. Correlates of fatigue in older adults with rheumatoid arthritis. Nurs Res. 1993;42(2):93–99.
- Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res. 2004;56(2):157–170.10.1016/S0022-3999(03)00371-4
- Sharpstone D, Neild P, Crane R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45(1):70–76.10.1136/gut.45.1.70
- Cottreau J, Tucker A, Crutchley R, et al. Crofelemer for the treatment of secretory diarrhea. Expert Rev Gastroenterol Hepatol. 2012;6(1):17–23.10.1586/egh.11.87
- EnteraGam [Product Information Sheet]. Cary, NC: Entera Health,; 2015. http://enteragam.com/assets/lib/EnteraGam_Product_Information.pdf. Accessed December 19, 2016.
- Awad A, Jasion VS. Use of a nutritional therapy, serum-derived bovine immunoglobulin/protein isolate (SBI), to achieve improvement in two different cases of colitis. Gastrointest Dig Syst. 2015;5:274.
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314.10.1038/nrgastro.2009.35
- Asmuth DM, Somsouk M, Hunt P, et al. Serum-derived bovine immunoglobulin protein isolate increases peripheral and mucosal CD4+ T-cell counts in patients with HIV enteropathy. 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention (AIDS 2015); MOAA02; 2015; July 19–22; Vancouver, Canada.
