Abstract
Introduction: The AIDS Clinical Trial Group (ACTG) 5257 clinical trial showed that raltegravir (RAL) was superior to atazanavir/ritonavir (ATV/r) and darunavir/ritonavir (DRV/r), when used in combination with emtricitabine/tenofovir DF (FTC/TDF), in a 96-week composite endpoint combining virologic efficacy and tolerability for treatment-naive adults with HIV-1 infection. This study aimed to estimate the efficiency associated with these three regimens in Spain.
Methods: An economic model was developed to estimate costs for antiretroviral drugs, adverse event management, and HIV care for individuals initiating first-line therapy. Antiretroviral drug costs were based on hospital costs with mandatory discounts applied. Adverse event management costs and HIV care costs were obtained from published sources and inflated to 2015 euros. Head-to-head efficacy and safety data (discontinuation rates, mean CD4 cell-count changes, adverse event incidence) up to 96 weeks for each regimen were obtained from the clinical trial. The efficiency of each regimen, as measured by the cost per successfully treated patient (i.e. on first-line therapy for 96 weeks), was estimated and examined in sensitivity analyses. All cost outcomes were discounted at 3.0% annually.
Results: Total costs per successfully treated patient were €22,377 for RAL, €26,629 for ATV/r, and €23,928 for DRV/r. These results were found to be robust in sensitivity analyses.
Discussion: RAL has the lowest cost per successfully treated patient when compared with DRV/r and ATV/r, each used in combination with FTC/TDF, for treatment-naive adults with HIV-1 infection in Spain. This economic evidence complements the clinical benefits of RAL reported in the ACTG 5257 clinical trial.
Introduction
As of 2013, an estimated 130,000–160,000 people were living with HIV-1 infection in Spain.Citation1 Vast improvements in the treatment of HIV-1 infection over past decades have allowed individuals to live longer, but concurrently, the cost of HIV care has risen. Because the decision about first-line therapy can have such a significant impact on an individual’s long-term treatment and care, it is of utmost importance to carefully evaluate the efficacy, safety, and economic implications of the various regimens available for individuals starting HIV treatment.
The AIDS Clinical Trial Group (ACTG) 5257 clinical trial is a large head-to-head study (1809 participants) that investigated the use of common integrase and protease inhibitors for treatment-naive individuals who do not receive efavirenz or other non-nucleoside reverse transcriptase inhibitors. Specifically, the aim of the study was to compare the efficacy and tolerability of raltegravir (RAL) 400 mg twice daily, atazanavir/ritonavir (ATV/r) 300 mg/100 mg once daily, and darunavir/ritonavir (DRV/r) 800 mg/100 mg once daily, all used in combination with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) 200 mg/300 mg once daily, among treatment-naive adults in the United States (US).Citation2,3 The ACTG 5257 clinical trial found that at 96 weeks, the RAL regimen exhibited favorable efficacy and safety results compared with the ATV/r and DRV/r regimens.Citation2,3
The ACTG 5257 clinical trial did not analyze the costs associated with these first-line HIV treatment regimens. However, cost information can be a critical additional component, when coupled with efficacy and safety information, in helping hospitals efficiently allocate their budgets. The aim of this study was to estimate the efficiency, as measured by the cost per successfully treated patient, associated with the three antiretroviral regimens examined in the ACTG 5257 clinical trial – RAL, ATV/r, or DRV/r – when used in combination with FTC/TDF for treatment-naive adults with HIV-1 infection from the Spanish health system perspective.
Materials and methods
Model overview
An economic model was developed to assess 96-week cost and health outcomes in Spain for the three first-line regimens examined in the ACTG 5257 clinical trial, utilizing some components of an earlier US cost analysis.Citation4 The model followed a cohort of treatment-naive individuals with HIV-1 infection from baseline through 96 weeks of treatment in the trial. Individuals entering the model were assigned to one of three first-line treatment arms. After treatment initiation, the model tracked changes in health status (via CD4 cell count) and incidence of adverse events (Tables and , respectively). Individuals were reassigned to alternative treatment if they discontinued their first-line regimen.
Table 1 ACTG 5257 Antiretroviral therapy regimens: efficacy, tolerability, and costs
Table 2 ACTG 5257 Adverse event incidence and costs per episode
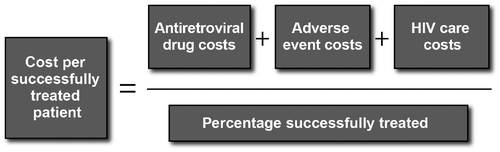
Over time, individuals progressed through the model and incurred costs for antiretroviral drug treatment, management of adverse events, and disease monitoring. HIV care costs for disease monitoring varied by health status, which was represented by seven health states based on CD4 cell count (<50, 50–199, 200–349, 350–499, 500–649, 650–799, ≥800 cells/μL). The model estimated per-person costs by category and in aggregate for each treatment arm. The cost categories are discussed in further detail in the sections to follow. The model also estimated the total cost per successfully treated patient for each treatment arm, where individuals remaining on their first-line regimen for 96 weeks were considered successfully treated (Figure ).
Figure 1 Economic analysis model overview
Input parameter values
The input parameters included in the economic analysis are summarized in the following sections.
Modeled population
Baseline characteristics of the modeled cohort were based on the pooled intention-to-treat population enrolled in the ACTG 5257 clinical trial.Citation2,3 The population was 76% male, and the median age was 37 years. The pooled baseline characteristics were also used to establish the distribution of the modeled cohort across the seven health states (<50: 11.9%; 50–199: 17.7%; 200–349: 29.2%; 350–499: 27.7%; 500–649: 8.5%; 650–799: 3.6%; ≥ 800 cells/μL: 1.4%) at treatment initiation.
Efficacy and tolerability data
Efficacy and tolerability data for the modeled cohort were also taken directly from the ACTG 5257 clinical trial.Citation2,3 These data were used to update the health and treatment status of the modeled cohort over the course of the trial period.
The probabilities of transitioning between CD4 cell-count health states were estimated using the CD4 cell-count increases from baseline at 24, 48, and 96 weeks (Table ). To estimate these transition probabilities, individuals were assumed to start at the midpoint of their initial CD4 cell-count range and were then shifted according to the mean CD4 cell-count change from baseline and further distributed according to the standard deviation of the CD4 cell-count change from baseline. In the model, these transition probabilities were used to update the distribution of individuals across the health states at 24, 48, and 96 weeks.
The model also allowed individuals to discontinue their ACTG 5257 clinical trial study drug after 24, 48, or 96 weeks of treatment. In the base-case analysis, treatment discontinuation was based on the primary, composite trial endpoint (Table ).Citation2,3 The composite endpoint captured discontinuation for virologic failure (confirmed HIV-1 ribonucleic acid level greater than 1000 copies/mL at or after 16 weeks and before 24 weeks from randomization or greater than 200 copies/mL at or after 24 weeks) or tolerability failure (time from randomization to discontinuation of the randomized regimen component for toxicity). At 96 weeks of follow-up, the ACTG 5257 clinical trial results showed that discontinuation due to virologic or tolerability failure was significantly lower for the RAL regimen (8.6%) than for the ATV/r (24.1%) and DRV/r (16.6%) regimens.Citation2,3
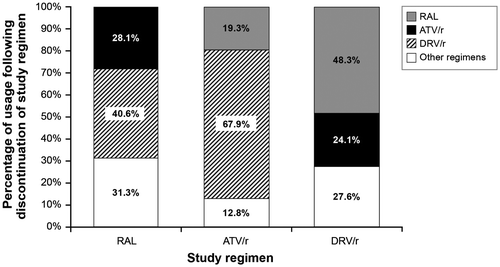
Individuals who remained on first-line treatment for all 96 weeks were considered successfully treated. Individuals who discontinued their first-line treatment transitioned in the model to various substitution regimens based on the percentage of participants who switched to each regimen in the ACTG 5257 clinical trial.Citation2,3 These regimens are summarized in Figure . The model assumed that individuals who switched to a substitution regimen received that regimen for the remainder of the economic analysis. An alternative treatment discontinuation endpoint based on discontinuation for any reasonCitation3 was tested in scenario analysis.
Figure 2 Substitution regimens following discontinuation of study Regimen
Adverse event incidence
Grade 2, 3, and 4 adverse events with an incidence of at least 5% in any arm of the ACTG 5257 clinical trial were included in the model.Citation2 Adverse event incidence is summarized in Table .
Costs
The model accounted for costs incurred for antiretroviral drug treatment, management of adverse events, and disease monitoring. The sections to follow discuss these cost categories in further detail. When necessary, cost estimates were inflated to 2015 euros.Citation5
Antiretroviral drug costs
The economic analysis assumed that antiretroviral drug costs were based on hospital costs (i.e. wholesaler selling price [WSP], which accounts for mandatory and commercial discounts) with additional RD-Law 8/2010 mandatory deductions applied.Citation6 Total daily regimen costs for each comparator first-line regimen were calculated by summing the cost of each individual component of the regimen. These total daily regimen costs are summarized in Table .
The total daily regimen cost for each substitution regimen was also calculated by summing the cost of each individual component of the regimen. A weighted average substitution regimen cost per day was then calculated using the percentage of participants who switched to each regimen after discontinuing their first-line regimen (Table , Figure ).
Total antiretroviral drug costs were then calculated using the daily regimen costs and the percentages of individuals on each first-line and substitution regimen at baseline and at weeks 24, 48, and 96. To account for first-line discontinuations occurring between these time points, average values were calculated for each time interval. Thus, for example, the total antiretroviral drug cost for the time interval between weeks 24 and 48 was estimated as the average of the daily cost at week 24 and at week 48, multiplied by the number of days in the 24-week time interval. This process was repeated for each of the time intervals (e.g. baseline to 24 weeks, 24–48 weeks, and 48–96 weeks) to estimate the 96-week total antiretroviral drug cost for each arm of the model.
Adverse event costs
The model applied a per-episode management cost (based on adverse event severity) to the percentage of individuals who experienced each adverse event. The model assumed that these costs were incurred within the first 48 weeks of treatment because adverse events that do occur generally do so soon after treatment initiation. Adverse event management costs were primarily taken from a prospective, multicenter study conducted in Spain that assessed the costs incurred by patients who developed drug-related adverse events that justified discontinuation.Citation7 Adverse event costs were also taken from a secondary Spanish cost database (e-Salud).Citation8 All adverse event costs were applied to the corresponding adverse event categories observed in the trial. Adverse events without a cost estimate in Llibre-Codina et al.Citation7 or e-SaludCitation8 had similar incidence between trial arms and therefore would have limited impact on incremental model results. Correspondingly, these adverse events were assumed to impose a €0 cost per episode. Costs per adverse event episode are summarized in Table .
HIV care costs
HIV care costs in the model included costs for disease monitoring and the treatment and prevention of opportunistic and other infections. Annual costs in Spain were estimated from results of a retrospective resource use study among individuals who received HIV care at one of the four outpatient clinics in the Canary Islands in 2003.Citation9 This study reported annual direct costs for inpatient care, outpatient care, and medications (including antiretroviral drug costs) by CD4 cell count and HIV/AIDS status. As antiretroviral drug costs were already captured by the model, appropriate steps were taken to exclude these costs from the total medication costs reported in the study (see Appendix 1). The HIV care costs used in the model, by cost category, are summarized in Table .
Table 3 Three-month HIV care costsTable Footnotea by CD4 cell-count range
Total HIV care costs were then calculated using the HIV care costs by CD4 cell-count range and the distribution of individuals across the CD4 cell-count ranges in the model at baseline and at weeks 24, 48, and 96. To account for CD4 cell-count changes occurring between these time points, average values were calculated for each time interval (e.g. baseline to 24 weeks, 24–48 weeks, and 48–96 weeks) using the same methodology employed to estimate antiretroviral drug costs. The total 96-week HIV care cost for each arm of the model was then estimated by appropriately summing the costs across all time intervals.
Analyses
Base-case analysis
The base-case analysis estimated the per-person 96-week cost associated with the three first-line regimens examined in the ACTG 5257 clinical trial, in total and by cost category. The total cost per successfully treated patient was then estimated for each treatment arm, where individuals were considered successfully treated if they did not discontinue their study regimen due to virologic or tolerability failure for at least 96 weeks. All cost outcomes were discounted using an annual discount rate of 3.0%.Citation10
Scenario and sensitivity analysis
To assess the robustness of the model results, scenario and sensitivity analyses were performed. In scenario analysis, the total cost per successfully treated patient was estimated for scenarios without discounting, after 48 weeks of treatment, with first-line treatment discontinuation based on the ACTG 5257 endpoint for discontinuation for any reason, with no HIV care costs, and with antiretroviral drug costs only.
A probabilistic sensitivity analysis (PSA) was performed to assess the joint impact of input parameter uncertainty on the model results by simultaneously varying all model input parameters for which there was sampling uncertainty. In each of the 10,000 Monte Carlo simulation runs, values for these model input parameters were sampled from appropriate probability distributions (see Appendix 1).
Results
Base-case results
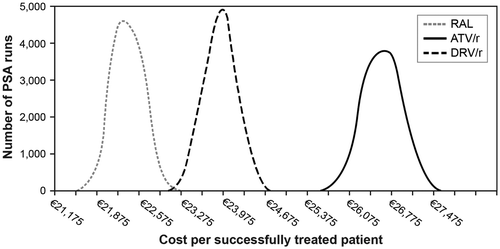
At 96 weeks, RAL was associated with higher antiretroviral drug costs, lower adverse event costs, and similar HIV care costs when compared with either ATV/r or DRV/r (Table ). Overall, the three first-line regimens exhibited similar total per-person costs over 96 weeks. RAL yielded the highest percentage of patients successfully treated (91.4%) compared with ATV/r (75.9%) or DRV/r (83.4%). Thus, RAL was associated with the lowest total cost per successfully treated patient (€22,377) compared with ATV/r (€26,629) or DRV/r (€23,928) (Table ).
Table 4 Base-case per-person results of 96-week economic analysis
Scenario analysis results
In all scenarios tested, the RAL regimen was found to have the lowest cost per successfully treated patient when compared with the ATV/r and DRV/r regimens (Table ). These scenarios also yielded relative differences between the RAL regimen and the ATV/r and DRV/r regimens that were similar to the relative differences observed in the base-case results.
Table 5 Results of additional scenario analysis
Probabilistic sensitivity analysis results
The PSA found RAL to have the lowest total cost per successfully treated patient at 96 weeks in all 10,000 simulations. The mean costs per successfully treated patient estimated by the PSA for each treatment arm were similar to the costs estimated in the base-case analysis. Additionally, the 95% confidence interval of the mean cost for RAL did not overlap with the confidence interval estimated for ATV/r and only slightly overlapped (€584) with the confidence interval estimated for DRV/r. ATV/r had the most variability in PSA results (i.e. the widest distribution) due to higher incidence of adverse events in this trial arm and the uncertainty around the associated costs per episode. These results are summarized in Figure , and Table further displays the distribution of PSA results for each treatment arm.
Table 6 Results of probabilistic sensitivity analysis
Discussion
RAL yielded the highest percentage of successfully treated patients and had a similar per-person total cost after 96 weeks of treatment compared with ATV/r and DRV/r when each was used in combination with FTC/TDF. Thus, RAL was associated with the lowest total cost per successfully treated patient when compared with ATV/r and DRV/r. These base-case results were found to be robust in scenario and sensitivity analyses. Specifically, RAL was found to have the lowest total cost per successfully treated patient in all scenarios tested. Additionally, the 95% confidence interval of total cost per successfully treated patient associated with RAL did not overlap with the confidence interval for ATV/r and only slightly overlapped with the confidence interval for DRV/r.
Most other published studies differed substantially from our study in comparators considered, time horizon, national setting, or patient population (e.g. treatment-experienced rather than treatment-naive). However, one study by Berenguer et al.Citation11 examined the use of RAL, ATV/r, DRV/r, and other comparators for treatment-naive individuals over a short-term time horizon in Spain. This study examined 48-week costs and cost-efficiency of all regimens recommended for first-line treatment in the 2015 GESIDA/Spanish AIDS National Plan guidelines, which included RAL, ATV/r, and DRV/r. The study used efficacy and safety data from a variety of published clinical trials, including ACTG 5257, where trials were selected if they included at least one of the regimens under evaluation and reported efficacy and safety data for at least 48 weeks. For each regimen, efficacy was calculated as the quotient of the total number of patients with undetectable viral load at week 48 (numerator) and the total number of patients who initiated antiretroviral therapy (denominator) pooled across all clinical trials that included each specific regimen. No formal meta-analyses were conducted to ensure robust indirect comparisons. Regimen costs at 48 weeks were based on WSP with mandatory discounts applied, but did not account for commercial discounts. RAL was found to have more favorable efficacy results than ATV/r and DRV/r, but ATV/r and DRV/r had slightly more favorable cost-efficiency results. Our efficacy results align with Berenguer et al.,Citation11 but our cost-efficiency results differ due to our application of updated WSPs and all appropriate mandatory and commercial discounts in our antiretroviral drug cost estimates.
This study has several key strengths. First, the economic model directly utilized the efficacy, tolerability, and safety data from the large, head-to-head ACTG 5257 clinical trial. This trial studied three relevant first-line regimens for individuals who do not receive efavirenz. Second, in focusing specifically on the 96-week time horizon, all clinical data for the model were obtained directly from the ACTG 5257 clinical trial, and the model therefore avoided the need for assumptions about lifetime treatment pathways and long-term efficacy. Finally, scenario and sensitivity analyses were conducted to ensure the robustness of the model’s results.
This analysis had the typical limitations of pharmacoeconomic analyses. The scope of this study was to estimate the cost and health outcomes for the three first-line regimens studied in the ACTG 5257 clinical trial. Therefore, this analysis did not account for other commonly used first-line therapies for HIV treatment. The ACTG 5257 clinical trial included US participants only, which may not be fully representative of a Spanish patient population. Clinical trial participants typically achieve very high levels of adherence, which may not be attainable in real-world clinical practice. Therefore, for all regimens, lower antiretroviral drug costs (due to lower adherence) and higher HIV care costs (due to resulting poorer health outcomes) may be observed in clinical practice than in our economic analysis. In estimating costs only through 96 weeks of treatment, the economic analysis did not capture the potential long-term benefits of RAL (e.g. potential long-term benefits of RAL’s favorable lipid profile, as reported in the ACTG 5257 clinical trialCitation2). Antiretroviral drug costs were estimated using approximate average commercial discounts across hospitals in Spain, but these discounts may vary by hospital and over time. Adverse event cost data and HIV care cost data were somewhat dated, but were the most recently available for this analysis. The HIV care costs obtained from Lopez-Bastida et al.Citation9 also were specific to the Canary Islands and may not be fully representative of costs in Spain.
In summary, RAL + FTC/TDF had a similar per-person total cost after 96 weeks of treatment and the lowest cost per successfully treated patient when compared with DRV/r + FTC/TDF and ATV/r + FTC/TDF, two other common first-line regimens for treatment-naive adults with HIV-1 infection in Spain. These results were found to be robust in scenario and sensitivity analyses. This economic evidence further complements the known clinical benefits of RAL as reported in the ACTG 5257 clinical trial. Further analysis should compare RAL treatment with other newer comparator treatments and study the long-term health and cost benefits of first-line RAL treatment.
Contributors
AD and AB contributed to the study design, data collection, analysis, and interpretation of results. BG contributed to the study design and interpretation of results. GN and VL contributed to the study design, data collection, and interpretation of results. All authors had full access to the data, made substantial contribution to the development of the manuscript, reviewed and commented upon drafts of the manuscript, had final responsibility for the decision to submit for publication, and approved the final article.
Conflict of interest
Funding for this study was provided to RTI Health Solutions by Merck & Co. Goodwin was an employee of Merck & Co. during the conduct of this study. Nocea and Lozano are employees of Merck Sharp & Dohme. Davis and Brogan are employees of RTI Health Solutions, an independent research organization, and maintained independent scientific control over the study design and analyses.
Funding
This work was supported by Merck & Co.
Contributors
AD and AB contributed to the study design, data collection, analysis, and interpretation of results. BG contributed to the study design and interpretation of results. GN and VL contributed to the study design, data collection, and interpretation of results. All authors had full access to the data, made substantial contribution to the development of the manuscript, reviewed and commented upon drafts of the manuscript, had final responsibility for the decision to submit for publication, and approved the final article.
Acknowledgments
The authors would like to thank the ACTG 5257 study team for providing unpublished data from their clinical trial study report for inclusion in our analysis.
Notes
* The listed dosing of TDF is different in Spain (245 mg) than in the US (300 mg) due to the fact that Spain does not include the weight of fumarate in the TDF molecular weight calculation. Therefore, the actual dosing is the same in both countries. The dosing of TDF will be referred to as 245 mg for the remainder of this analysis.
References
- Ministerio de Sanidad, Servicos Sociales, e Igualdad. Informe Nacional Sobre Los Progresos Realizados En La Aplicación Del Ungass España: Enero de 2013 – Diciembre de 2013. http://www.unaids.org/sites/default/files/country/documents//ESP_narrative_report_2014.pdf. Last updated March 31, 2014. Accessed October 30, 2015.
- Lennox JL, Landovitz RJ, Ribaudo HJ, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: A randomized, controlled equivalence trial. Ann Intern Med. 2014;161(7):461–471.10.7326/M14-1084
- Ribaudo H, Wang H, Na L. A phase III comparative study of three non-nucleoside reverse transcriptase inhibitor (NNRTI)-sparing antiretroviral regimens for treatment-naïve HIV-1 infected volunteers (The ARDENT Study). A5257 Core Primary Analysis Report. Data on file. September 24, 2013.
- Davis AE, Brogan AJ, Goodwin BB. Cost analysis of raltegravir versus atazanavir/r or darunavir/r for treatment-naive adults with HIV-1 infection in the United States. Presented at: ISPOR 21st Annual International Meeting; May 2016; Washington DC.
- INEbase. Indice de Precios de Consumo, Base 2011. http://www.ine.es/dynt3/inebase/en/index.htm?padre=1936&capsel=1940. Accessed September 21, 2015.
- Consejo General de Colegios Oficiales de Farmacéuticos. BotPlus Web. https://botplusweb.portalfarma.com/. Accessed June 15, 2015.
- Llibre-Codina JM, Casado-Gomez MA, Sanchez-de la Rosa R, et al. Costes de la toxicidad asociada a los análogos de nucleósidos inhibidores de la transcriptasa inversa en pacientes con infección por el VIH-1. Enferm Infecc Microbiol Clin. 2007;25(2):98–107.10.1157/13098570
- Spanish Healthcare Costs Database e-Salud. Oblikue Consulting. http://www.oblikue.com/bddcostes/. Accessed May 2015.
- Lopez-Bastida J, Oliva-Moreno J, Perestelo-Perez L, Serrano-Aguilar P. The economic costs and health-related quality of life of people with HIV/AIDS in the Canary Islands, Spain. BMC Health Serv Res. 2009;9(55).
- Lopez-Bastida J, Oliva J, Antonanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–520.10.1007/s10198-010-0244-4
- Berenguer J, Rivero A, Blasco AJ, et al. Costs and cost-effectiveness analysis of 2015 GESIDA/Spanish AIDS national plan recommended guidelines for initial antiretroviral therapy in HIV-infected adults. Enferm Infecc Microbiol Clin. 2015;34(6):361–371.
Appendix 1. Short-term cost and efficiency analysis of raltegravir versus atazanavir/ritonavir or darunavir/ritonavir for treatment-naive adults with HIV-1 infection in Spain
HIV Care Costs
Annual HIV care costs in Spain for disease monitoring and the treatment and prevention of opportunistic and other infections were estimated from the results of a retrospective resource use study among individuals who received HIV care at one of four outpatient clinics in the Canary Islands in 2003.Citation1 Among other costs, this study reported annual direct costs for medications (including antiretroviral drugs) stratified by CD4 cell-count range and by HIV/AIDS status. As antiretroviral drug costs were already captured separately by the cost-analysis model, non-antiretroviral medication costs were estimated by excluding antiretroviral drug costs from the total medication costs. Total medication costs were reported by CD4 cell-count range and by HIV/AIDS status, but antiretroviral drug costs were reported only by HIV/AIDS status. Therefore, antiretroviral drug costs by CD4 cell-count range were estimated by mapping HIV/AIDS status to CD4 cell-count ranges. The model assumed that asymptomatic individuals had CD4 cell counts greater than 200 cells/µL, symptomatic individuals had CD4 cell counts between 51 and 200 cells/µL, and individuals with AIDS had CD4 cell counts less than 50 cells/µL. The resulting non-antiretroviral medication costs, and all other HIV care cost estimates, were inflated to 2015 eurosCitation2 and are summarized in Table 3 of the paper.
Probability Distributions Used in the Probabilistic Sensitivity Analysis
A probabilistic sensitivity analysis was performed to assess the joint impact of input parameter uncertainty on the model results by simultaneously varying all model input parameters for which there was sampling uncertainty. In each of the 10,000 Monte Carlo simulation runs, values for these model input parameters were sampled from the probability distributions summarized in Table A-1.
Table A-1 Input parameters and probability distributions used in the probabilistic sensitivity analysis
PSA = probabilistic sensitivity analysis.
aThe Dirichlet distribution is the multivariate generalization of the beta distribution and can be used when there are more than two categories that must sum to 100%.
Appendix references
| (1) | Lopez-Bastida J, Oliva-Moreno J, Perestelo-Perez L, Serrano-Aguilar P. The economic costs and health-related quality of life of people with HIV/AIDS in the Canary Islands, Spain. BMC Health Services Research. 2009;9(55). | ||||
| (2) | INEbase. Indice de Precios de Consumo, Base 2011. http://www.ine.es/dynt3/inebase/en/index.htm?padre=1936&capsel=1940. Accessed September 21, 2015. | ||||
| (3) | Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, et al. Efficacy and tolerability of three nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med. 2014 Oct 7;161(7):461-471. | ||||
| (4) | Ribaudo H, Wang H, Na L. A phase III comparative study of three non-nucleoside reverse transcriptase inhibitor (NNRTI)-sparing antiretroviral regimens for treatment-naïve HIV-1 infected volunteers (The ARDENT Study). A5257 Core Primary Analysis Report. Data on file. September 24, 2013. | ||||
| (5) | Llibre-Codina JM, Casado-Gomez MA, Sanchez-de la Rosa R, Perez-Elias MJ, Santos-Gonzales J, Miralles-Alverez C, et al. Costes de la toxicidad asociada a los análogos de nucleósidos inhibidores de la transcriptasa inversa en pacientes con infección por el VIH-1. Enferm Infecc Microbiol Clin. 2007;25(2):98-107. | ||||
| (6) | Spanish Healthcare Costs Database e-Salud. Oblikue Consulting. http://www.oblikue.com/bddcostes/. Accessed May 2015. | ||||