Abstract
Background: Exercise training has been shown to be an effective strategy to balance oxidative stress status; however, this is underexplored in people living with HIV/AIDS (PLWHA).
Objective: To evaluate the effects of exercise training on oxidative stress in PLWHA receiving antiretroviral therapy.
Methods: Patients performed 24 sessions (3 times per week, 8 weeks) of either aerobic (AT), resistance (RT), or concurrent training (CT). Glutathione disulphide to glutathione ratio (GSSG/GSH) in circulating erythrocytes and thiobarbituric acid–reactive substances (TBARS) in plasma samples were assessed as oxidative stress markers. Eight PLWAH completed the training protocol (AT =3, RT =3, CT =2). The GSSG/GSH and TBARS values were logarithmically transformed to approximate a normal distribution. A paired t-test was used to determine the differences between baseline and post-training values.
Results: Data-pooled analysis showed a decrease in GSSG/GSH and TBARS after the training period: log GSSG/GSH= –1.26 ± 0.57 versus –1.54 ± 0.65, p = .01 and log TBARS =0.73 ± 0.35 versus 0.43 ± 0.21, p = .01. This was paralleled by a rise in peak oxygen uptake (VO2peak = 29.14 ± 5.34 versus 32.48 ± 5.75 ml kg−1 min−1, p = .04). All the subjects who performed resistance exercises showed an average gain of 37 ± 8% in muscle strength with no difference between performing single or multiple sets in terms of muscle strength gain. The results reinforce the clinical importance of exercise as a rehabilitation intervention for PLWHA and emphasizes the safety of exercise at the physiological level with the potential to mediate health outcomes.
Introduction
People living with HIV/AIDS (PLWHA) usually present higher levels of oxidative stress, even those receiving highly active antiretroviral therapy (HAART).Citation 1 This condition may contribute to several aspects of HIV disease, including viral replication, imbalances of inflammatory responses, decreased immune cell proliferation,Citation 1 and increased cardiovascular risk.Citation 2
Considering a strategy to alleviate oxidative stress, exercise training (including for short periods) has been shown to be effective in healthy and in different chronic disease populations.Citation 3 However, few studies have investigated the effects of exercise training on oxidative stress in PLWHA. Therefore, the main goal of this study was to evaluate the effect of exercise training on oxidative stress in PLWHA receiving HAART.
Materials and methods
Participants
Patients from a Public Health Centre in Southern Brazil were invited to participate of this clinical trial. Eligible volunteers were PLWHA (18–59 years old), on a stable HAART regimen and without regular physical exercise for at least 6 months before the start of the training protocol. Subjects with physical and/or neurological limitations that could restrict the performance of physical exercise, antioxidant supplements, tobacco and/or drug use, and pregnant women were excluded from the study. Participants were advised to maintain their dietary pattern and not to use antioxidant supplements during the protocol period.
All patients signed an informed written consent and the study was approved by the Federal University of Health and Sciences of Porto Alegre Institutional Review Boards (n° 951/09) and was in accordance with the Declaration of Helsinki. The study is registered in Brazilian Clinical Trials Registry (RBR-7FNBZ7).
Cardiopulmonary exercise testing (CPET)
The CPET was performed on a treadmill using a ramp protocol.Citation 4 Ventilatory and metabolic parameters were collected breath-by-breath using Metalyzer 3B (Cortex, Germany). Two independent evaluators determined the ventilatory anaerobic threshold (VAT)Citation 5 and the respiratory compensation point (RCP).Citation 4
Muscular strength testing
Resistance and concurrent training groups were conducted to assess the muscular strength by the one-repetition maximum test (1-RM).Citation 6
Exercise training protocol
Participants were allocated into three training groups: (a) Aerobic (AT), (b) Resistance (RT), or (c) Concurrent (CT) training. Subjects performed three sessions per week for 8 weeks (24 sessions). CPET and 1-RM were performed before the exercise protocol (Baseline), after the 12th (Evaluation) and 24th (After) exercise sessions. For all the groups exercise intensity was adjusted in the 13th session based on the Evaluation data.
Aerobic training group comprised a 40 min period on a treadmill. Exercise intensity was monitored by heart rate (HR). In the first to sixth sessions, HR was ≈10% higher than the VAT; in the 7th–18th, HR was ≈20% higher than the VAT and from the session 19 to 24, HR was ≈10% lower than the RCP.
The RT group performed three sets of 10–12 repetitions of six exercises for major muscle groups. Between the 1st and 12th sessions, the RT group performed 60–65% of 1-RM and in the sessions 13th–24th, 70–75% of 1-RM.
The CT group performed 20 min of aerobic exercise on a treadmill and a single set of the same six exercises as per the RT group, at same intensity as in AT and RT groups.
Venous collection and general blood processing procedures
A blood sample was collected from each participant after a 12-h fasting period, before and after the training protocol. T CD4+ and T CD8+ lymphocytes were quantified by flow cytometry, using FACSCalibur™ system (BD Biosciences). Viral load (bDNA) was measured by the VERSANT HIV-1 RNA 3.0 Assay (Siemens, Germany). Glutathione disulphide to glutathione ratios (GSSG/GSH, an index of redox status) were measured in blood erythrocyte samples while thiobarbituric acid reactive substances (TBARS) were measured in plasma samples as previously described.Citation 7 , Citation 8
Statistical analysis
Pooled data’s normal distribution was tested using the Shapiro–Wilk test. GSSG/GSH ratios and TBARS values were logarithmically transformed to approximate a normal distribution. A paired Student’s t-test was used to compare the training protocol values. Kruskal–Wallis test was used to compare the three groups at baseline and after training protocol (p ≤ .05). GraphPad Prism version 5.00 for Windows (GraphPad Software) was used for statistical analysis.
Results
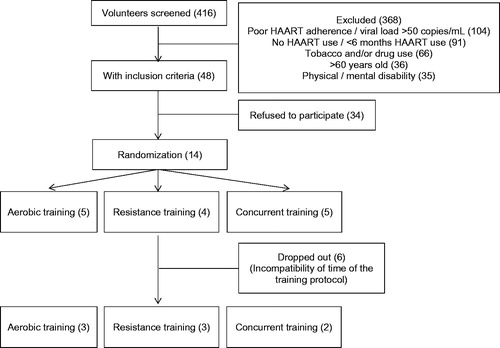
Four hundred and sixteen volunteers were screened and 368 were excluded. shows the screening and randomization diagram of the groups. Fourteen volunteers started the training protocol; five subjects were randomized to AT, 4 to RT and 5 to CT. Two subjects of the AT group, one from the RT group and three from the CT group dropped out of the training protocol, all of them due to schedule incompatibility. All included participants performed 24 exercise sessions. No injury or side effects related to exercise training were observed or reported during the training protocol.
Baseline and after-training protocol subjects’ characteristics are shown in . There were no differences between subjects from the different groups at baseline. Likewise, no difference was observed when comparing baseline values with those after exercise training in body mass, TCD4+ and TCD8+.
Table 1 Clinical characteristics of participants before and after training protocol
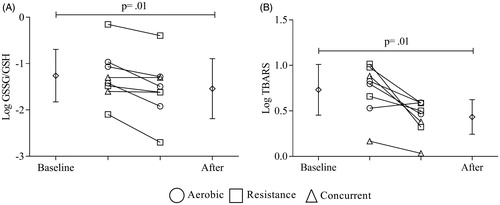
GSSG/GSH ratios and TBARS values decreased after all training protocols (log GSSG/GSH: –1.26 ± 0.57 versus –1.54 ± 0.65, p = .01; ; log TBARS: 0.73 ± 0.35 versus 0.43 ± 0.21, p = .01, ).
Figure 2 Effects of exercise training on oxidative stress. All subjects were considered on comparison. Diamonds represent the average values. Circle, square and triangle represent individual data before and after for aerobic, resistance and concurrent training, respectively. (A) log GSSG/GSH values and (B) log TBARS values before and after training protocol. A paired t-test was used to determine the differences between baseline and post-training values. Statistical significance was defined as p ≤ .05.
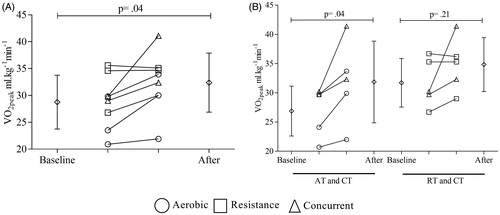
Pooled data’s analysis showed an increase in aerobic power after the training protocols (VO2peak: 29.1 ± 5.3 versus 32.5 ± 5.7 ml kg−1 min−1; p = .04, ). However, these results were more relevant when aerobic exercise was performed (AT and CT VO2peak: 26.9 ± 4.3 versus 31.9 ± 7.0 ml kg−1 min−1; p = .04; RT and CT VO2peak: 31.7 ± 4.2 versus 34.8 ± 4.6; p = .21; ). All subjects who performed resistance exercises (RT and CT) showed increases in muscle strength after the training protocol. The sum of average 1-RM strength increase was 37 ± 8% (baseline to after, p < .01) and there were no differences between performing single (36 ± 1%) or multiple sets (38 ± 10%) to gain muscle strength.
Figure 3 VO2peak values before and after training protocol. Diamonds represent the average values. Circles, squares and triangles represent individual data before and after for aerobic, resistance, and concurrent training, respectively. (A) All subjects were considered on comparison. (B) Leftmost part presents comparison between AT (aerobic training) plus CT (concurrent training) subjects whereas the rightmost partpresents comparison RT (resistance training) plus CT subjects. A paired t-test was used to determine the differences between baseline and post-training values. Statistical significance was defined as p ≤ .05.
Discussion
This study clearly shows that exercise training contributed to improve the redox status by decreasing GSSG/GSH and TBARS levels while enhancing physical fitness in PLWHA.
To the best of our knowledge, only two studiesCitation 9 , Citation 10 have evaluated the effects of exercise training on oxidative stress in PLWHA. In a previous study, our group showed that enzymatic antioxidant response to acute exercise is similar in PLWHA and healthy subjects,Citation 9 suggesting a positive effect of the exercise training to control oxidative stress, which was confirmed in this study. Moreover, the results presented here for TBARS levels are similar to those shown by Garcia et al. Citation 10 with a decrease in TBARS levels after the 20-week training protocol.
Additionally, improvement of redox status in trained patients may be promoted by exercise-induced expression of the 70 kDa family of heat shock proteins (HSP70) which, although not evaluated here, are well known to block nuclear factor-κB (NF-κB) having anti-inflammatory properties that are translated into lower oxidative stress.Citation 11 HSP70 inducers have been found to block NF-κB activation, and consequently, HIV-1 replication, in a process that is completely dependent on HSP70 expression.Citation 12 , Citation 13 Hence, one cannot discard the possibility that exercise training, a potent HSP70 inducer,Citation 11 have also beneficial effects on PLWHA by a combined action on antioxidant systems and viral machinery through the induction of HSP70.
In agreement with the literature, aerobic training promoted increase in VO2peak in participants of the AT and CT groupsCitation 14 and muscular strength in both groups that performed resistance exercise.Citation 15 The results presented herein showed no differences between performing single or multiple sets in terms of muscular strength gain. This is expected in untrained subjects because muscular strength gain, in the early stages, is mainly due to neuromuscular adaptation and a single-set of resistance exercises can promote this improvement.Citation 16
The high dropout rate of exercise training found in this study is recurrent in controlled trials with PLWHA.Citation 17–19 However, as the benefits of exercise training are similar to those reported in non-HIV subjects, combined exercise training with psychological support might be included to obtain exercise-induced benefits.Citation 20
The small sample size and the absence of a control group is a limitation of this study. Furthermore, the exclusion criteria were stringent to minimize other potential factors of oxidative stress and only virologically suppressed PLWHA were included in the protocol, which may limit the generalized application of the findings.
In summary, the results of this study indicate that exercise training improves aerobic and muscular fitness and suggest benefits on oxidative stress in PLWHA, emphasizing that exercise training may be an auxiliary antioxidant strategy thus complementing the medical management of this population, although more studies are needed to confirm these results.
Acknowledgments
We would like to thank all volunteers for their commitment to make this study possible.
Disclosure statement
Luís Fernando Deresz was supported by CAPES fellowship. No potential conflict of interest was reported by the remaining authors.
Additional information
Funding
References
- Sharma B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res. 2014;12(1):13–21.
- Masia M, Padilla S, Bernal E, et al. Influence of antiretroviral therapy on oxidative stress and cardiovascular risk: a prospective cross-sectional study in HIV-infected patients. Clin Ther. 2007;29(7):1448–1455.
- de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2016;47:277–293.
- Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225.
- Higa MN, Silva E, Neves VF, Catai AM, Gallo L, Silva de Sá MF. Comparison of anaerobic threshold determined by visual and mathematical methods in healthy women. Braz J Med Biol Res. 2007;40(4):501–508.
- Brzycki M. Strength testing - predicting a one-rep max from reps-to-fatigue J Phys Educ Recreation Dance. 1993;64(1):88–90.
- Kolberg A, Rosa TG, Puhl MT, et al. Low expression of MRP1/GS-X pump ATPase in lymphocytes of Walker 256 tumour-bearing rats is associated with cyclopentenone prostaglandin accumulation and cancer immunodeficiency. Cell Biochem Funct. 2006;24(1):23–39.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358.
- Deresz LF, Sprinz E, Kramer AS, et al. Regulation of oxidative stress in response to acute aerobic and resistance exercise in HIV-infected subjects: a case-control study. AIDS Care. 2010;22(11):1410–1417.
- Garcia A, Fraga GA, Vieira RC, et al. Effects of combined exercise training on immunological, physical and biochemical parameters in individuals with HIV/AIDS. J Sports Sci. 2014;32(8):785–792.
- Heck TG, Schöler CM, de Bittencourt PI. HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct. 2011;29(3):215–226.
- Rossi A, Kapahi P, Natoli G, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403(6765):103–108.
- Rozera C, Carattoli A, De Marco A, Amici C, Giorgi C, Santoro MG. Inhibition of HIV-1 replication by cyclopentenone prostaglandins in acutely infected human cells. Evidence for a transcriptional block. J Clin Invest. 1996;97(8):1795–1803.
- O'Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis. 2016;16(1):182.
- O'Brien K, Tynan AM, Nixon S, Glazier RH. Effects of progressive resistive exercise in adults living with HIV/AIDS: systematic review and meta-analysis of randomized trials. AIDS Care. 2008;20(6):631–653.
- Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674–688.
- Engelson ES, Agin D, Kenya S, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metab Clin Exp. 2006;55(10):1327–1336.
- Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med Sci Sports Exerc. 2006;38(3):411–417.
- Driscoll SD, Meininger GE, Lareau MT, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. Aids. 2004;18(3):465–473.
- Petróczi A, Hawkins K, Jones G, Naughton DP. HIV Patient Characteristics that Affect Adherence to Exercise Programmes: An Observational Study. Open AIDS J. 2010;4:148–155.